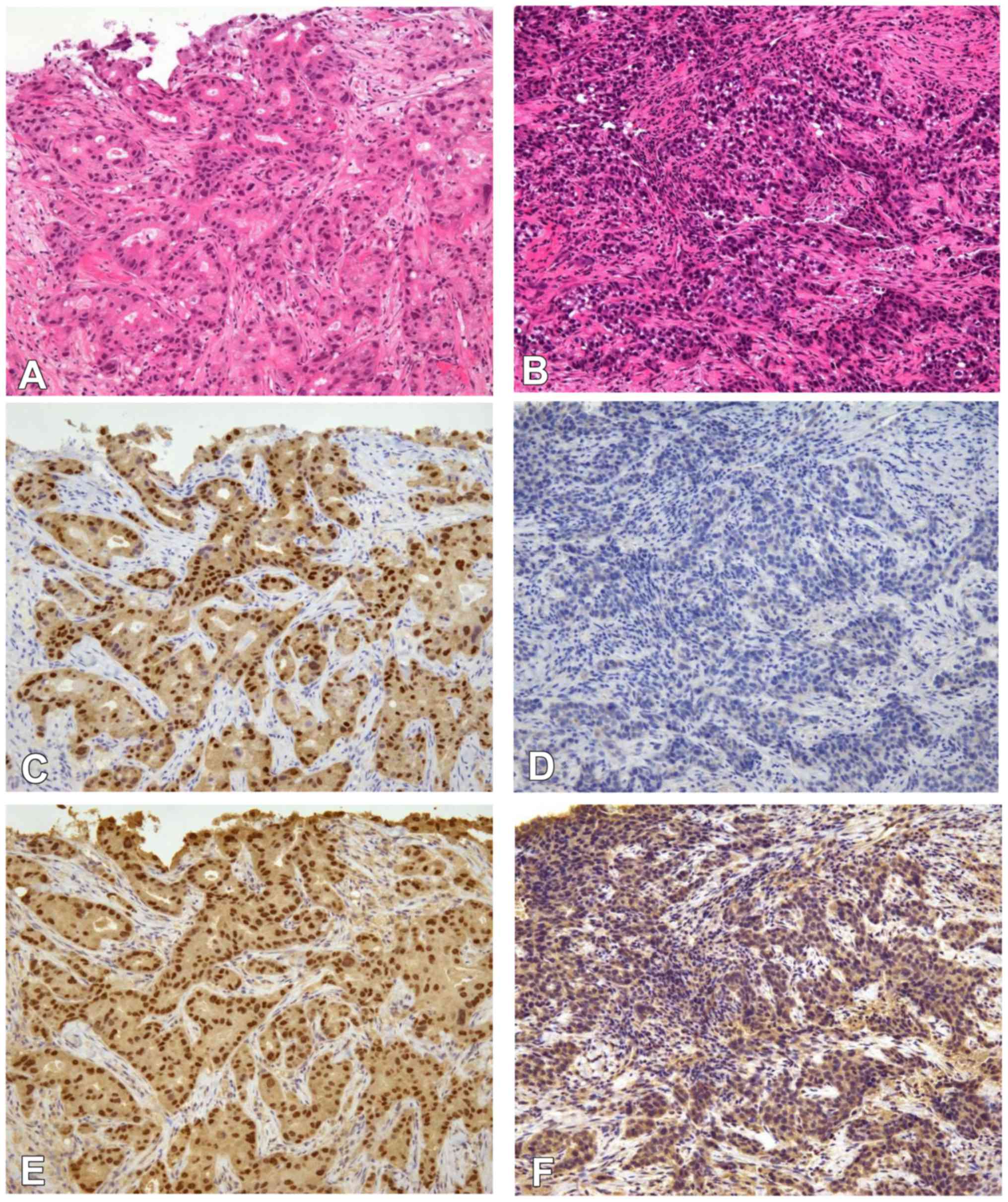
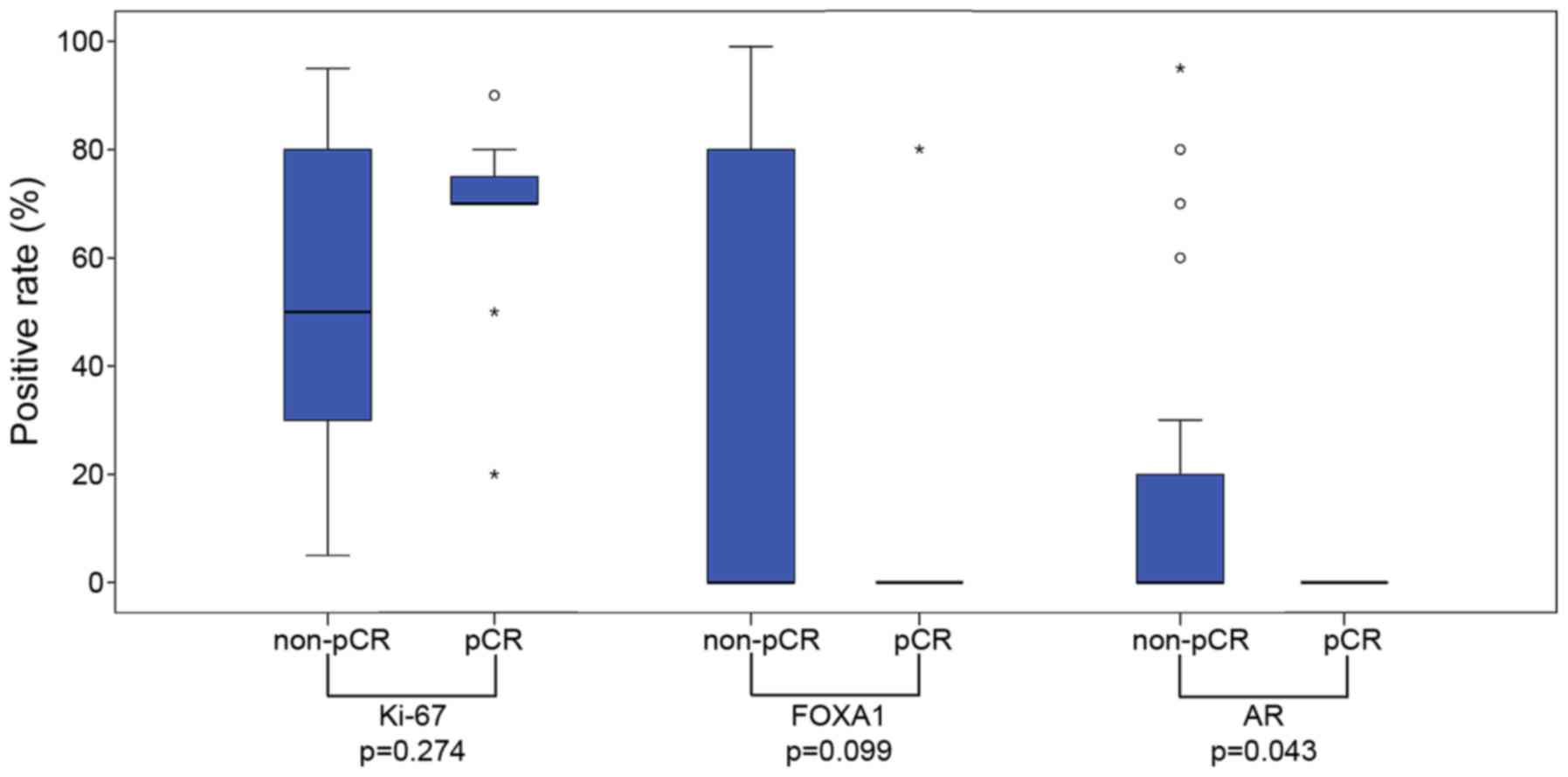
|
1
|
Bauer KR, Brown M, Cress RD, Parise CA and
Caggiano V: Descriptive analysis of estrogen receptor
(ER)-negative, progesterone receptor (PR)-negative, and
HER2-negative invasive breast cancer, the so-called triple-negative
phenotype: A population-based study from the California cancer
registry. Cancer. 109:1721–1728. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Carey LA, Dees EC, Sawyer L, Gatti L,
Moore DT, Collichio F, Ollila DW, Sartor CI, Graham ML and Perou
CM: The triple negative paradox: Primary tumor chemosensitivity of
breast cancer subtypes. Clin Cancer Res. 13:2329–2334. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Palma G, Frasci G, Chirico A, Esposito E,
Siani C, Saturnino C, Arra C, Ciliberto G, Giordano A and D'Aiuto
M: Triple negative breast cancer: Looking for the missing link
between biology and treatments. Oncotarget. 6:26560–26574. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lehmann BD, Bauer JA, Chen X, Sanders ME,
Chakravarthy AB, Shyr Y and Pietenpol JA: Identification of human
triple-negative breast cancer subtypes and preclinical models for
selection of targeted therapies. J Clin Invest. 121:2750–2767.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lehmann BD, Jovanović B, Chen X, Estrada
MV, Johnson KN, Shyr Y, Moses HL, Sanders ME and Pietenpol JA:
Refinement of triple-negative breast cancer molecular subtypes:
Implications for neoadjuvant chemotherapy selection. PLoS One.
11:e01573682016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rouzier R, Perou CM, Symmans WF, Ibrahim
N, Cristofanilli M, Anderson K, Hess KR, Stec J, Ayers M, Wagner P,
et al: Breast cancer molecular subtypes respond differently to
preoperative chemotherapy. Clin Cancer Res. 11:5678–5685. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nwaogu I, Fayanju O, Jeffe D and
Margenthaler J: Predictors of pathological complete response to
neoadjuvant chemotherapy in stage II and III breast cancer: The
impact of chemotherapeutic regimen. Mol Clin Oncol. 3:1117–1122.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liedtke C, Mazouni C, Hess KR, André F,
Tordai A, Mejia JA, Symmans WF, Gonzalez-Angulo AM, Hennessy B,
Green M, et al: Response to neoadjuvant therapy and long-term
survival in patients with triple-negative breast cancer. J Clin
Oncol. 26:1275–1281. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
von Minckwitz G, Untch M, Blohmer JU,
Costa SD, Eidtmann H, Fasching PA, Gerber B, Eiermann W, Hilfrich
J, Huober J, et al: Definition and impact of pathologic complete
response on prognosis after neoadjuvant chemotherapy in various
intrinsic breast cancer subtypes. J Clin Oncol. 30:1796–1804. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dawood S, Broglio K, Kau SW, Green MC,
Giordano SH, Meric-Bernstam F, Buchholz TA, Albarracin C, Yang WT,
Hennessy BT, et al: Triple receptor-negative breast cancer: The
effect of race on response to primary systemic treatment and
survival outcomes. J Clin Oncol. 27:220–226. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Masuda H, Baggerly KA, Wang Y, Zhang Y,
Gonzalez-Angulo AM, Meric-Bernstam F, Valero V, Lehmann BD,
Pietenpol JA, Hortobagyi GN, et al: Differential response to
neoadjuvant chemotherapy among 7 triple-negative breast cancer
molecular subtypes. Clin Cancer Res. 19:5533–5540. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
The Japanese Breast Cancer Society:
General Rules for Clinical and Pathological Recording of Breast
Cancer. 17th. Kanehara & Co., Ltd.; Tokyo, Japan: 2012
|
|
13
|
Japan Radiology Society Japanese Society
of Radiologic Technology: Mammography GuidelineJapan Central
Organization on Quality Assurance of Breast Cancer Screening (ed).
3rd. IGAKU-SHOIN Ltd.; Tokyo, Japan: 2010
|
|
14
|
Sobin LH, Gospodarowicz MK and Wittekind
C: International Union Against Cancer: TNM Classification of
Malignant Tumors. 7th. Wiley-Blackwell; New York: 2011
|
|
15
|
Donepudi MS, Kondapalli K, Amos SJ and
Venkanteshan P: Breast cancer statistics and markers. J Cancer Res
Ther. 10:506–511. 2014.PubMed/NCBI
|
|
16
|
Dnistrian AM, Schwartz MK, Greenberg EJ,
Smith CA and Schwartz DC: Evaluation of CA M26, CA M29, CA 15-3 and
CEA as circulating tumor markers in breast cancer patients. Tumour
Biol. 12:82–90. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ichihara S and Aoyama H: Intraductal
carcinoma of the breast associated with high levels of circulating
tumor-associated antigens (CA 15-3 and NCC-ST-439). Cancer.
73:2181–2185. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wolff AC, Hammond ME, Hicks DG, Dowsett M,
McShane LM, Allison KH, Allred DC, Bartlett JM, Bilous M,
Fitzgibbons P, et al: Recommendations for human epidermal growth
factor receptor 2 testing in breast cancer: American Society of
Clinical Oncology/College of American Pathologists clinical
practice guideline update. J Clin Oncol. 31:3997–4013. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Greenwood HI, Heller SL, Kim S, Sigmund
EE, Shaylor SD and Moy L: Ductal carcinoma in situ of the breasts:
Review of MR imaging features. Radiographics. 33:1569–1588. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li J, Chen C, Gu Y, Di G, Wu J, Liu G and
Shao Z: The role of mammographic calcification in the neoadjuvant
therapy of breast cancer imaging evaluation. PLoS One.
9:e888532014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bae MS, Park SY, Song SE, Kim WH, Lee SH,
Han W, Park IA, Noh DY and Moon WK: Heterogeneity of
triple-negative breast cancer: Mammographic, US and MR imaging
features according to androgen receptor expression. Eur Radiol.
25:419–427. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Asano Y, Kashiwagi S, Onoda N, Kurata K,
Morisaki T, Noda S, Takashima T, Ohsawa M, Kitagawa S and Hirakawa
K: Clinical verification of sensitivity to preoperative
chemotherapy in cases of androgen receptor-expressing positive
breast cancer. Br J Cancer. 114:14–20. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hennessy BT, Gonzalez-Angulo AM,
Stemke-Hale K, Gilcrease MZ, Krishnamurthy S, Lee JS, Fridlyand J,
Sahin A, Agarwal R, Joy C, et al: Characterization of a naturally
occurring breast cancer subset enriched in
epithelial-to-mesenchymal transition and stem cell characteristics.
Cancer Res. 69:4116–4124. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Denkert C, Loibl S, Müller BM, Eidtmann H,
Schmitt WD, Eiermann W, Gerber B, Tesch H, Hilfrich J, Huober J, et
al: Ki67 levels as predictive and prognostic parameters in
pretherapeutic breast cancer core biopsies: A translational
investigation in the neoadjuvant GeparTrio trial. Ann Oncol.
24:2786–2793. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tokunaga E, Hisamatsu Y, Tanaka K,
Yamashita N, Saeki H, Oki E, Kitao H and Maehara Y: Molecular
mechanisms regulating the hormone sensitivity of breast cancer.
Cancer Sci. 105:1377–1383. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sasahara M, Matsui A, Ichimura Y, Hirakata
Y, Murata Y and Marui E: Overexpression of androgen receptor and
forkhead-box A1 protein in apocrine breast carcinoma. Anticancer
Res. 34:1261–1267. 2014.PubMed/NCBI
|
|
27
|
Miyashita M, Sasano H, Tamaki K, Chan M,
Hirakawa H, Suzuki A, Tada H, Watanabe G, Nemoto N, Nakagawa S, et
al: Tumor-infiltrating CD8+ and FOXP3+ lymphocytes in
triple-negative breast cancer: Its correlation with pathological
complete response to neoadjuvant chemotherapy. Breast Cancer Res
Treat. 148:525–534. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ono M, Tsuda H, Shimizu C, Yamamoto S,
Shibata T, Yamamoto H, Hirata T, Yonemori K, Ando M, Tamura K, et
al: Tumor-infiltrating lymphocytes are correlated with response to
neoadjuvant chemotherapy in triple-negative breast cancer. Breast
Cancer Res Treat. 132:793–805. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang K, Xu J, Zhang T and Xue D:
Tumor-infiltrating lymphocytes in breast cancer predict the
response to chemotherapy and survival outcome: A meta-analysis.
Oncotarget. 7:44288–44298. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Adams S, Gray RJ, Demaria S, Goldstein L,
Perez EA, Shulman LN, Martino S, Wang M, Jones VE, Saphner TJ, et
al: Prognostic value of tumor-infiltrating lymphocytes in
triple-negative breast cancers from two phase III randomized
adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin
Oncol. 32:2959–2966. 2014. View Article : Google Scholar : PubMed/NCBI
|