Introduction
Leukemia is the cancer of the body's blood-forming
tissues, including the bone marrow and the lymphatic system
(1). Despite advances in the
understanding of the etiology of leukemia and the
sub-classifications of the disease (2), the number of treatment alternatives for
patients remain limited. Bone marrow transplantation has curative
potential; however, this is only an option for a limited number of
patients. Typical chemotherapy may be associated with poor
efficacy, high toxicity and a limited survival time benefit
(3). Traditional Chinese medicine
(TCM), which originates from botanical, animal and mineral sources,
has provided a rich resource for anticancer drug discovery that is
attracting an increasing amount of interest (4).
Compound banmao capsule (CBC) is a traditional
Chinese medicinal formula composed of extracts from 11 organisms,
including Mylabris phalerata (banmao), Panax ginseng
(radix ginseng), Astragalus membranaceus (radix astragali),
Eleutherococcus senticosus (radix acanthopanax senticosus),
Rhizome sparganii, Scutellaria barbata, Curcuma
zedoaria, Cornus officinalis, Ligustrum lucidum
ait, Herba galii aparinis and Glycyrrhiza glabra L.
(radix glycyrrhizae). As the main component of CBC, banmao extract
shows potential for anti-tumor capability as it has previously been
demonstrated to directly kill tumor cells (5). Banmo has therefore been applied for the
treatment of primary liver and lung cancer, rectal carcinoma,
malignant lymphoma and gynecological tumors (5). However, the underlying mechanism for
anti-tumor activity by CBC remains unknown.
Seropharmacology is a novel method for
pharmacological study on Chinese materia medicain vitro
using drug-containing serum (6). The
essence of seropharmacology is the administration of drug to an
experimental animal (for example, a rabbit, rat or mouse), followed
by harvesting the animal blood and conducting in vitro
pharmacological experiments with the drug-containing animal serum.
The method has the same convenience as a typical in vitro
experiment. Seropharmacology also provides a bioactive metabolite
with the true pharmacological potency, having undergone
biotransformation in the body of the laboratory animal. Therefore,
it can be employed to study the biological effects of CBC.
In the present study, drug-containing animal serum
was prepared, and insight into the mechanism underlying the
anti-tumor effect of CBC was gained using the seropharmacological
method.
Materials and methods
Drugs and reagents
CBC (Shaanxi Huaxi Pharmaceutical Co., Ltd., Baoji,
China) consists of 11 traditional Chinese drugs including Mylabris
phalerata extract (banmao), radix ginseng, radix astragali, radix
acanthopanax senticosus, Rhizomasparganii, Scutellaria barbata,
Curcuma zedoaria, Cornus officinalis, Ligustrum lucidum ait. and
Herba galii aparinis extracts, andradix glycyrrhizae. A total of
one capsule of CBC was dissolved in 1 ml of PBS.
RPMI-1640 medium and fetal bovine serum (FBS) were
obtained from Gibco (Thermo Fisher Scientific, Inc., Waltham, MA,
USA). 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, propidium
iodide (PI), RNase, trypsin and
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
(MTS) were from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). The
Annexin V-Fluorescein Isothiocyanate Apoptosis Detection kit I was
obtained from BD Pharmingen (San Diego, CA, USA).
Cell culture
K562 and HL60 human leukemia cells were obtained
from the Institute of Biochemistry and Cell Biology, Chinese
Academy of Sciences (Shanghai, China). Cells were maintained in
RPMI-1640 medium supplemented with 10% FBS, 100 U/ml penicillin and
100 µg/ml streptomycin in 5% CO2 at 37°C. At confluency,
the cells were harvested with 0.25% trypsin in EDTA, counted and
seeded. Confluent cells were used for the present study.
Preparation of rat sera
The animal experiments were performed according to
the Guidelines for the Care and Use of Laboratory Animals (Zhejiang
University, Zhejiang, China). The present study was approved by the
Ethics Committee of The First Affiliated Hospital, College of
Medicine, Zhejiang University. Female Wistar rats weighing 200–250
g, aged 5–6 months were provided by the Shanghai Research Center
for Model Organisms (China), and housed in a room with 22–25°C
temperature, 50–60% relative humidity and a 12 h light/12 h dark
cycle. All animals had free access to food and water. A total of 16
rats were used for drug containing serum preparation. Rats were
randomly divided into vehicle control (n=8) and CBC (n=8) groups,
and intragastrical administration of PBS and CBC (3x rat weight/65
kg) was then performed for 7 days. Blood was obtained from the
heart of the rats (before sacrificing) following the last
administration and serum was acquired by blood centrifugation at
2,000 × g for 20 min at 4°C. Following two filtration procedures
with 0.22-µm cellulose acetate membranes, the sera were bottled,
heated to inactivate at 56°C for 30 min and stored at −20°C until
use.
Assessment of relative viability with
an MTS assay
K562 and HL60 cells were seeded into a 96-well plate
at a density of 104 cells/well and cultured in RPMI-1640
containing 10% drug-containing serum, control serum or control
serum with 10 µg/ml norcantharidin (Shanghai Tauto Biotech Co.,
Ltd., Shanghai, China) for up to 72 h in the previously described
conditions. The relative viability of the cells was quantified by
measuring the amount of MTS converted by the cells. After 24, 48 or
72 h incubation with the treatment, 20 µl MTS was added to each
well, incubated for another 2 h at 37°C and 5% CO2, and
25 µl/well of 10% SDS was subsequently added. The absorbance of
each plate at 490 nm was measured with a spectrophotometer. All
experiments were performed ≥3 times.
Detection of apoptosis with Annexin
V-fluorescein isothiocyanate (FITC)/PI staining
The rate of apoptosis in the K562 and HL60 cells was
detected using flow cytometry. The cells were maintained in
RPMI-1640 medium with 10% drug-containing or control serum for 72 h
in the previously described conditions. Subsequently, cells were
harvested, washed twice with PBS and resuspended in the binding
buffer to a final concentration of 106 cells/ml.
Following the addition of Annexin V-FITC/PI solution, the cells
were kept in the dark for 20 min at room temperature to stain the
damaged DNA of the apoptotic cells. Subsequent to filtration using
a 300-µm mesh strainer, the cells underwent apoptosis analysis by
flow cytometry (BD Biosciences, Franklin Lakes, NJ, USA; Canto II).
All experiments were performed ≥3 times.
Cell cycle distribution
assessment
Subsequent to incubating the K562 and HL60 cells
with drug-containing or control serum for 72 h, the harvested cells
were seeded to a concentration of 106 cells/ml, fixed
with 70% ice-cold ethanol, incubated with 0.25% RNase at 37°C for
30 min and stained with 50 µg/ml PI according to the manufacturer's
protocol. Subsequent to filtration with a 300-µm mesh strainer, the
cells underwent cell cycle analysis by flow cytometry. All
experiments were performed independently ≥3 times.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Subsequent to incubating human leukemia cell lines
with drug-containing or control serum for 72 h, the total RNA from
the harvested cells was extracted using TRIzol reagent (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. RT-qPCR was performed with a One Step SYBR®
PrimeScript™ RT-qPCR kit (Takara Biotechnology Co., Ltd., Dalian,
China) and an iQ5 Real-time PCR Detection system (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). The procedure was as
follows: 95°C for 1 min for 40 cycles (95°C for 10 sec and 60°C 1
min). The mRNA of VEGF-A and chemotactic and inflammatory genes was
detected with specific primers (Sangon Biotech Co., Ltd, Shanghai,
China), as listed in Table I.
Expression of the GAPDH gene was assessed simultaneously in all
samples as an internal control. Relative gene expression was
determined using the 2−ΔΔCq method (7). All examinations were repeated three
times.
 | Table I.The primers used in the reverse
transcription-quantitative polymerase chain reaction for detection
of relative expression levels of genes. |
Table I.
The primers used in the reverse
transcription-quantitative polymerase chain reaction for detection
of relative expression levels of genes.
| Genes | Primers (5′ to
3′) |
|---|
| VEGF-F |
CGCAGCTACTGCCATCCAAT |
| VEGF-R |
GTGAGGTTTGATCCGCATAATCT |
| CCR1-F |
TCCTGCTGACGATTGACAGGTA |
| CCR1-R |
GTGCCCGCAAGGCAAAC |
| CCR2-F |
GCGTTTAATCACATTCGAGTGTTT |
| CCR2-R |
CCACTGGCAAATTAGGGAACAA |
| CCR4-F |
CAATACTGTGGGCTCCTCCAA |
| CCR4-R |
ATCCATGGTGGACTGCGTGTA |
| CCR5-F |
GCTGGTCATCCTCATCCTGATAA |
| CCR5-R |
ATGGCCAGGTTGAGCAGGTA |
| CCL5-F |
TCCCGAACCCATTTCTTCTCT |
| CCL5-R |
CCCAGCAGTCGTCTTTGTCA |
| CXCL1-F |
AGGGAATTCACCCCAAGAAC |
| CXCL1-R |
ACTATGGGGGATGCAGGATT |
| CCND1-F |
GGCGGAGGAGAACAAACAGA |
| CCND1-R |
TGGCACAAGAGGCAACGA |
| DDX1-F |
AGCCAAGATGCAGGAAAGATG |
| DDX1-R |
GCTATAAAGGCCATGTGGATATTTTG |
| DDK3-F |
GAAACTGCTCTGGTCTTCACTAGCT |
| DDK3-R |
CTCCTCGTCCATCAGGGATCT |
| HMGB2-F |
CTTGGCACGATATGCAGCAA |
| HMGB2-R |
CAGCCAAAGATAAACAACCATATGA |
| IL6-F |
TGCGTCCGTAGTTTCCTTCT |
| IL6-R |
GCCTCAGACATCTCCAGTCC |
Statistical analysis
All statistical analyses were performed using SPSS
17.0 software (SPSS Inc., Chicago, IL, USA). Data are presented as
the mean ± standard deviation. A Student's t test was employed to
identify the significance of the difference between groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
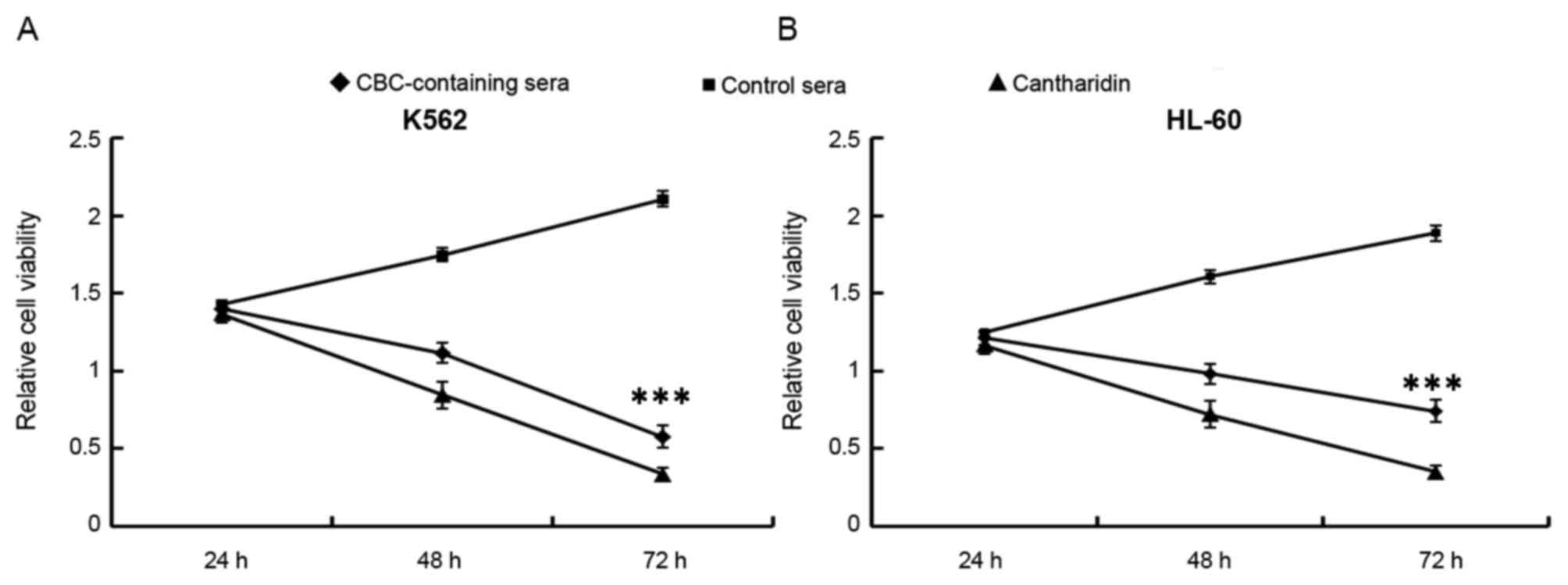
Treatment with CBC-containing serum
reduces the relative viability of human leukemia cells
As demonstrated in Fig.
1, the relative viability of human leukemia K562 (P=0.00198;
Fig. 1A) and HL60 (P=0.00266;
Fig. 1B) cells at 72 h were
significantly reduced by treatment with CBC-containing serum
compared with treatment with control serum. The relative viability
rates of K562 and HL60 cells treated with CBC-containing serum at
72 h were 80.35±2.64 and 76.43±2.31%, respectively.
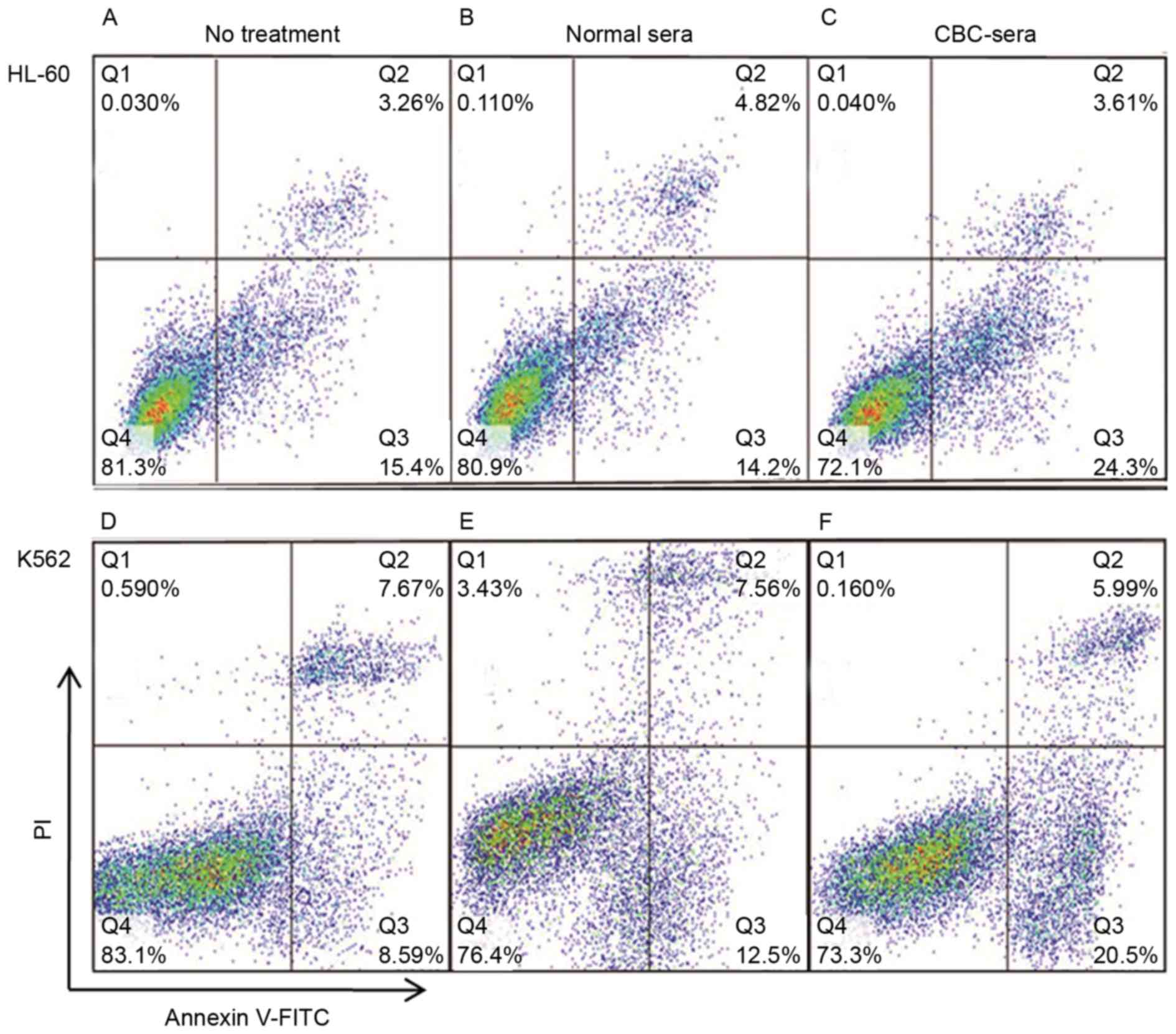
Treatment with CBC-containing serum
increases the rate of apoptosis in human leukemia cells
Subsequent to incubating with CBC-containing or
control serum for 72 h, the rate of apoptosis in HL60 and K562
cells was analyzed using flow cytometry (Fig. 2). In HL60 cells, the apoptosis rate
was 9.10±2.01% for FBS-treated cells (Fig. 2A), 18.33±3.13% for the control
serum-treated cells (Fig. 2B) and
28.99±1.33% for the CBC-containing serum-treated cells (Fig. 2C). In K562 cells, a similar trend to
that observed in HL60 cells was identified; the rate was 6.88±1.12%
for FBS treated cells (Fig. 2D),
10.25±3.22% for control serum-treated cells (Fig. 2E) and 31.38±4.45% for the
CBC-containing serum-treated cells (Fig.
2F).
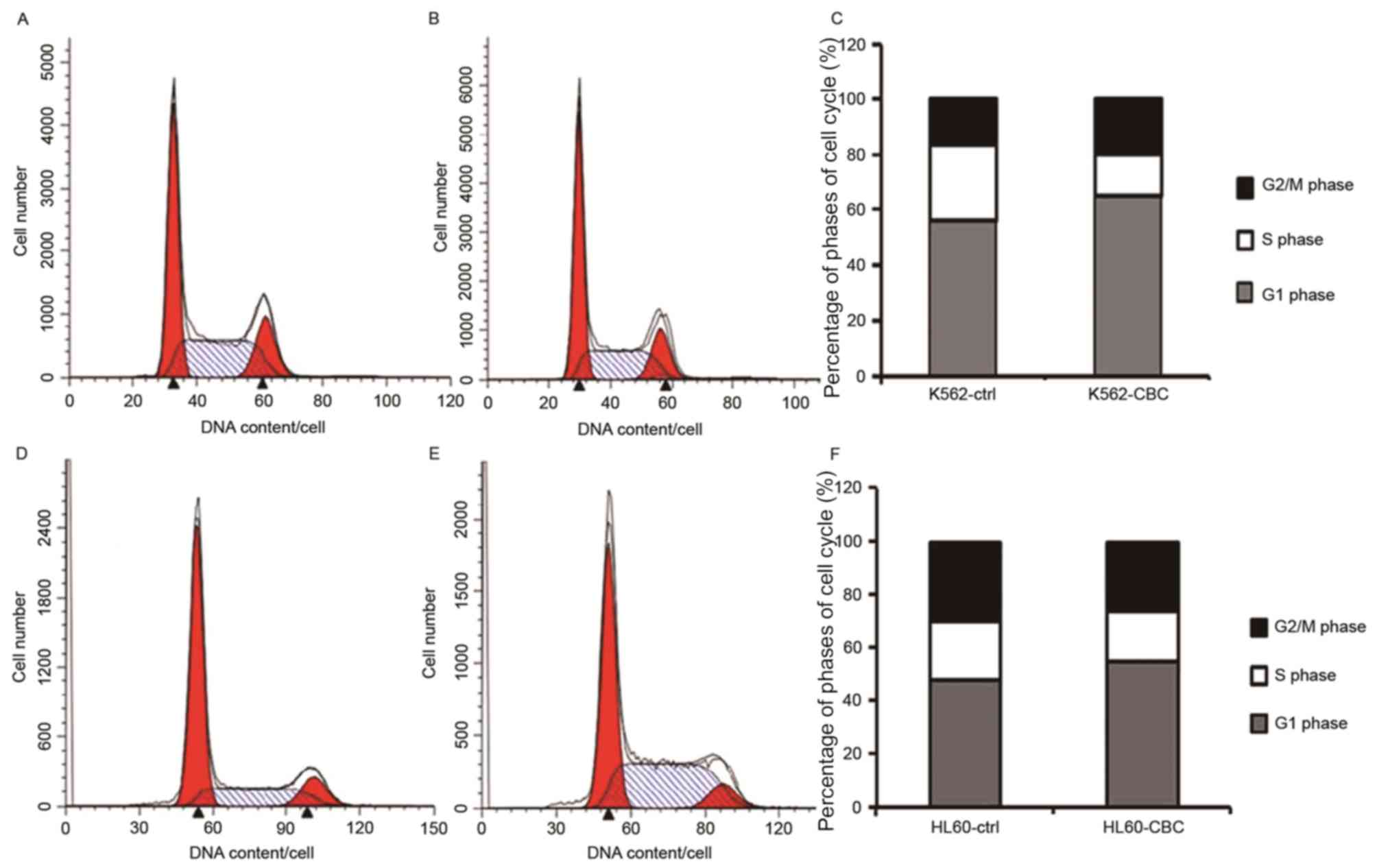
Treatment with CBC-containing serum
alters the cell cycle distribution of human leukemia cells
Subsequent to incubation with CBC-containing serum,
a cell cycle arrest was observed in HL60 and K562 cells at 48–72 h
(Fig. 3). For HL60 cells, the
proportion of S phase cells was 19.22% in CBC-containing
serum-treated group and 22.33% in the control serum group (Fig. 3C). In K562 cells, the ratio of S phase
cells was 15.42% in CBC-containing serum-treated group and 27.79%
in the control serum group (Fig.
3F).
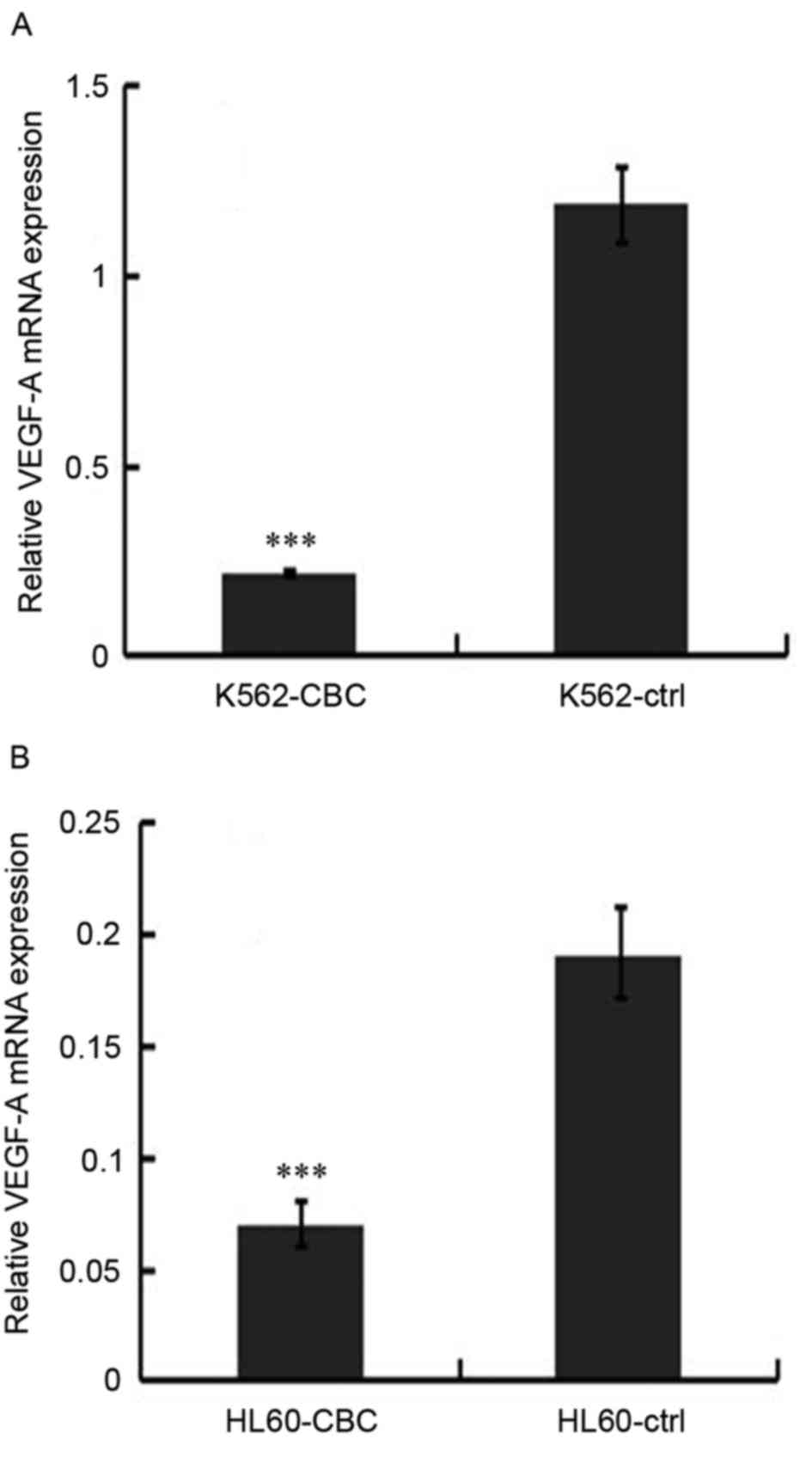
Treatment with CBC-containing serum
decreases the mRNA expression of VEGF-A in human leukemia
cells
As VEGF-A is an important protein in the promotion
of angiogenesis, its mRNA expression level was assessed using
RT-qPCR. As demonstrated in Fig. 4,
decreased gene expression of VEGF-A mRNA was observed in HL60 and
K562 cells treated by CBC-containing serum; the fold changes were
5.67 for K562 cells (Fig. 4A) and 2.7
for HL60 cells (Fig. 4B).
Effect of CBC-containing serum on the
mRNA expression of chemotactic and inflammatory genes in human
leukemia cells
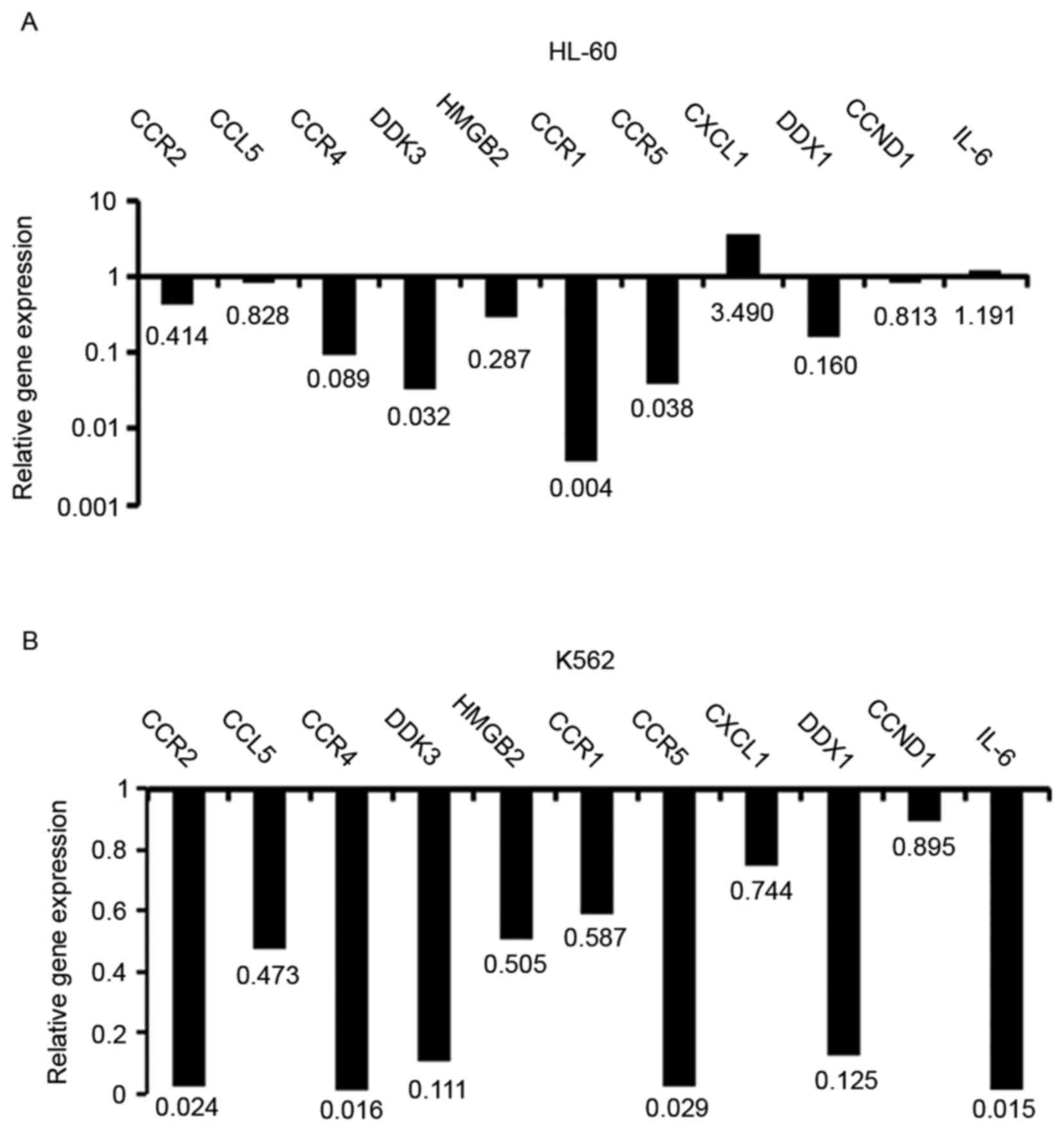
The mRNA expression of chemotactic and inflammatory
genes, including C-C motif chemokine receptor (CCR)2, C-C motif
chemokine ligand (CCL)5, CCR4, Dickkopf 3 (DDK3), high mobility
group box (HMGB)2, CCR1, CCR5, C-X-C motif chemokine ligand
(CXCL)1, DEAD-box helicase (DDX)1, cyclin D1 (CCND1) and
interleukin (IL)-6, were detected using RT-qPCR. As demonstrated by
Fig. 5, decreased mRNA expression of
CCR2, CCR4, DDK3, HMGB2, CCR1, CCR5 and DDX1 and increased
expression of CXCL1 were identified in HL60 cells following
treatment with CBC-containing serum (Fig.
5A). Decreased mRNA expression of CCR2, CCL5, CCR4, DDK3, CCR5,
DDX1, CCND1 and IL-6 was identified in K562 cells treated with
CBC-containing serum (Fig. 5B).
Discussion
Seropharmacology is a novel method for the
pharmacological study of Chinese materia medica (8). It has the advantages of in vitro
experiment, including convenient control of the experimental
conditions and the exclusion of interference of internal factors,
which facilitate the in-depth study of the mechanism of action for
a drug. Seropharmacology reflects the drug metabolism process
(including absorption, distribution, metabolism and excretion) of
the body, and the drug in the animal serum is in the form of
bioactive metabolites with the real pharmacological functions of
interest. A number of substances (including secreted cell factor,
hormones, antibodies and complement) induced by drug in the body
are then retained in the animal serum and can be applied to an
in vitro experiment, important for the investigation of
indirect effects of a drug. In addition, Chinese herbal preparation
is likely to be affected by various factors (including chemicals,
ions, pH, purity and osmotic pressure) that could result in changes
of the outcome of an in vitro experiment. Therefore,
seropharmacology is quite suitable for pharmacological study on
Chinese herbal preparation (9,10). For
this reason, CBC-containing serum was prepared for the present
study.
CBC is a traditional Chinese medicinal preparation
consisting of 11 TCM drugs. Among these 11 drugs, banmao and radix
acanthopanax senticosus have been widely applied in TCM cancer
therapy (11). Banmao is the dried
body of the Chinese blister beetle, which has been used from
antiquity to the present day in folk medicine (12,13).
Banmao can directly inhibit the proliferation of tumor cells. The
active constituent of Banmao is cantharidin, which occurs in the
dried body at a concentration of 0.6–1.9%. A previous study
demonstrated that cantharidin is a strong protein phosphatase and
phosphatase 2A inhibitor, and can be used to inhibit cAMP
phosphodiesterase activity in hepatoma cells (14). Cantharidin has been used to treat
various types of tumor, including bowel (15), mammary (16) and gallbladder (17) cancer and leukemia, through inhibiting
cellular proliferation and angiogenesis, and inducing cell cycle
arrest and apoptosis. In primary hepatic carcinoma cells,
cantharidin has been demonstrated to exhibit greater cytotoxic
effects against tumor cells compared with those observed in normal
cells, and may induce a G2/M cell cycle arrest in carcinoma cells
(18). Dorn et al (19) reported that cantharidin induced
apoptosis, inhibited proliferation and caused cell cycle arrest in
Jurkat cells. Cantharidin has also been reported to exhibit an
antitumor effect on acute and chronic myeloid leukemia cells
through the induction of apoptosis and the inhibition of
proliferation (20).
Distinct from the anticancer mechanism of Banmao,
stimulation (21,22) and suppression (23,24) of the
immune response have been proposed as mechanisms for the antitumor
effect of radix acanthopanax senticosus, a plant stem extract, the
main anticancer constituents of which are glucosides and
polysaccharides. Yoon et al (25) investigated the antitumor activity of
an aqueous acanthopanax senticosus solution; its anti-tumor effect
was reported to be associated with the activation of macrophages
and natural killer cells (25). In
TCM clinical treatment, banmao is used to cure patients of their
cancer, whereas acanthopanax senticosus is used for cancer
prevention (26). In the present
study, a significant reduction in relative cell viability, an
increase in the rate of apoptosis, and a
G0/G1 cell cycle arrest were identified.
Therefore, in addition to previous reports that CBC-containing
serum can exert an antitumor effect on liver carcinoma cells
(27,28), its efficacy on leukemia cells has now
been demonstrated.
In the present study, a decreasing level of VEGF-A,
the main subtype of VEGF, was identified in HL60 and K562 cells
subsequent to treatment with CBC-containing serum. According to
previous reports, VEGF-associated angiogenesis performs a critical
role in the pathogenesis of hematopoietic malignancies (29,30). VEGF
expression may mediate apoptosis resistance and thus, radiotherapy
resistance (28). Therefore, an
effect against VEGF could be an important mechanism for the
treatment of leukemia. Our previous study demonstrated that
angiogenesis performed a critical role in the pathological process
of acute leukemia, and that the expression level of VEGF was
closely associated with the degree of angiogenesis and the disease
progression in patients with leukemia (31). At present, a number of drugs targeting
angiogenesis have been successful in the treatment of acute and
chronic myeloid leukemia (32).
A number of studies (33–35) have
demonstrated that inflammatory and chemotactic genes are closely
associated with hematopoietic malignancies, which is why it was
considered necessary to assess the status of inflammatory and
chemotactic genes in the present study. Downregulation of chemokine
gene mRNA expression was identified in HL60 and K562 cells treated
with CBC-containing serum; however, differential expression of IL6
was identified between HL60 and K562 cells. This may be due to the
different types of leukemia represented by HL60 and K562, as HL60
was established from acute myeloid leukemia and K562 from chronic
myeloid leukemia.
In conclusion, a seropharmacological method was used
to prepare CBC-containing rat serum to evaluate its effect on human
leukemia HL60 and K562 cells. A reduction in cell viability,
increased apoptosis and cell cycle arrest were all observed
following treatment with CBC-containing serum. Furthermore, the
mRNA expression of VEGF-A and chemotactic and inflammatory genes
was attenuated following treatment with CBC-containing serum.
Additional studies are required to investigate the efficacy of the
combination of CBC with other anticancer drugs in the clinical
practice.
Acknowledgements
The present study was supported by Chinese Medicine
Scientific Research Foundation of Zhejiang Province (grant no.
2010B501720), Zhejiang Provincial Department of Education (grant
no. Y201120523) and Zhejiang Provincial Administration of
Traditional Chinese Medicine (grant no. Y201120523).
References
|
1
|
Vardiman JW: The World Health Organization
(WHO) classification of tumors of the hematopoietic and lymphoid
tissues: An overview with emphasis on the myeloid neoplasms. Chem
Biol Interact. 184:16–20. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li FF, Yi S, Wen L, He J, Yang LJ, Zhao J,
Zhang BP, Cui GH and Chen Y: Oridonin induces NPM mutant protein
translocation and apoptosis in NPM1c+ acute myeloid leukemia cells
in vitro. Acta Pharmacol Sin. 35:806–813. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hu XM, Yuan B, Tanaka S, Song MM, Onda K,
Tohyama K, Zhou AX, Toyoda H and Hirano T: Arsenic
disulfide-triggered apoptosis and erythroid differentiation in
myelodysplastic syndrome and acute myeloid leukemia cell lines.
Hematology. 19:352–360. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Man S, Gao W, Wei C and Liu C: Anticancer
drugs from traditional toxic Chinese medicines. Phytother Res.
26:1449–1465. 2012.PubMed/NCBI
|
|
5
|
Liu D and Chen Z: The effects of
cantharidin and cantharidin derivates on tumour cells. Anticancer
Agents Med Chem. 9:392–396. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xu H, Wu Q, Peng C and Zhou L: Study on
the antiviral activity of San Huang Yi Gan Capsule against
hepatitis B virus with seropharmacological method. BMC Complement
Altern Med. 13:2392013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bochu W, Liancai Z and Qi C: Primary study
on the application of Serum Pharmacology in Chinese traditional
medicine. Colloids Surf B Biointerfaces. 43:194–197. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu Q, Rong X, Huang P, Han J and Xu H:
Study on inhibitory actions of san huang yi gan capsule (SHYGC) on
HBeAg with seropharmacological method. Zhong Yao Cai. 23(2):
75–278. 2000.(In Chinese).
|
|
10
|
Zhang YH, Liu JT, Wen BY and Liu N:
Mechanisms of inhibiting proliferation of vascular smooth muscle
cells by serum of rats treated with Dahuang Zhechong pill. J
Ethnopharmacol. 124:125–129. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Han SB, Yoon YD, Ahn HJ, Lee HS, Lee CW,
Yoon WK, Park SK and Kim HM: Toll-like receptor-mediated activation
of B cells and macrophages by polysaccharide isolated from cell
culture of Acanthopanax senticosus. Int Immunopharmacol.
3:1301–1312. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang GS: Medical uses of mylabris in
ancient China and recent studies. J Ethnopharmacol. 26:147–162.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tagwireyi D, Ball DE, Loga PJ and Moyo S:
Cantharidin poisoning due to ‘Blister beetle’ ingestion. Toxicon.
38:1865–1869. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Graziano MJ, Pessah IN, Matsuzawa M and
Casida JE: Partial characterization of specific cantharidin binding
sites in mouse tissues. Mol Pharmacol. 33:706–712. 1988.PubMed/NCBI
|
|
15
|
Peng F, Wei YQ, Tian L, Yang L, Zhao X, Lu
Y, Mao YQ, Kan B, Lei S, Wang GS, et al: Induction of apoptosis by
norcantharidin in human colorectal carcinoma cell lines:
Involvement of the CD95 receptor/ligand. J Cancer Res Clin Oncol.
128:223–230. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang Y, Liu Q, Liu K, Yagasaki K and
Zhang G: Suppression of growth of highly-metastatic human breast
cancer cells by norcantharidin and its mechanisms of action.
Cytotechnology. 59:2092009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fan YZ, Fu JY, Zhao ZM and Chen CQ:
Inhibitory effect of norcantharidin on the growth of human
gallbladder carcinoma GBC-SD cells in vitro. Hepatobiliary Pancreat
Dis Int. 6:72–80. 2007.PubMed/NCBI
|
|
18
|
Huan SK, Lee HH, Liu DZ, Wu CC and Wang
CC: Cantharidin-induced cytotoxicity and cyclooxygenase 2
expression in human bladder carcinoma cell line. Toxicology.
223:136–143. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dorn DC, Kou CA, Png KJ and Moore MA: The
effect of cantharidins on leukemic stem cells. Int J Cancer.
124:2186–2199. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yi SN, Wass J, Vincent P and Iland H:
Inhibitory effect of norcantharidin on K562 human myeloid leukemia
cells in vitro. Leuk Res. 15:883–886. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Schmeda-Hirschmann G, Villaseñor-García
MM, Lozoya X and Puebla-Pérez AM: Immunomodulatory activity of
Chilean Cyttaria species in mice with L5178Y lymphoma. J
Ethnopharmacol. 77:253–257. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schmolz MW, Sacher F and Aicher B: The
synthesis of Rantes, G-CSF, IL-4, IL-5, IL-6, IL-12 and IL-13 in
human whole-blood cultures is modulated by an extract from
Eleutherococcus senticosus L. roots. Phytother Res. 15:268–270.
2001. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jeong HJ, Koo HN, Myung NI, Shin MK, Kim
JW, Kim DK, Kim KS, Kim HM and Lee YM: Inhibitory effects of mast
cell-mediated allergic reactions by cell cultured Siberian Ginseng.
Immunopharmacol Immunotoxicol. 23:107–117. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yi JM, Kim MS, Seo SW, Lee KN, Yook CS and
Kim HM: Acanthopanax senticosus root inhibits mast cell-dependent
anaphylaxis. Clin Chim Acta. 312:163–168. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yoon TJ, Yoo YC, Lee SW, Shin KS, Choi WH,
Hwang SH, Ha ES, Jo SK, Kim SH and Park WM: Anti-metastatic
activity of Acanthopanax senticosus extract and its possible
immunological mechanism of action. J Ethnopharmacol. 93:247–253.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang Q, Wang Y, Liu R, Yan X, Li Y, Fu H,
Bi K and Li Q: Comparison of the effects of Mylabris and
Acanthopanax senticosus on promising cancer marker polyamines in
plasma of a Hepatoma-22 mouse model using HPLC-ESI-MS. Biomed
Chromatogr. 27:208–215. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chang C, Zhu YQ, Mei JJ, Liu SQ and Luo J:
Involvement of mitochondrial pathway in NCTD-induced cytotoxicity
in human hepG2 cells. J Exp Clin Cancer Res. 29:1452010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zheng LH, Bao YL, Wu Y, Yu CL, Meng X and
Li YX: Cantharidin reverses multidrug resistance of human hepatoma
HepG2/ADM cells via down-regulation of P-glycoprotein expression.
Cancer Lett. 272:102–109. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Avramis IA, Kwock R and Avramis VI:
Taxotere and vincristine inhibit the secretion of the angiogenesis
inducing vascular endothelial growth factor (VEGF) by wild-type and
drug-resistant human leukemia T-cell lines. Anticancer Res.
21:2281–2286. 2001.PubMed/NCBI
|
|
30
|
Katoh O, Tauchi H, Kawaishi K, Kimura A
and Satow Y: Expression of the vascular endothelial growth factor
(VEGF) receptor gene, KDR, in hematopoietic cells and inhibitory
effect of VEGF on apoptotic cell death caused by ionizing
radiation. Cancer Res. 55:5687–5692. 1995.PubMed/NCBI
|
|
31
|
Ye X, Wang LJ, Lin MF and Ding W: The
clinical significance of angiogenesis in the bone marrow of acute
leukemia patients. Zhonghua Nei Ke Za Zhi. 42:486–489. 2003.(In
Chinese). PubMed/NCBI
|
|
32
|
Ye XJ and Lin MF: Homoharringtonine
induces apoptosis of endothelium and down-regulates VEGF expression
of K562 cells. J Zhejiang Univ Sci. 5:230–234. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Aggarwal BB, Shishodia S, Sandur SK,
Pandey MK and Sethi G: Inflammation and cancer: How hot is the
link? Biochem Pharmacol. 72:1605–1621. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gasparini C, Celeghini C, Monasta L and
Zauli G: NF-κB pathways in hematological malignancies. Cell Mol
Life Sci. 71:2083–2102. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Candido J and Hagemann T: Cancer-related
inflammation. J Clin Immunol. 33:(Suppl 1). S79–S84. 2013.
View Article : Google Scholar : PubMed/NCBI
|