Introduction
Pancreatic cancer (PC) is one of the most aggressive
types of malignancy worldwide with notably poor prognosis (1) It is characterized by invasive growth and
early metastasis, and is also highly resistant to chemotherapy and
radiation therapy (2). Surgical
resection remains the only potentially curative treatment for local
patients with PC. However, >85% of PC cases are diagnosed at an
advanced stage. Even following radical surgical resection, the
patients usually succumb to recurrence or metastasis after a short
period of time (3). Therefore, PC
represents one of the major challenges in oncology to date. It is
necessary to investigate the mechanisms involved in PC in order to
develop an effective treatment strategy.
Increasing evidence has highlighted the significance
of Krüppel-like factor 8 (KLF8) in tumor progression and suggested
that it may be a promising target for cancer treatment (4,5). As a
GT-box (CACCC) binding dual-transcription factor, KLF8 regulates
the transcription of numerous genes by recruiting the C-terminal
binding protein (CtBP) corepressor (6–10) or p300
and P300/CtBP-associated factor histone acetyltransferase
co-activators (8,11–14) in
order to target gene promoters. It is aberrantly overexpressed in a
number of cancer subtypes and has been implicated in the
initiation, development and progression of these cancer subtypes,
including hepatocellular carcinoma (15–17),
gastric cancer (18–21), breast cancer (22,23),
ovarian cancer (14,24), renal cancer (22,25) and
glioma (26). It is worth noting that
KLF8 induced tumor cell epithelial-to-mesenchymal transition (EMT)
and maintained the invasive potential of cancer, which appeared to
serve a critical role in the metastatic progression of cancer cells
(6,27). Various target genes of KLF8 and
signaling pathways associated with cancer have been identified
(14,22,27–29).
However, the role of KLF8 in PC remains to be elucidated and less
is known about the mechanisms underlying KLF8-modulated expression
of its target genes.
Four and a half LIM-only protein 2 (FHL2) is a
member of the FHL family of proteins, which functions as a
coactivator of numerous transcription factors, including β-catenin
(30,31). It is expressed in a cell- and
tissue-specific manner, and contributes to the regulation of
important cell functions, including cell adhesion, cell
transcription and signal transduction (32–36). It
has been reported that FHL2 was overexpressed in PC tissue samples
and critically involved in cell survival, radio-resistance,
proliferation and apoptosis in PC cells (32). FHL2 directly interacts with Snail2 and
downregulates E-cadherin transcriptional activity, thereby inducing
colorectal cancer cell EMT and invasion (27,37,38). The
upregulated expression level of FHL2 may be a trigger or mediator
of EMT in cancer cells and is essential to maintain their malignant
phenotype. Previous studies have indicated that FHL2 and KLF8 were
overexpressed in numerous types of cancer, including colorectal
cancer (27), PC (39,40),
breast cancer (36,41) and ovarian cancer (42,43). It is
plausible to consider that they may have a synergistic effect on
cellular processes in association with malignancy. Notably, it was
suggested in a more recent study that KLF8 induced FHL2-mediated
EMT and potentially promoted colorectal cancer cell metastasis
(27). However, confirmation of its
role, and the possible mechanisms underlying the role KLF8 may
serve in FHL2-mediated cell EMT, invasion and metastasis have not
yet been investigated.
The present study firstly demonstrated that aberrant
co-overexpression of KLF8 and FHL2 was associated with PC
metastasis. Subsequently, the present study revealed that exogenous
overexpression of KLF8 in PC cells induced cell EMT, and invasive
and metastatic phenotypes. Furthermore, the present study
determined that FHL2 was a direct target for transcriptional
activation by KLF8, and FHL2 knockdown by short interfering RNA
(siRNA) may partially reverse these effects induced by
overexpressed KLF8. These results provided evidence supporting an
important role of FHL2 downstream of KLF8 in promoting PC cell EMT
and invasion. The results of the present study further supported a
potentially important role that KLF8 serves in human PC
development.
Materials and methods
Reagents and antibodies
The following antibodies and reagents were used:
Anti-E-cadherin (cat. no. sc-8426), anti-Slug (cat. no. sc-166476;
both Santa Cruz Biotechnology, Inc., Dallas, TX, USA), anti-FHL2
(cat. no. SAB2500398), anti-GAPDH (cat. no. G8795; Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany), Boyden Chambers, polycarbonate
membranes (8-µm pore size; Neuro Probe, Inc., Gaithersburg, MD,
USA), lipofectamine and oligofectamine (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). The human Panc-1, SW1990,
Capan-1, Bxpc-3 and Miapaca-2 PC cell lines were obtained from the
American Type Culture Collection (Manassas, VA, USA) and cultured
as previously described (32,44).
Tissue samples
The present study was approved by the Ethics
Committee of Xiangya Hospital, Central Southern University
(Changsha, China; no. 201406373). Written informed consent was
obtained from all the patients prior to enrollment in the present
study. A total of 34 pairs of surgically resected specimens,
including tumor tissue samples and their noncancerous counterparts,
were obtained at the Department of General Surgery, Xiangya
Hospital between September 2010 and June 2015. The patients
included 20 males and 14 females, with a mean age of 56 (range,
33–76) years. Among them, 18 patients did not exhibit lymph node
metastasis, with a male to female ratio of 8:10 and a mean age of
58.4 (range, 35–75) years. The remaining 16 patients presented with
lymph node metastasis, with a male to female ratio of 9:7 and a
mean age of 53.4 (range, 33–76) years.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA extraction and RT-qPCR were performed as
previously described (32,44). The primer sequences used were as
follows: KLF8 sense, 5′-TTCAGAAGGTGGCTCAATGC-3′ and KLF8 antisense,
5′-GGAGTGTTGGAGAAGTCATATTAC-3′; FHL2 sense,
5′-TCCATACTGCCTGACCTGC-3′ and FHL2 antisense,
5′-TTGGCGTTCCTCGAAAGAG-3′; and GAPDH sense,
5′-TGACTTCAACAGCGACACCCA-3′ and GAPDH antisense,
5′-CACCCTGTTGCTGTAGCCAAA-3′. For each sample, triplicate
determinations were performed, and mean values were used for
further calculations, with GAPDH detected as the internal control.
The relative quantitative expression of the target gene compared
with that of GAPDH using the 2−ΔΔCq method (45).
Western blotting (WB)
WB was performed as described previously (44). Briefly, pancreatic cells (including
Panc-1, Sw1990, Bxpc-3, Capan-1, Miapaca-2) were harvested and
lysed in lysis buffer. Subsequently, total protein (50 µg) was
subjected to 10% SDS-PAGE and transferred onto polyvinylidene
fluoride membranes (EMD Millipore, Billerica, MA, USA). Primary
antibodies were diluted according to the manufacturer's
recommendations. The relative content of each protein was detected
by enhanced chemiluminescence (GE Healthcare, Chicago, IL, USA).
Endogenous GAPDH was used as an internal control.
Cell transfection and clone
selection
pcDNA3.1 vectors expressing KLF8 or mock messenger
RNA (mRNA) were constructed, as previously described (17). KLF8 or mock vectors were transfected
into Panc-1 cells using Lipofectamine 2000 reagent (Invitrogen;
Thermo Fisher Scientific, Inc.). The transfected cells were
screened with 400 µg/ml G418 (Merck KGaA) for 4–6 weeks. Clones of
stably transected cells were obtained by the limited dilution
method, and the overexpression efficiency was confirmed by WB.
Transfections of FHL2 siRNA (5′-GCCAAUUGGAACCAAGAGUTT-3′) were
performed as previously described (32). The scrambled (scr) siRNA
(5′-TTCTCCGAACGTGTCACGT-3′) was used as the negative control.
Following a 24-h incubation at 37°C, cells were used for further
experiments. The knockdown efficiency was detected by WB.
Cell invasion analysis
An invasion assay was performed as previously
described (46). Transwell
polycarbonate filters (8-µm pore size; Corning Incorporated,
Corning, NY, USA) were used. A total of 2×105 Panc-1
cells were plated in the top chamber of the Transwell with a
Matrigel-coated polycarbonate membrane. The bottom wells of the
system were filled with 10% fetal bovine serum complete medium
(Gibco; Thermo Fisher Scientific). Following a 24-h incubation at
37°C, the number of transmigrated cells was counted, as previously
described (46).
Promoter reporter and dual luciferase
assays
For the validation of FHL2 as a direct target of
KLF8, a luciferase reporter assay was performed. Briefly,
55-basepair fragments of the FHL2 promoter upstream of the
transcription start site were cloned into the pGL3 basic vector
(Promega Corporation, Madison, WI, USA), and the pLuc55 mutant
construct was created by site-directed mutagenesis of the FHL2
promoter vector (Stratagene; Agilent Technologies, Inc., Santa
Clara, CA, USA), as previously described (27). The primer sequences were listed as
follows: pLuc55 forward (KpnI),
5′-GGGGTACCGGGGGGTGCACAGAGGTGGAGC-3′ and reverse (XhoI),
5′-TCCGCTCGAGCGAGGCCTCATATTTTCCAG-3′; and pLuc55-MT (site-directed
mutagenesis) forward, 5′-CCTAATCTGGGGAGTACACAGAGGTGGAGCTGAGCAGCC-3′
and reverse, 5′-GGCTGCTCAGCTCCACCTCTGTGTACTCCCCAGATTAGG-3′.
Wild-type or mutant reporter constructs were co-transfected with
Lipofectamine 2000 transfection reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) into the Panc-1 cells with KLF8 or mock vectors,
according to the manufacturer's protocol. After a 48 h incubation
at 37°C, the luciferase activity was evaluated with the
Dual-Luciferase® Reporter Assay System (Promega
Corporation). The relative luciferase activity was normalized to
that of firefly luciferase. The transcriptional activity at the
promoter was presented as the fold change of relative luciferase
units compared with those of the basic pGL3 vector control.
Statistical analysis
Data are presented as the mean ± standard deviation.
The comparison between the test and control group was evaluated by
Student's t-test. All statistical analyses were performed using
SPSS version 15.0 software (SPSS, Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference. Assays were performed ≥3 times with triplicate samples
independently.
Results
Aberrant co-overexpression of KLF8 and
FHL2 in PC is associated with tumor metastasis
In order to investigate the association between
aberrant expression of KLF8 and FHL2 in PC, RT-qPCR and WB were
performed to detect the expression levels of these genes and
proteins, respectively, in 34 pairs of cancer tissues and adjacent
normal pancreatic tissues. Of note, patients with PC and lymph node
metastasis exhibited higher KLF8 and FHL2 mRNA expression levels
compared with those exhibited by patients with PC without lymph
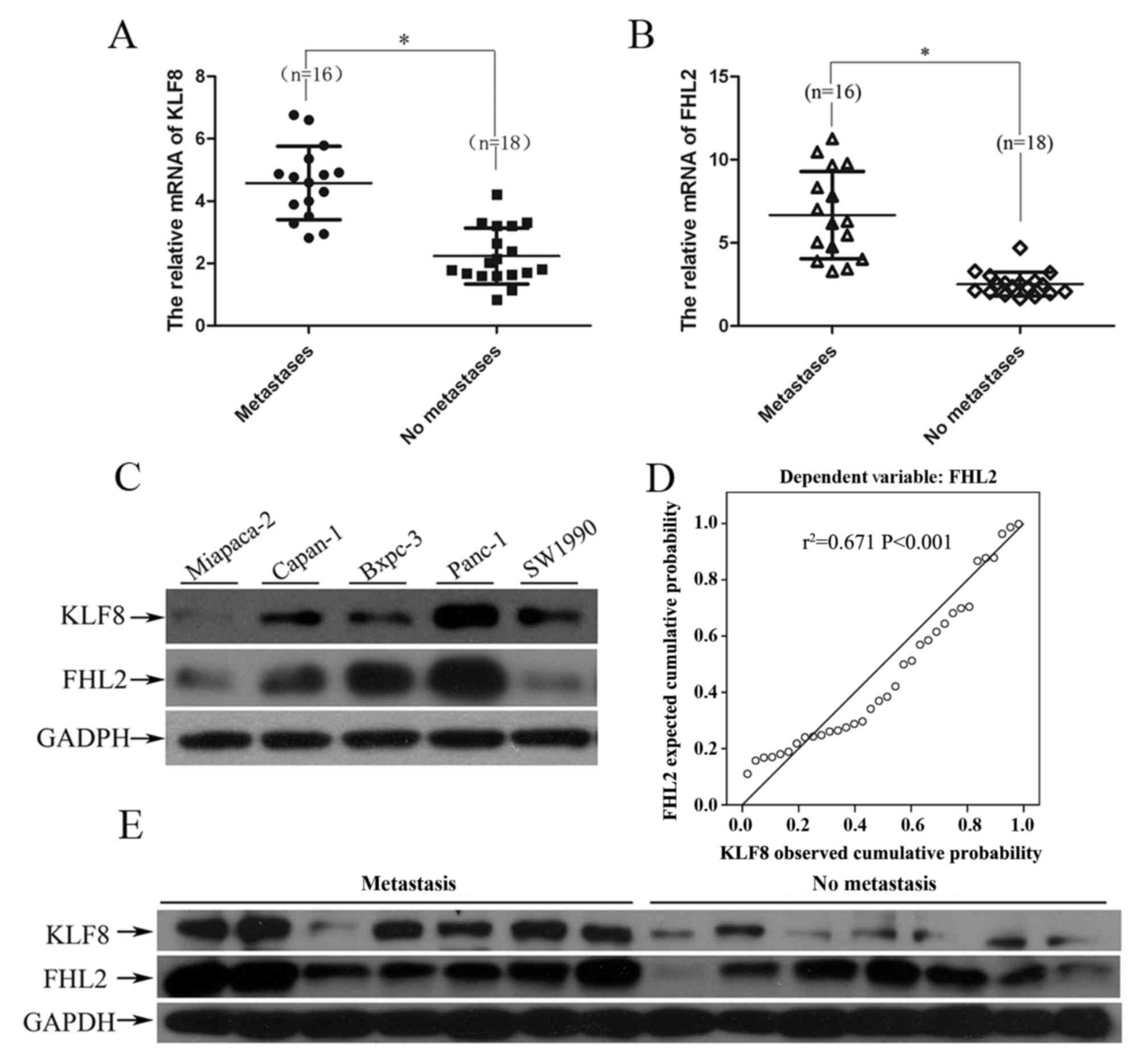
node metastasis (P<0.01; Fig. 1A and
B). Furthermore, KLF8 mRNA was positively correlated with FHL2
mRNA expression level (r2=0.673 P<0.001) in PC tissue
samples (P<0.01, Fig. 1D).
Additionally, relative higher protein expression levels of KLF8 and
FHL2 were also observed in the resected tumor tissue samples with
lymph node metastasis in comparison with those in tissue samples
without lymph node metastasis, as determined by WB (Fig. 1E).
In order to validate our findings in vitro,
the expression levels of KLF8 and FHL2 protein were investigated in
five PC cell lines: Panc-1, Capan-1, Bxpc-3, Miapaca-2 and SW1990.
As expected, the expression levels of KLF8 and FHL2 were positively
associated with the metastatic potential of the PC cell lines. As
presented in Fig. 1C, KLF8 was
expressed at a relatively high level in Panc-1, Capan-1, SW1990 and
Bxpc-3 cells, and at a relatively low level in Miapaca-2 cells. The
FHL2 expression level was similar to that of KLF8 in the five cell
lines, with the exception of SW1990 cells. Panc-1 cells, with the
highest metastatic potential in the five aforementioned cell lines
(47), expressed significantly higher
expression levels of KLF8 compared with those expressed by the
other PC cell lines. These results indicated that aberrant
co-overexpression of KLF8 and FHL2 was associated with tumor
metastasis in PC.
KLF8 upregulates FHL2 expression
levels to induce EMT and promote cell invasion in a human PC cell
line
In order to investigate the biological role of KLF8
in PC, the present study first determined whether KLF8 was
sufficient to promote invasion using exogenous introduction of KLF8
in Panc-1 cells. Overexpression of KLF8 protein in the stably
transfected cells was confirmed by WB (Fig. 2A).
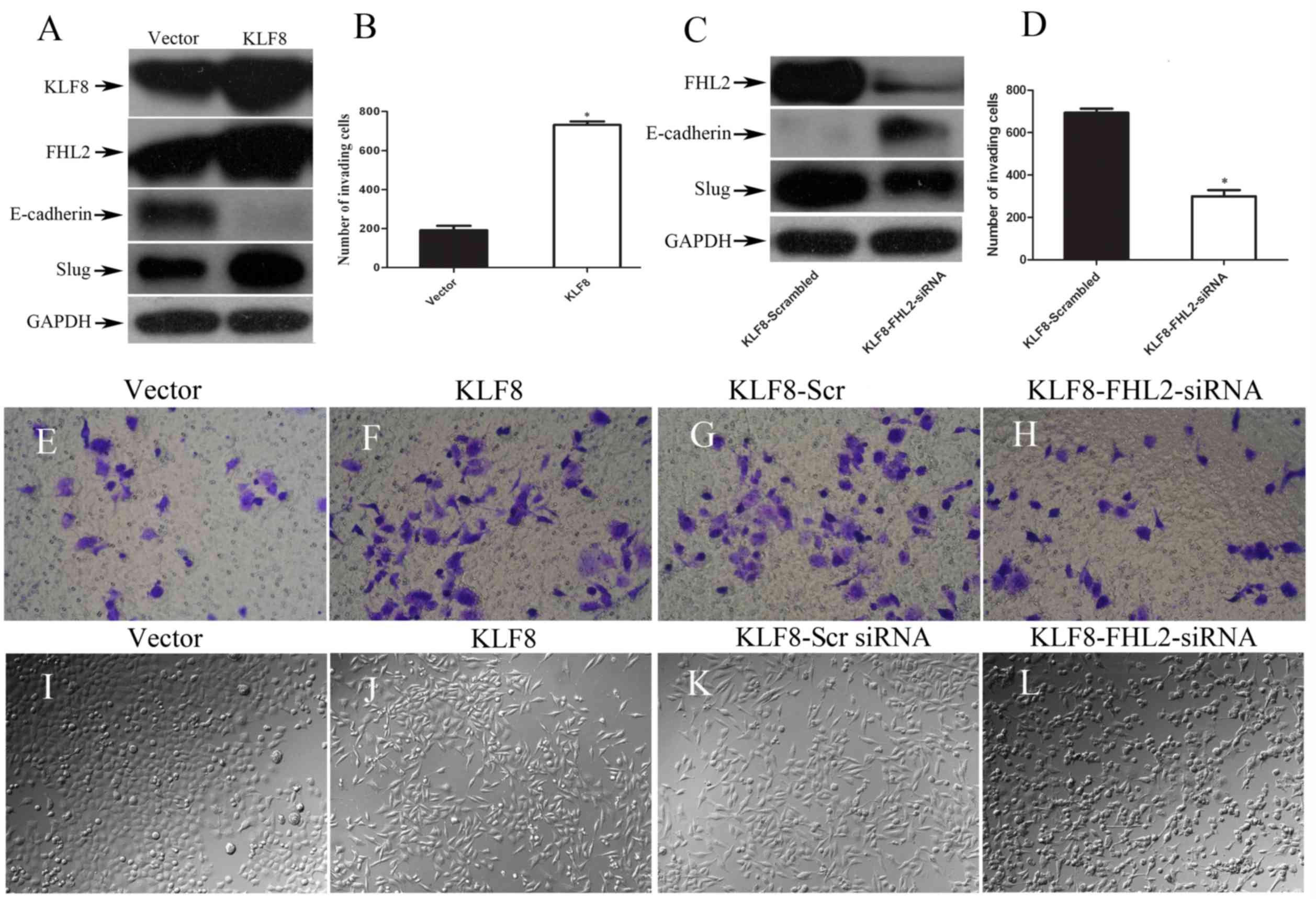 | Figure 2.KLF8 upregulates FHL2 expression to
induce EMT and promote cell invasion. (A) KLF8, FHL2, E-cadherin
and Slug expression levels in stable KLF8 transfectants in Panc-1
cells were detected by WB. GAPDH was used as the internal control.
(B) Quantification of an invasion assay performed with or without
KLF8 transfection. (C) Expression of epithelial-to-mesenchymal
transition-associated biomarkers, including E-cadherin, Slug and
FHL2, were detected by WB 72 h following transfection with KLF8-scr
or KLF8-FHL2 siRNA. (D) Quantification of an invasion assay
performed with KLF8-scr or KLF8-FHL2 siRNA. Invasive potential of
Panc-1 stable transfectants following transfection with (E) vector,
(F) KLF8, (G) KLF8-scr siRNA and (H) KLF8-FHL siNRA. Morphology of
Panc-1 cells transfected with (I) vector, (J) KLF8, (K) KLF8-scr
siRNA and (L) KLF8-FHL2-siRNA cells, visualized by phase-contrast
microscopy. Magnification, ×100. *P<0.05 vs. other group. KLF8,
Krüppel-like factor 8; FHL2, four and a half LIM-only protein 2;
EMT, epithelial-to-mesenchymal transition; scr, scrambled; siRNA,
short interfering RNA; WB, western blotting. |
In order to investigate the effect of KLF8 on EMT
induction, morphological examination was conducted and the
expression levels of EMT markers were analyzed. It was also
demonstrated that overexpression of KLF8 in Panc-1 cells resulted
in a shift in EMT marker expression levels by WB. Upregulation of
mesenchymal markers (Slug) and downregulation of the epithelial
marker E-cadherin were observed by WB in the stable vector
transfectants. The results demonstrated that induction of KLF8
expression induced a significant increase in FHL2 protein
expression (Fig. 2A). In addition,
the present study revealed that exogenous induction of KLF8
expression resulted in a ≥3-fold increase in cell invasiveness
(Fig. 2B-D). The present study also
investigated the morphological features of Panc-1 cells. The stable
KLF8 transfectants exhibited spindle-like, fibroblastic morphology,
which is one of the main characteristics of EMT (6). Dendritic-like or long cytoplasmic
processes were observed under a phase-contrast microscope; however,
the stable vector transfectants (control) presented a round or flat
morphology with a short cytoplasmic process (Fig. 2E and F).
These results may suggest the effect of
co-overexpression of FHL2 and KLF8 on EMT and cell invasion. Based
on the in vitro overexpression experiments, KLF8 appeared to
be a potent inducer of EMT.
FHL2 is required for KLF8-mediated EMT
and invasive phenotypes
According to previous studies, FHL2 may induce tumor
cell EMT and maintain the invasive phenotype of cancer cells
(27); however, the role of FHL2 in
KLF8-induced EMT in PC cells remains elusive. Downregulation of
FHL2 expression by siRNA was performed in KLF8-overexpressing
Panc-1 cells and resulted in the conversion from the expression of
the mesenchymal marker Slug to the epithelial marker E-cadherin
when compared with that of the control FHL2-scr siRNA cells
(Fig. 2G). Cell invasion analysis
indicated that the number of invasive cells in the
FHL2-siRNA-treated group decreased significantly compared with
those in the control group (Fig. 2H, I
and J). Morphological examination demonstrated that FHL2
downregulation in KLF8-overexpressing cells induced partial
reversal of the EMT process, i.e. the mesenchymal-to-epithelial
transition process (Fig. 2K and L).
Taken together, a critical role for FHL2 on the EMT and invasion
phenotypes induced by abnormal KLF8 expression in PC cells is
indicated.
FHL2 is a direct transcriptional
activation target of KLF8
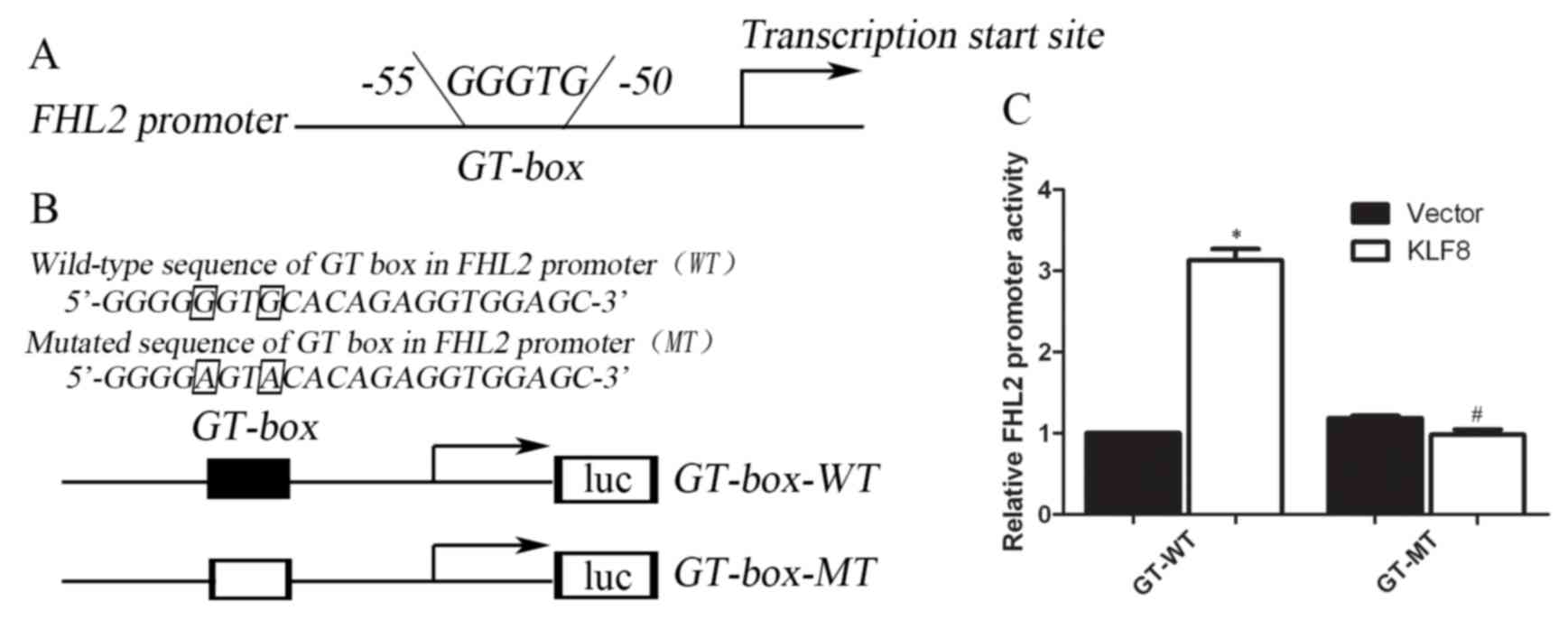
The present study assessed whether the FHL2 promoter
may be directly activated by the KLF8 protein using a
dual-luciferase reporter system. The promoter regions containing
the GT-box (−50 to −55), which are the potential binding sites, of
human FHL2 were cloned upstream of a luciferase gene in a reporter
plasmid (Fig. 3A and B).
Subsequently, transient transfections were performed to explore
whether the FHL2 promoter was activated by overexpressed KLF8. The
results revealed that the luciferase activity increased by
3.02±0.12-fold compared with that of the vector control
(P<0.001) (Fig. 3C).
Correspondingly, the present study also mutated two nucleotides of
the identified GT-box binding region to generate a FHL2
3′-untranslated region (UTR) mutant in the KLF8 target region to
disrupt binding (Fig. 3B).
Conversely, the increased effect of luciferase activity was not
observed in cells carrying the mutant-type construct of the FHL2
3′-UTR (Fig. 3C). These results
suggested that FHL2 may be a direct transcriptional activation
target of KLF8, and the proximal GT-box at −55 is the main
KLF8-binding site in the FHL2 promoter (Fig. 3).
Discussion
PC is known for its aggressive growth; mortality
usually results from tissue invasion and early metastasis (2,3). Similarly
to the majority of other carcinoma types, PC progression is
associated with EMT (48). It has
been widely accepted that EMT frequently occurs in PC and is
involved in tumorigenesis, cancer progression and metastasis
(49). Therefore, identification of
critical factors associated with tumor progression and EMT, and
their underlying mechanisms are particularly important.
Loss of expression of E-cadherin, an epithelial
marker, is a hallmark of EMT (6,48,50). An increasing number of transcription
factors have been implicated in the repression of E-cadherin
expression, including KLF transcription factor family proteins
(6,23). KLF8 is a relatively new member of this
family and is emerging as a crucial regulator of cancer initiation
and progression (10). With
accumulating evidence demonstrating its overexpression in numerous
types of aggressive human cancer and its vital role during a
variety of cellular processes in cancer, KLF8 has become a novel
focus in cancer research and a potential target for the treatment
of cancer (4,5). Notably, a number of previous studies
have revealed that KLF8 induces tumor cell EMT and maintains the
invasive potential of cancer, which serves a critical role in the
regulation of various cancer-associated cellular processes favoring
tumor metastatic progression (6,23,24). Our previous study demonstrated that
KLF8 was upregulated in PC tissues and cell lines, which serves a
critical role in PC cell proliferation by promoting the
G2/M phase progression via up- or downregulating
numerous cell cycle-associated proteins (50). However, to the best of our knowledge,
whether the mechanisms underlying KLF8 serve a role in cancer cell
invasion and metastasis has not previously been investigated.
The present study demonstrated that KLF8 was
overexpressed in highly metastatic PC cell lines and in patients
with lymphatic metastasis, which indicated that KLF8-positive tumor
cells may have more aggressive phenotypes than KLF8-negative tumor
cells. Based on the exogenous overexpression experiments in
vitro, KLF8 was revealed to be a potent inducer of EMT. In
agreement with our finding, a number of previous studies have
demonstrated that ectopic expression of KLF8 repressed E-cadherin
expression, induced tumor cell EMT, and increased cell invasion and
metastasis in breast cancer (6,22) and
hepatocellular carcinoma (17).
Furthermore, downregulation of KLF8 may inhibit cell invasion and
metastasis in gastric cancer (23,24).
Of note, the present study identified the
KLF8-mediated upregulation of FHL2 as a novel mechanism for
induction of EMT and promotion of invasion in human PC cells.
Firstly, the present study identified a strong association between
the co-expression of KLF8 and FHL2 in PC tumors with lymph node
metastasis. Secondly, the present study demonstrated that KLF8
expression promotes FHL2 transcription in human PC cells. Finally,
the present study provided evidence that FHL2 was required for
KLF8-induced EMT and metastatic phenotypes in vitro. These
results suggested a potentially significant role for the
cooperative association between FHL2 and KLF8 in promoting PC
invasion and metastasis, and may shed new light on the underlying
molecular mechanisms.
It is known that FHL2 is aberrantly upregulated in
invasive PC tissues (32), as
revealed in present study. Notably, the present study identified
FHL2 as a novel target of transcriptional activation by KLF8, which
was demonstrated by a dual-luciferase reporter system that
identified that KLF8 directly binds to the FHL2 promoter at the
GT-box (−50 to −55). Of note, previous studies have revealed that
KLF8 directly bound to a GT-box located in the E-cadherin promoter,
repressed its expression and triggered the subsequent EMT process
in the MDCK canine kidney epithelial cell line, and in the MCF-10A
and MDA-MB-231 human mammary epithelial cell lines (6,51). In
addition, this process does not appear to depend upon other EMT
inducer proteins such as Snail. This may explain why the EMT
phenotype of KLF8-overexpressing PC cells was partially reversed
following FHL2 knockdown. KLF8-associated target genes involved in
EMT and how KLF8 regulates the EMT process require further
investigation.
The present study demonstrated that FHL2 was highly
overexpressed in PC tumor tissues, and suggested that FHL2 was
involved in KLF8-induced EMT and invasion in vitro; however,
the molecular mechanisms underlying the aberrant high expression
level of FHL2 in PC, and whether and how FHL2 serves a role in
vivo for the progression of PC require further elucidation.
Previous studies have revealed that FHL2 was overexpressed in PC
and served an important role in the survival and radiosensitivity
of PC cell lines grown using three-dimensional cultures of
laminin-rich extracellular matrix (32). However, the role of FHL2 in cancer
remains ambivalent because FHL2 binds to various proteins with
distinct functions (31). The roles
of KLF8 and FHL2 in PC are unknown. The present study demonstrated
that FHL2 protein expression level was highly associated with KLF8
expression level in PC tumor tissues with lymph node metastasis.
Furthermore, FHL2 repression resulted in a marked reversion of the
KLF8-induced cell EMT phenotype in vitro. Therefore, the
present study suggested that KLF8 and FHL2 may cooperatively exert
roles in PC progression. Further experiments are required to
investigate the detailed mechanism.
In conclusion, the present study identified
KLF8/FHL2 as a novel signaling pathway responsible for EMT and
invasion in PC cells, and opened a new avenue in the research of
KLF8 and FHL2. Notably, the expression level of KLF8 is barely
detectable in normal epithelial cells (6), and in addition to FHL2, KLF8 also
targeted E-cadherin and other factors associated with invasion and
metastasis. Therefore, KLF8 may represent a novel promising target
for intervention against PC cell invasion and metastasis.
Acknowledgements
The present study was supported by The Freedom
Exploration Program of Central South University (grant no.
2011QNZT153), Hunan Provincial Natural Science Foundation of China
(grant no. 14JJ6001) and The Foundation of Science and Technology
Department of Hunan Province (grant no. 2014SK3268). Dr Xiaoping Yi
is a Postdoctoral Fellow in Postdoctoral Research Workstation of
Pathology and Pathophysiology, Basic Medical Sciences, Xiangya
Hospital, Central South University. The authors thank all the
members of the laboratory of Yixiong Li for the useful discussions
and technical assistance provided.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Garrido-Laguna I and Hidalgo M: Pancreatic
cancer: From state-of-the-art treatments to promising novel
therapies. Nat Rev Clin Oncol. 12:319–334. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hartwig W, Werner J, Jäger D, Debus J and
Büchler MW: Improvement of surgical results for pancreatic cancer.
Lancet Oncol. 14:e476–e485. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lu XJ, Shi Y, Chen JL and Ma S:
Krüppel-like factors in hepatocellular carcinoma. Tumour Biol.
36:533–541. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lahiri SK and Zhao J: Krüppel-like factor
8 emerges as an important regulator of cancer. Am J Transl Res.
4:357–363. 2012.PubMed/NCBI
|
|
6
|
Wang X, Zheng M, Liu G, Xia W,
McKeown-Longo PJ, Hung MC and Zhao J: Krüppel-like factor 8 induces
epithelial to mesenchymal transition and epithelial cell invasion.
Cancer Res. 67:7184–7193. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hu JH, Navas P, Cao H, Stamatoyannopoulos
G and Song CZ: Systematic RNAi studies on the role of Sp/KLF
factors in globin gene expression and erythroid differentiation. J
Mol Biol. 366:1064–1073. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wei H, Wang X, Gan B, Urvalek AM,
Melkoumian ZK, Guan JL and Zhao J: Sumoylation delimits KLF8
transcriptional activity associated with the cell cycle regulation.
J Biol Chem. 281:16664–16671. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang P, Basu P, Redmond LC, Morris PE,
Rupon JW, Ginder GD and Lloyd JA: A functional screen for
Krüppel-like factors that regulate the human gamma-globin gene
through the CACCC promoter element. Blood Cells Mol Dis.
35:227–235. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
van Vliet J, Turner J and Crossley M:
Human Krüppel-like factor 8: A CACCC-box binding protein that
associates with CtBP and represses transcription. Nucleic Acids
Res. 28:1955–1962. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lloyd JA: KLF8 sets the pace for the cell
cycle through interactions with p300 and PCAF. Cell Cycle.
9:650–651. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Urvalek AM, Wang X, Lu H and Zhao J: KLF8
recruits the p300 and PCAF co-activators to its amino terminal
activation domain to activate transcription. Cell Cycle. 9:601–611.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mehta TS, Lu H, Wang X, Urvalek AM, Nguyen
KH, Monzur F, Hammond JD, Ma JQ and Zhao J: A unique sequence in
the N-terminal regulatory region controls the nuclear localization
of KLF8 by cooperating with the C-terminal zinc-fingers. Cell Res.
19:1098–1109. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhao J, Bian ZC, Yee K, Chen BP, Chien S
and Guan JL: Identification of transcription factor KLF8 as a
downstream target of focal adhesion kinase in its regulation of
cyclin D1 and cell cycle progression. Mol Cell. 11:1503–1515. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang T, Cai SY, Zhang J, Lu JH, Lin C,
Zhai J, Wu MC and Shen F: Krüppel-like factor 8 is a new
Wnt/beta-catenin signaling target gene and regulator in
hepatocellular carcinoma. PLoS One. 7:e396682012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Han S, Han L, Sun H, Zan X, Zhou Z, Xu K,
Yao Y and Liu Q: Krüppel-like factor expression and correlation
with FAK, MMP9 and E-cadherin expression in hepatocellular
carcinoma. Mol Med Rep. 8:81–88. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li JC, Yang XR, Sun HX, Xu Y, Zhou J, Qiu
SJ, Ke AW, Cui YH, Wang ZJ, Wang WM, et al: Up-regulation of
Krüppel-like factor 8 promotes tumor invasion and indicates poor
prognosis for hepatocellular carcinoma. Gastroenterology.
1392146–2157. (e12)2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen G, Yang W, Jin W, Wang Y, Tao C and
Yu Z: Lentivirus-mediated gene silencing of KLF8 reduced the
proliferation and invasion of gastric cancer cells. Mol Biol Rep.
39:9809–9815. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu L, Liu N, Xu M, Liu Y, Min J, Pang H,
Zhang N and Zhang H and Zhang H: Lentivirus-delivered Krüppel-like
factor 8 small interfering RNA inhibits gastric cancer cell growth
in vitro and in vivo. Tumour Biol. 33:53–61. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hsu LS, Wu PR, Yeh KT, Yeh CM, Shen KH,
Chen CJ and Soon MS: Positive nuclear expression of KLF8 might be
correlated with shorter survival in gastric adenocarcinoma. Ann
Diagn Pathol. 18:74–77. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang WF, Li J, Du LT, Wang LL, Yang YM,
Liu YM, Liu H, Zhang X, Dong ZG, Zheng GX and Wang CX: Krüppel-like
factor 8 overexpression is correlated with angiogenesis and poor
prognosis in gastric cancer. World J Gastroenterol. 19:4309–4315.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang X, Lu H, Urvalek AM, Li T, Yu L,
Lamar J, DiPersio CM, Feustel PJ and Zhao J: KLF8 promotes human
breast cancer cell invasion and metastasis by transcriptional
activation of MMP9. Oncogene. 30:1901–1911. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu N, Wang Y, Zhou Y, Pang H, Zhou J,
Qian P, Liu L and Zhang H: Krüppel-like factor 8 involved in
hypoxia promotes the invasion and metastasis of gastric cancer via
epithelial to mesenchymal transition. Oncol Rep. 32:2397–2404.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang H, Liu L, Wang Y, Zhao G, Xie R, Liu
C, Xiao X, Wu K, Nie Y, Zhang H and Fan D: KLF8 involves in
TGF-beta-induced EMT and promotes invasion and migration in gastric
cancer cells. J Cancer Res Clin Oncol. 139:1033–1042. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fu WJ, Li JC, Wu XY, Yang ZB, Mo ZN, Huang
JW, Xia GW, Ding Q, Liu KD and Zhu HG: Small interference RNA
targeting Krüppel-like factor 8 inhibits the renal carcinoma 786-0
cells growth in vitro and in vivo. J Cancer Res Clin Oncol.
136:1255–1265. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schnell O, Romagna A, Jaehnert I, Albrecht
V, Eigenbrod S, Juerchott K, Kretzschmar H, Tonn JC and Schichor C:
Krüppel-like factor 8 (KLF8) is expressed in gliomas of different
WHO grades and is essential for tumor cell proliferation. PLoS One.
7:e304292012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yan Q, Zhang W, Wu Y, Wu M, Zhang M, Shi
X, Zhao J, Nan Q, Chen Y, Wang L, et al: KLF8 promotes
tumorigenesis, invasion and metastasis of colorectal cancer cells
by transcriptional activation of FHL2. Oncotarget. 6:25402–25417.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lu H, Hu L, Yu L, Wang X, Urvalek AM, Li
T, Shen C, Mukherjee D, Lahiri SK, Wason MS and Zhao J: KLF8 and
FAK cooperatively enrich the active MMP14 on the cell surface
required for the metastatic progression of breast cancer. Oncogene.
33:2909–2917. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li T, Lu H, Mukherjee D, Lahiri SK, Shen
C, Yu L and Zhao J: Identification of epidermal growth factor
receptor and its inhibitory microRNA141 as novel targets of
Krüppel-like factor 8 in breast cancer. Oncotarget. 6:21428–21442.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cao CY, Mok SW, Cheng VW and Tsui SK: The
FHL2 regulation in the transcriptional circuitry of human cancers.
Gene. 572:1–7. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kleiber K, Strebhardt K and Martin BT: The
biological relevance of FHL2 in tumour cells and its role as a
putative cancer target. Anticancer Res. 27:55–61. 2007.PubMed/NCBI
|
|
32
|
Zienert E, Eke I, Aust D and Cordes N:
LIM-only protein FHL2 critically determines survival and
radioresistance of pancreatic cancer cells. Cancer Lett. 364:17–24.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Al-Nomani L, Friedrichs J, Schüle R,
Buttner R and Friedrichs N: Tumoral expression of nuclear cofactor
FHL2 is associated with lymphatic metastasis in sporadic but not in
HNPCC-associated colorectal cancer. Pathol Res Pract. 211:171–174.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gullotti L, Czerwitzki J, Kirfel J,
Propping P, Rahner N, Steinke V, Kahl P, Engel C, Schüle R,
Buettner R and Friedrichs N: FHL2 expression in peritumoural
fibroblasts correlates with lymphatic metastasis in sporadic but
not in HNPCC-associated colon cancer. Lab Invest. 91:1695–1705.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Qiao L, Wang Y, Pang R, Wang J, Dai Y, Ma
J, Gu Q, Li Z, Zhang Y, Zou B, et al: Oncogene functions of FHL2
are independent from NF-kappaBIalpha in gastrointestinal cancer.
Pathol Oncol Res. 15:31–36. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gabriel B, Fischer DC, Orlowska-Volk M,
zur Hausen A, Schüle R, Müller JM and Hasenburg A: Expression of
the transcriptional coregulator FHL2 in human breast cancer: A
clinicopathologic study. J Soc Gynecol Investig. 13:69–75. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ding L, Wang Z, Yan J, Yang X, Liu A, Qiu
W, Zhu J, Han J, Zhang H, Lin J, et al: Human four- and -a-half LIM
family members suppress tumor cell growth through a TGF-beta-like
signaling pathway. J Clin Invest. 119:349–361. 2009.PubMed/NCBI
|
|
38
|
Zhang W, Wang J, Zou B, Sardet C, Li J,
Lam CS, Ng L, Pang R, Hung IF, Tan VP, et al: Four and a half LIM
protein 2 (FHL2) negatively regulates the transcription of
E-cadherin through interaction with Snail1. Eur J Cancer.
47:121–130. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
McGrath MJ, Binge LC, Sriratana A, Wang H,
Robinson PA, Pook D, Fedele CG, Brown S, Dyson JM, Cottle DL, et
al: Regulation of the transcriptional coactivator FHL2 licenses
activation of the androgen receptor in castrate-resistant prostate
cancer. Cancer Res. 73:5066–5079. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
He HJ, Gu XF, Xu WH, Yang DJ, Wang XM and
Su Y: Krüppel-like factor 8 is a novel androgen receptor
co-activator in human prostate cancer. Acta Pharmacol Sin.
34:282–288. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li T, Lu H, Shen C, Lahiri SK, Wason MS,
Mukherjee D, Yu L and Zhao J: Identification of epithelial stromal
interaction 1 as a novel effector downstream of Kruppel-like factor
8 in breast cancer invasion and metastasis. Oncogene. 33:4746–4755.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gabriel B, Mildenberger S, Weisser CW,
Metzger E, Gitsch G, Schüle R and Müller JM: Focal adhesion kinase
interacts with the transcriptional coactivator FHL2 and both are
overexpressed in epithelial ovarian cancer. Anticancer Res.
24:921–927. 2004.PubMed/NCBI
|
|
43
|
Wang X, Urvalek AM, Liu J and Zhao J:
Activation of KLF8 transcription by focal adhesion kinase in human
ovarian epithelial and cancer cells. J Biol Chem. 283:13934–13942.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yi XP, Han T, Li YX, Long XY and Li WZ:
Simultaneous silencing of XIAP and survivin causes partial
mesenchymal-epithelial transition of human pancreatic cancer cells
via the PTEN/PI3K/Akt pathway. Mol Med Rep. 12:601–608. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Han T, Yi XP, Liu B, Ke MJ and Li YX:
MicroRNA-145 suppresses cell proliferation, invasion and migration
in pancreatic cancer cells by targeting NEDD9. Mol Med Rep.
11:4115–4120. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Deer EL, González-Hernández J, Coursen JD,
Shea JE, Ngatia J, Scaife CL, Firpo MA and Mulvihill SJ: Phenotype
and genotype of pancreatic cancer cell lines. Pancreas. 39:425–435.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Beuran M, Negoi I, Paun S, Ion AD, Bleotu
C, Negoi RI and Hostiuc S: The epithelial to mesenchymal transition
in pancreatic cancer: A systematic review. Pancreatology.
15:217–225. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Jiang JH, Liu C, Cheng H, Lu Y, Qin Y, Xu
YF, Xu J, Long J, Liu L, Ni QX and Yu XJ: Epithelial-mesenchymal
transition in pancreatic cancer: Is it a clinically significant
factor. Biochim Biophys Acta. 1855:43–49. 2015.PubMed/NCBI
|
|
50
|
Yi X, Li Y, Zai H, Long X and Li W: KLF8
knockdown triggered growth inhibition and induced cell phase arrest
in human pancreatic cancer cells. Gene. 585:22–27. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|