Introduction
Gastric cancer is the fourth most common type of
cancer and the second leading cause of cancer mortality worldwide
(1). The most common type of gastric
cancer is adenocarcinoma, which is classified into intestinal and
diffuse types (2), which develop
through distinct pathways (3).
Although treating receptor tyrosine-protein kinase erb-2
(Her-2/neu/H2 N/HER2)-overexpressing gastric cancers with
trastuzumab has significantly improved patient survival (4), the prognosis of patients with advanced
gastric adenocarcinoma is poor; the 5-year survival rate is <20%
(5), which may in part be due to the
lack of prognostic and diagnostic biomarkers. Molecular biomarker
expression provides prognostic value and prompts the development of
more effective molecular targeted drugs. Genetic and epigenetic
alterations of proto-oncogenes and tumor-suppressor genes,
including epidermal growth factor receptor and those involved in
the phosphatidylinositol 3-kinase/protein kinase B/mechanistic
target of rapamycin signaling pathway, have been associated with
gastric cancer (6,7).
The cyclin D protein family regulates cell cycle
progression, which is mediated by their interactions with
cyclin-dependent kinases 2, 4 and 6 (8). There have been three isoforms, cyclin D1
(CCND1); cyclin D2 (CCND2); and cyclin D3 (CCND3), identified in
humans (9,10). Overexpression of CCND1 is
correlated with tumor differentiation, poor survival and increased
metastasis (11–13). Amplification of CCND1 has been
associated with non-small cell lung cancers (14,15), head
and neck squamous cell carcinomas (16–18) and
pancreatic carcinomas (19). CCND1,
CCND2 and CCND3 serve differential roles in tumor cell
carcinogenesis that are cell and tissue-type specific (20). High levels of CCND2 expression
were observed in ovarian and testicular tumors (21,22), and
overexpression of CCND2 has been associated with gastric
cancer progression (23,24). In addition, CCND3 has been associated
with cell proliferation as well as induction and/or maintenance of
terminal differentiation (25).
Furthermore, overexpression of CCND1 and CCND3 has
been identified in malignant melanomas (26), pancreatic cancer (27) and ductal carcinoma of the breast
(28). To understand the contribution
of CCND1, CCND2 and CCND3 to tumor progression, a detailed analysis
of their expression levels in gastric cancer must be explored.
The aim of the present study was to evaluate the
expression of CCND1 protein and its correlation with the clinical
outcome of patients with gastric cancer. In order to identify the
effects of CCND1, CCND2 and CCND3 in cancer cells, their
differential roles in gastric cancer were examined. It was
hypothesized that increased CCND1 expression may be used as
biomarker in patients with gastric cancer. Therefore, data on
CCND1, CCND2 and CCND3 mRNA expression were
extracted from the Oncomine database (29) for gastric cancer, and the effect of
CCND1, CCND2 and CCND3 expression level on
overall survival (OS) and progression-free survival (PFS) was
examined by Kaplan-Meier analysis.
Materials and methods
Patients
Fresh specimens were collected from 32 patients with
gastric adenocarcinoma who underwent radical resection at National
Cheng Kung University Hospital (Tainan, Taiwan) between August 2003
and August 2008. The mean age was 60±11 years old (range, 35–82
years old; 20 males, 12 females). A total of 32 pairs of cancerous
and matched adjacent normal gastric mucosa tissues were collected
and analyzed as previously described (30). The Tumor, Node, Metastasis system by
the American Joint Committee on Cancer was used for the
classification, grading and staging of gastric cancer (31). The specimens were preserved in the
Human Biobank within the Research Center of Clinical Medicine of
the National Cheng Kung University Hospital (Tainan, Taiwan). All
the patients provided written informed consent, and the study was
approved by the Institutional Review Board of National Cheng Kung
University Hospital (approval no., ER-97-148).
Western blot analysis of CCND1
protein
Total cell lysates were prepared and analyzed by 10%
SDS-PAGE as previously described (30,32,33).
Membranes were blocked with 5% (w/v) skimmed milk (Merck KGaA,
Darmstadt, Germany) for 1 h at room temperature and incubated with
the following primary antibodies overnight at 4°C: Anti-CCND1 (cat.
no., 2922; dilution, 1:2,000; Cell Signaling Technology, Inc.,
Danvers, MA, USA) and anti-β-actin (cat. no., GTX26276; dilution,
1:5,000; GeneTex, Inc., Irvine, CA, USA). Membranes were then
incubated for 1 h at room temperature with peroxidase-conjugated
goat anti-rabbit IgG (cat. no. 7074S; 1:3,000; Cell Signaling
Technology, Inc.) or peroxidase-conjugated sheep anti-mouse IgG
antibody (ECL anti-mouse IgG; cat. no. NA931V; 1:3,000) (Amersham
Pharmacia Biosciences, Buckinghamshire, U.K.). Immunodetection was
performed using the horseradish peroxidase-based SuperSignal
Chemiluminescent Substrate (Pierce; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). For quantification, the bands were measured with
the AlphaImager 2200 Imaging System (Alpha Innotech; Bio-Techne,
Minneapolis, MN, USA), and the densities of the CCND1 bands were
normalized to those of the β-actin bands. CCND1 expression was
quantified and described as a ratio to β-actin expression
(CCND1/β-actin ratio).
Bioinformatics and statistical
analysis
A search of the Oncomine database (http://www.oncomine.com) (34) was initially conducted to
systematically assess the expression level of the CCND1,
CCND2 and CCND3 genes in gastric cancer. For this,
normal vs. cancer tissues were compared in the differential
analysis. The results were analyzed for their P-values, fold change
and cancer subtype. The prognostic value of the CCND1,
CCND2 and CCND3 genes in gastric cancer was also
analyzed using the Kaplan-Meier Plotter (http://kmplot.com/analysis/), as described previously
(35). The following settings were
used for the analysis: ‘Overall survival’; ‘progression-free
survival’; ‘autoselect best cutoff’; ‘censore at threshold all’
(patients surviving over the selected threshold are censored
instead of excluded); ‘tumor stage all’; ‘tumor stage T all’;
‘tumor stage N all’; ‘tumor stage M all’; ‘grade all’; ‘Lauren
classification all’ (2);
‘differentiation all’; and ‘moderate and poor differentiation’.
Tumors were classified according to WHO histopathological type
(36). Three cyclin D genes probe
sets were available: 208712_at at CCND1, 200953_s_at at
CCND2 and 201700_at at CCND3, and patients were split
according to median expression or to expression at best cut-off for
each probe. A total of 1,065 patients with gastric cancer were
assessed using a Kaplan-Meier plot (36) and HER2 status was identified using the
gene chip probe set 216836_s_at, as previously described (37). The hazard ratio (HR) 95% confidence
intervals and logrank P-values were calculated and described.
P<0.05 was considered to indicate a statistically significant
difference. The data were extracted from the Oncomine database and
Kaplan-Meier Plotter between March 2015 and August 2015. Finally,
the association between CCND1 protein expression, assessed
according to previously published protocols (30,38)
(CCND1/β-actin ratio), and differentiation type (moderate and poor
differentiation) in fresh specimens derived from patients with
gastric adenocarcinoma was assessed using the Student's t-test. The
statistical differences between two groups were assessed, and
P<0.05 was considered to indicate a statistically significant
difference. Statistical analysis was performed using GraphPad Prism
5 (GraphPad Software, Inc., La Jolla, CA, USA).
Results
Analysis of CCND1, CCND2 and CCND3
gene expression
Data for CCND1, CCND2 and CCND3
transcript expression were extracted from the Oncomine database for
gastric cancer, focusing on cancer vs. normal patient datasets. The
statistical significance, fold change, patient number, and type of
tissues that displayed upregulation or downregulation of CCND1,
CCND2 and CCND3 gene expression were also analyzed in
normal vs. cancer tissues from the Oncomine database. The Derrico
and Cho datasets obtained from Oncomine is embedded in the NCBI GEO
database (https://www.ncbi.nlm.nih.gov/geo/) at accession
numbers GSE13911 and GSE13861, respectively (Fig. 1). Overexpression and downregulation of
the CCND1, CCND2 and CCND3 genes were identified in
gastric cancer (Fig. 1). To determine
the clinical relevance of CCND1, CCND2 and
CCND3 expression in human gastric cancer, their expression
profiles in the oncomine cancer microarray database were analyzed.
Information on the expression of CCND1, CCND2 and
CCND3 in normal and cancerous gastric tissues was compiled
from all of the microarray studies in the database (39,40). The
histological type of gastric adenocarcinoma was divided into
intestinal, diffuse and mixed types (2). As demonstrated in Fig. 1A, CCND1 expression was
significantly increased in gastric intestinal-type adenocarcinoma
of gastric cancer (40). CCND2
expression was significantly increased in several types of gastric
cancer, including diffuse gastric adenocarcinoma, gastric
intestinal-type adenocarcinoma and gastric mixed adenocarcinoma
(Fig. 1B-E) (39,40). By
contrast, CCND3 expression was significantly decreased in gastric
mixed adenocarcinoma (Fig. 1F)
(39). Oncomine analysis of
neoplastic vs. normal tissue revealed that CCND1 and
CCND2 were overexpressed in gastric cancer from the GSE13911
and GSE13861 datasets, respectively.
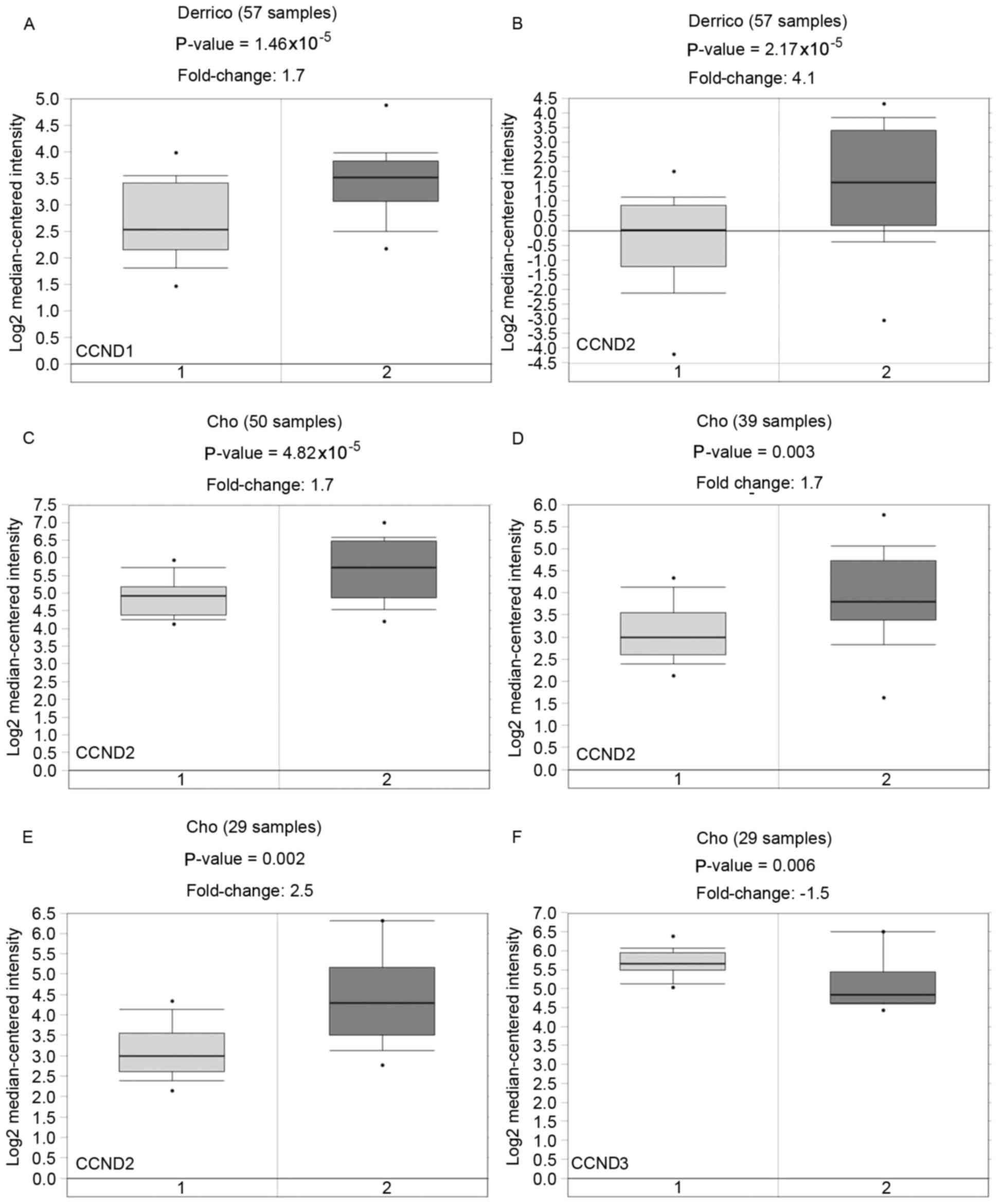 | Figure 1.Gene expression of CCND1,
CCND2 and CCND3 from Oncomine database in gastric
cancer. CCND1 was overexpressed in the Derrico dataset.
CCND2 was overexpressed in the Derrico and Cho datasets.
CCND3 was underexpressed in the Cho dataset. The expression
patterns of (A) CCND1, (B-E) CCND2 and (F)
CCND3 in gastric cancer datasets were obtained from the
Oncomine database. (A and B) 1, gastric mucosa; 2, gastric
intestinal type adenocarcinoma. (C) 1, gastric tissue; 2, diffuse
gastric adenocarcinoma. (D) 1, gastric tissue; 2, gastric
intestinal type adenocarcinoma. (E and F) 1, gastric tissue; 2,
gastric mixed adenocarcinoma. CCND, cyclin D. |
Association of CCND1, CCND2 and CCND3
expression with OS and PFS in patients with gastric cancer
To analyze the association of CCND1,
CCND2 and CCND3 expression with gastric cancer
patient survival, Kaplan-Meier survival curves were constructed
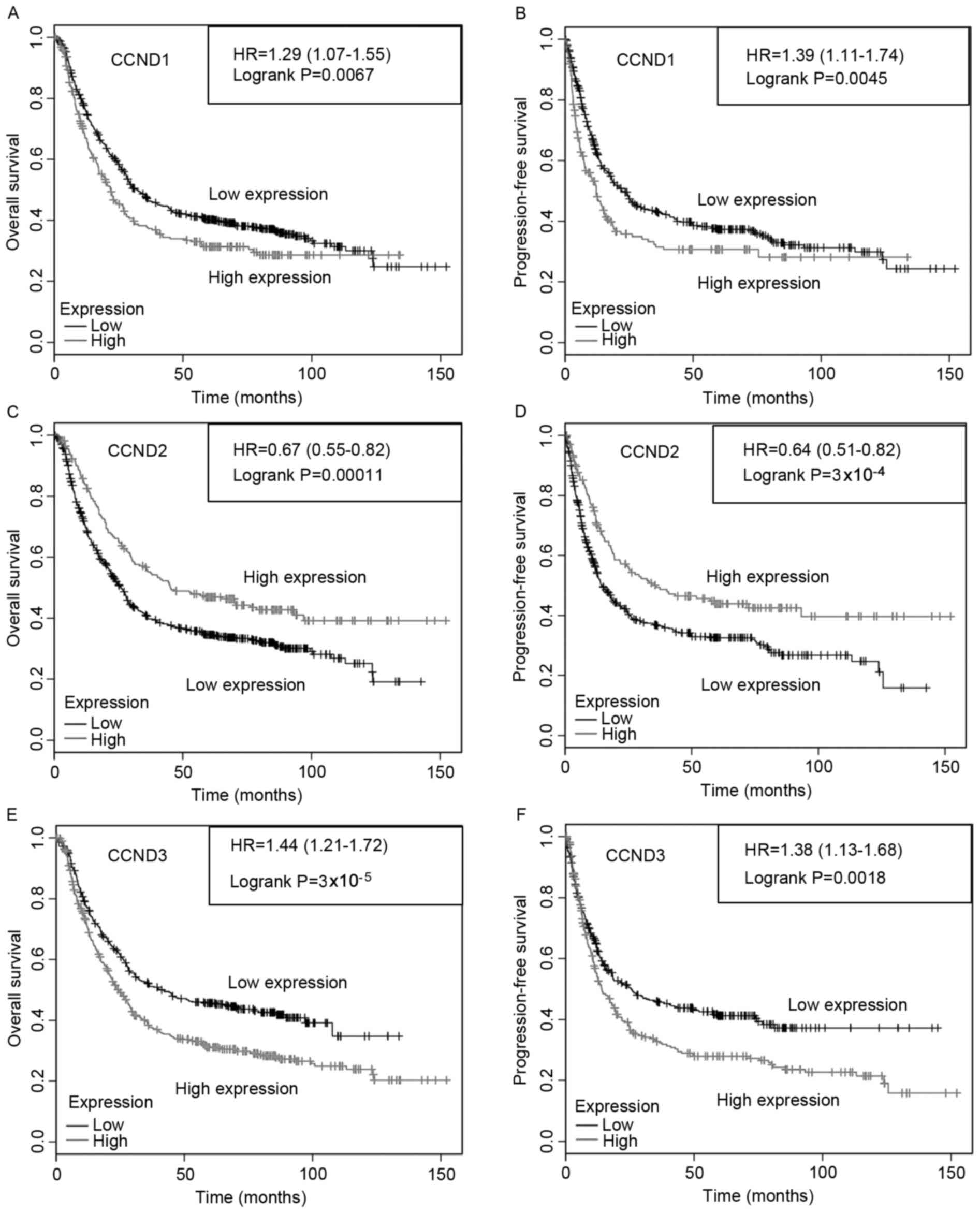
(Fig. 2). A significant association
was identified between CCND1, CCND2 and CCND3
mRNA and survival (P<0.05, log-rank test). Overexpression of
CCND1 (Fig. 2A and B) was
correlated with lower OS and PFS. By contrast, CCND2
overexpression was correlated with increased survival (Fig. 2C and D). Overexpression of
CCND3 was correlated with lower OS and PFS (Fig. 2E and F).
Effect of CCND1, CCND2 and CCND3
expression on gastric cancer patient survival by differentiation
types
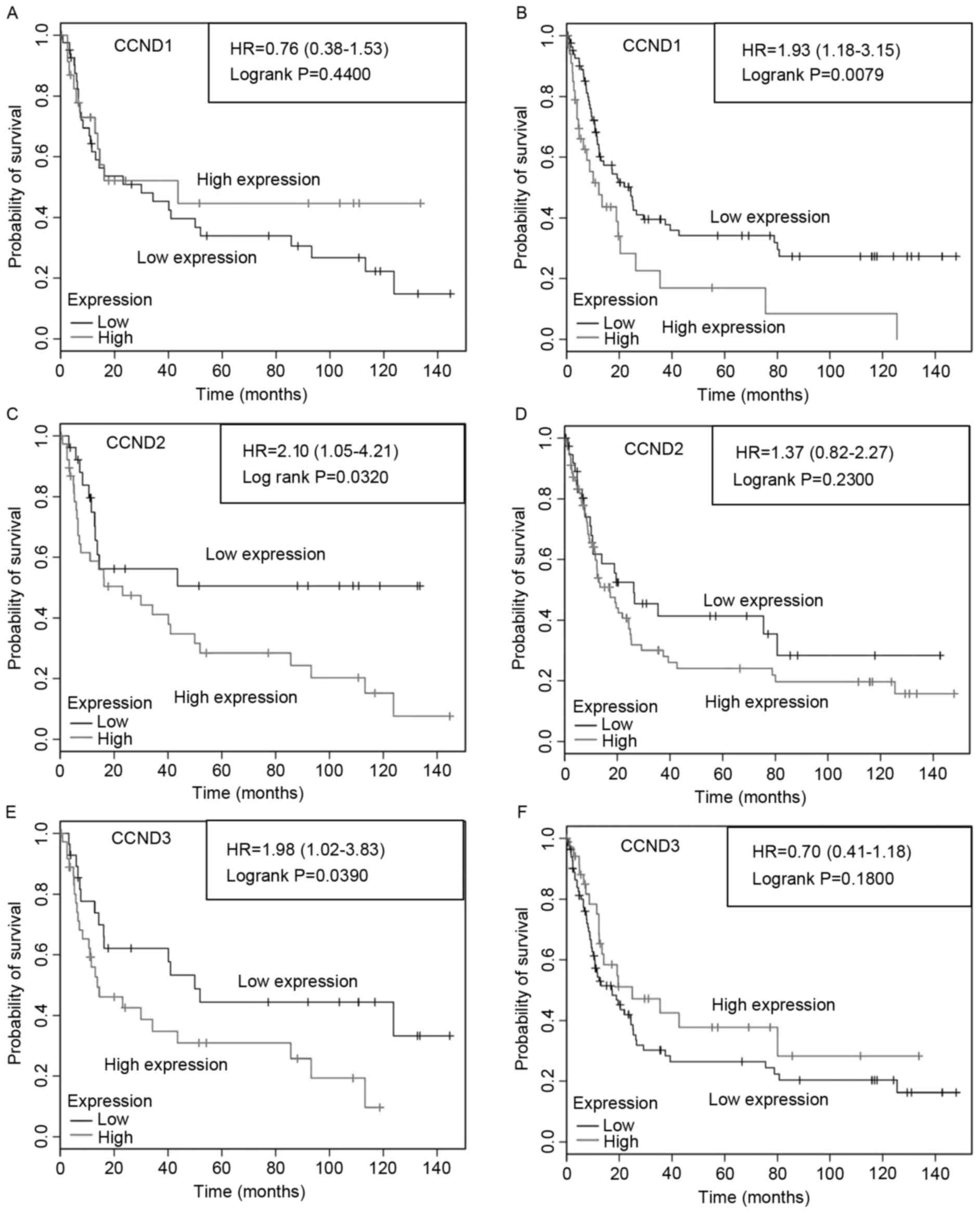
When the analysis was restricted by differentiation
type, significant differences in OS and PFS were observed for the
expression of the CCND1, CCND2 and CCND3 genes in
patients with moderately and poorly differentiated tumors.
Specifically, high CCND1 expression was not associated with PFS
compared with that of patients with low CCND1 expression in
patients with moderately differentiated gastric cancer (Fig. 3A), but in low CCND1 expression was
significantly (P=0.0079) longer associated with PFS compared with
that of patients with high CCND1 expression in patients with poorly
differentiated tumors (Fig. 3B). By
contrast, high CCND2 expression was associated with a significantly
poorer (P=0.032) PFS in patients with moderately differentiated
tumors (Fig. 3C), but not in those
with poorly differentiated gastric cancer (Fig. 3D). Additionally, low CCND3 expression
was associated with significantly (P=0.039) longer PFS in patients
with moderately differentiated tumors (Fig. 3E), but not in patients with poorly
differentiated gastric cancer (Fig.
3F). The results demonstrated that the high expression of CCND1
was significantly correlated with poor differentiation and poor
survival, while high expression of CCND2 and CCND3 was
significantly correlated with moderate differentiation and poor
survival.
Association of CCND1, CCND2 and CCND3
expression with gastric cancer patient survival by HER2 status
HER2 overexpression has been correlated with poor
outcomes and a more aggressive disease (41); however, the association between HER2
status and the prognosis of patients with gastric cancer remains
controversial (42). To analyze the
association of CCND1, CCND2 and CCND3 expression and HER2 status
with survival, Kaplan-Meier PFS curves of PFS stratified by CCND1,
CCND2 and CCND3 mRNA expression in HER2-negative and HER2-positive
tumors were constructed. Overexpression of CCND1 (Fig. 4A and B) was associated with reduced
PFS in HER2-negative and HER2-positive tumors. By contrast, reduced
CCND2 expression was associated with lower PFS in patients with
either HER2-negative or HER2-positive tumors (Fig. 4C and D). Overexpression of
CCND3 (Fig. 4E and F) was
associated with reduced PFS in HER2-negative and HER2-positive
tumors. These results indicated that CCND1, CCND2 and
CCND3 expression, and HER2 status were associated with
survival.
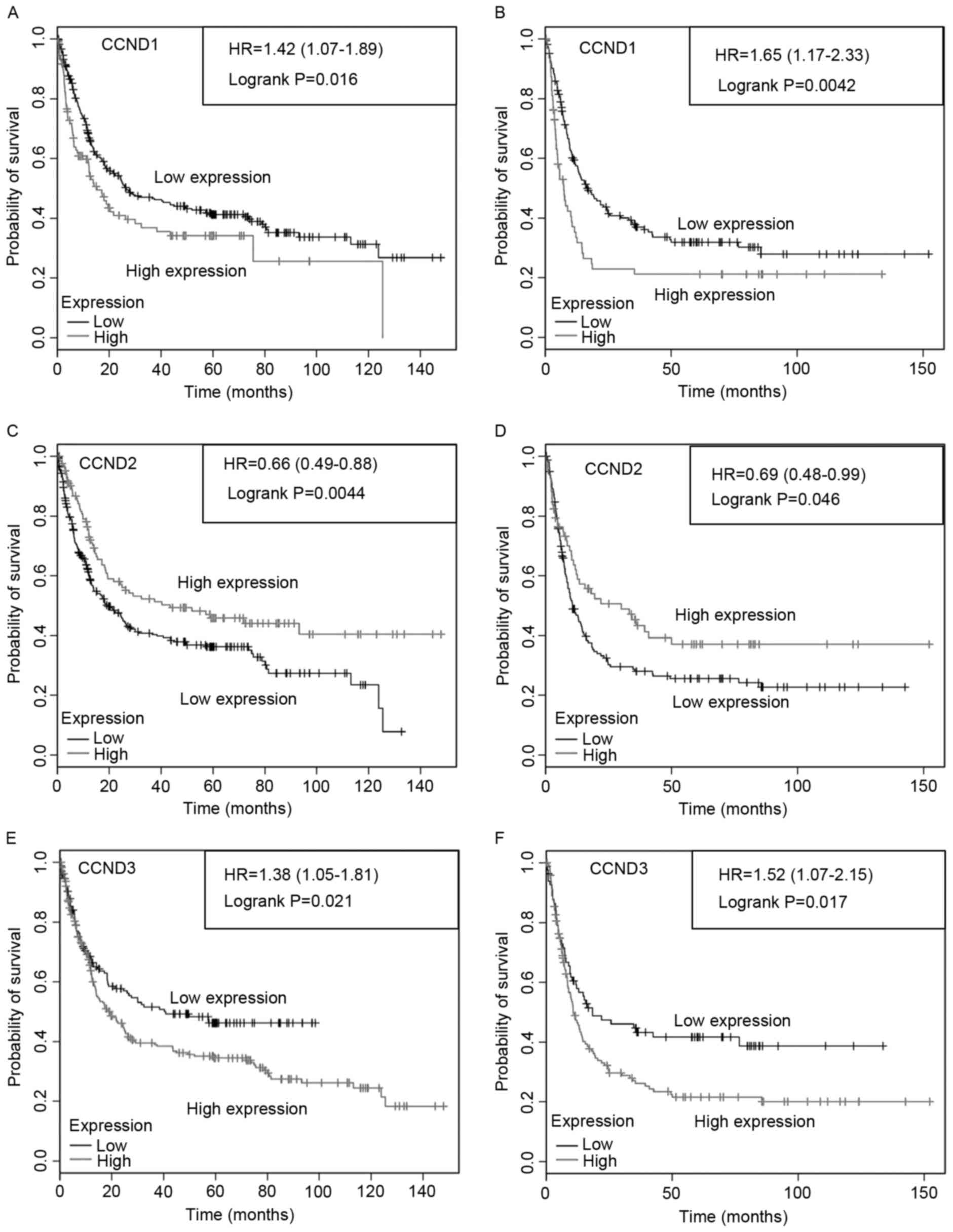 | Figure 4.Progression-free survival of patients
with gastric cancer with by HER2 status, and CCND1, CCND2
and CCND3 gene expression. Progression-free survival of
patients with (A, C and E) HER2-negative or (B, D and F)
HER2-positive tumors by (A and B) CCND1, (C and D)
CCND2 and (E and F) CCND3 expression. The total
number of patients in the low- and high-expression groups, as well
as the HR and P-values (logrank), are included. CCND, cyclin
D; HR, hazard ratio; HER2, receptor tyrosine-protein kinase
erb-2. |
Effects of CCND1, CCND2 and CCND3
expression on the PFS of patients with poorly differentiated tumors
by HER2 status
When the analysis was limited to those patients with
gastric cancer with poorly differentiated tumors and was restricted
by HER2 status, significant differences in PFS were observed
between high and low expression levels of CCND1,
CCND2 and CCND3 (Fig.
5). Low CCND1 expression was associated with
significantly longer PFS in patients with poorly differentiated,
HER2-negative tumors (Fig. 5A), but
not in those with poorly differentiated, HER2-positive tumors
(Fig. 5B). By contrast, low CCND2
expression was associated with significantly poorer PFS in patients
with poorly differentiated, HER2-negative tumors (Fig. 5C), but not in those with poorly
differentiated, HER2-positive tumors (Fig. 5D). PFS was not affected by CCND3 gene
expression level in poorly differentiated, HER2-negative or
HER2-positive tumors (Fig. 5E and F,
respectively). However, moderately differentiated tumors were not
analyzed in the present study due to the small number of patients.
Overexpression of CCND1 was significantly correlated with
HER2-negative tumors, poor differentiation and poor survival.
Additionally, downregulation of CCND2 was significantly correlated
with poor differentiation, HER2-negative tumor status and poor
survival. Taken together, these results suggest that overexpression
of CCND1 is predictive of a poor prognosis and serves an important
role in poorly differentiated, HER2-negative gastric tumors.
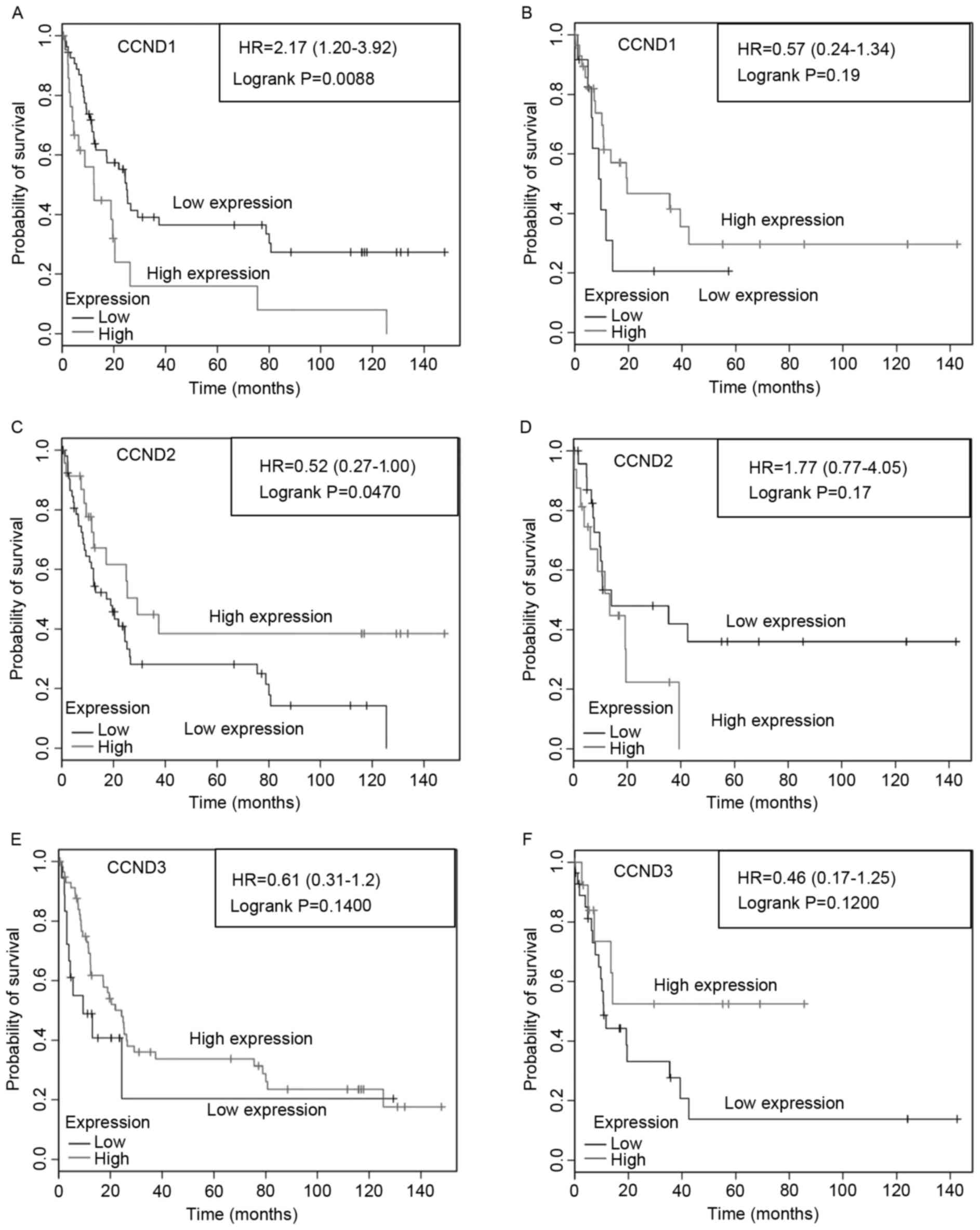 | Figure 5.Progression-free survival of patients
with poorly differentiated gastric cancer by HER2 expression, and
CCND1, CCND2 and CCND3 gene expression.
Progression-free survival of patients with (A, C and E) poorly
differentiated, HER2-negative or (B, D and F) poorly
differentiated, HER2-positive gastric cancer by (A and B)
CCND1, (C and D) CCND2 and (E and F) CCND3
expression. The total number of patients in the low- and
high-expression groups, as well as the HR and P-values (logrank),
are included. CCND, cyclin D; HR, hazard ratio; HER2,
receptor tyrosine-protein kinase erb-2. |
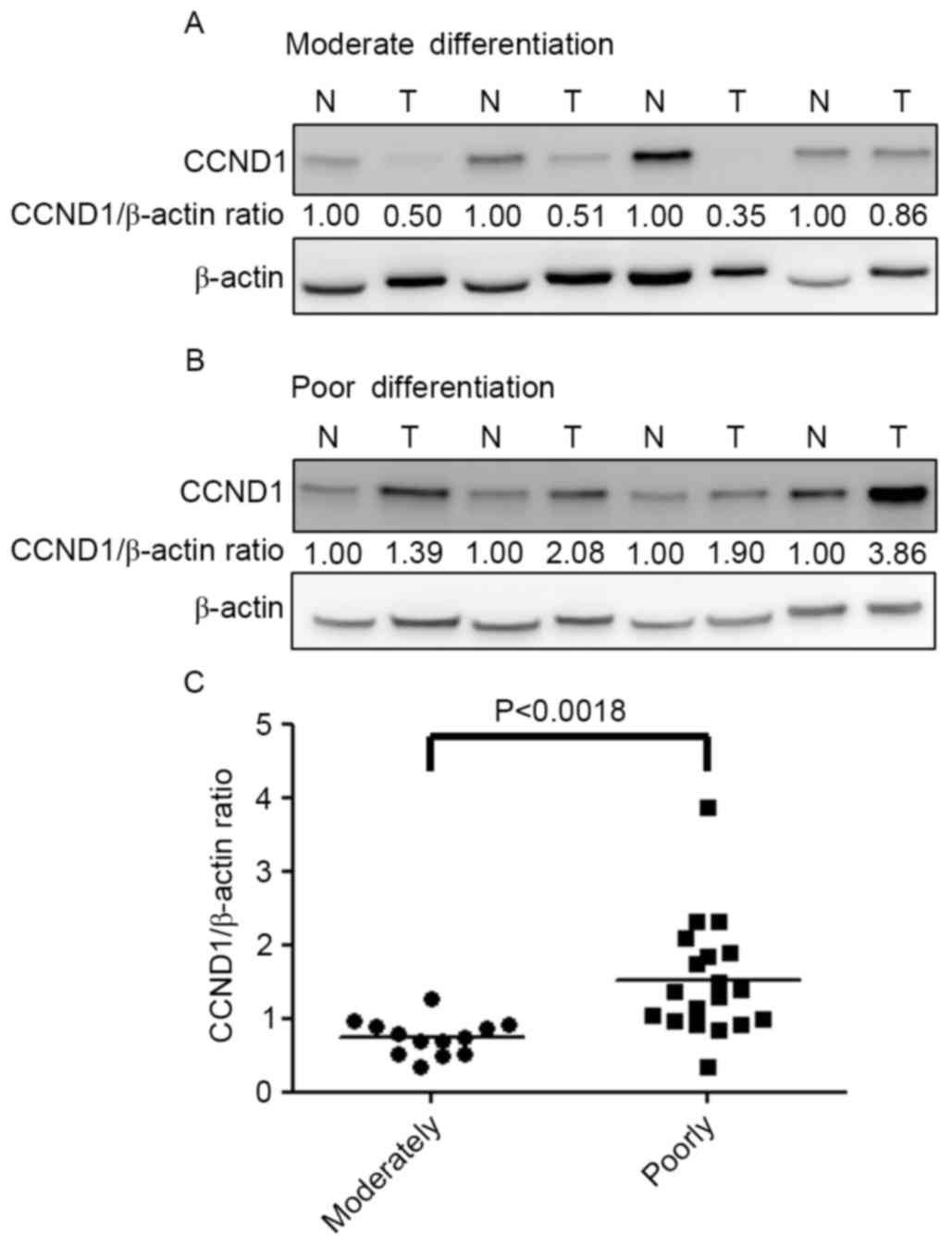
CCND1 protein expression in clinical
samples of gastric cancer tissues
CCND1 expression was also compared in moderately and
poorly differentiated gastric cancer, and CCND1 protein expression
was examined by western blot analysis in tumor and adjacent normal
gastric tissues of 32 patients. All 32 cases of gastric cancer were
adenocarcinomas, including 13 moderately differentiated and 19
poorly differentiated tumors (Table
I). Analysis of the relative expression of CCND1 in moderately
(Fig. 6A) and poorly (Fig. 6B) differentiated tissues indicated
that the CCND1/β-actin ratio in poorly differentiated samples was
significantly greater compared with that in moderately
differentiated samples (P<0.0018) (Fig. 6C). In summary, the overexpression of
CCND1 is associated with poorly differentiated gastric cancer.
 | Table I.Demographics and histopathological
data of patients with gastric cancer. |
Table I.
Demographics and histopathological
data of patients with gastric cancer.
| Characteristic | No. of patients
(%) |
|---|
| Patients with
gastric cancer | 32 (100) |
| Mean age ± standard
deviation, years | 60±11 |
| Sex |
|
Male | 20 (62) |
|
Female | 12 (38) |
| Histological
differentiation |
|
Moderate | 13 (40) |
|
Poor | 19 (60) |
| Lauren's
classification |
|
Intestinal | 15 (47) |
|
Diffuse | 12 (37) |
|
Mixed | 5 (16) |
| American Joint
Committee on cancer tumor node metastasis stage |
| I | 7 (22) |
| II | 9 (28) |
|
III | 12 (38) |
| IV | 4 (12) |
Discussion
Analysis of CCND1, CCND2 and CCND3
expression in human gastric cancer identified that overexpression
of CCND1 was associated with poor survival of patients with
poorly differentiated gastric cancer. In addition, the effect of
CCND1 overexpression in patients with HER2-negative tumors
was correlated with poor outcomes. The present study suggests that
the overexpression of CCND1, but not of CCND2 or
CCND3, in poorly differentiated gastric cancer is closely
associated with lower survival rates. CCND2 and CCND3 expression
were associated with moderate differentiation. Consistent with
these results, the present study identified that the CCND1 protein
is overexpressed in poorly differentiated gastric tumors. Thus,
CCND1 serves a prognostic role in tumor progression, and is
involved in the regulation of tumor cell differentiation.
Oncomine analysis identified a strong correlation
between CCND1 and CCND2 gene expression and certain
subtypes of gastric cancer in the present study. Histologically,
gastric cancer is divided into two types in the Lauren
classification: Intestinal and diffuse (2). In the present study, gastric
intestinal-type adenocarcinomas were associated with CCND1
expression, while diffuse gastric adenocarcinoma, gastric
intestinal-type adenocarcinoma and gastric mixed adenocarcinoma
types were associated with CCND2 expression. Notably, a
previous study suggested that promoter hypermethylation of
CCND2 caused the loss of CCND2 function in gastric cell
lines and primary gastric carcinomas (43). In addition, CCND2 protein expression
was not detected in KATOIII, AGS, MKN45 or N87 cell lines (43). It has been suggested that
hypermethylation of the CCND2 promoter occurs in breast
(44), prostate (45) and gastric cancer (43). To date, there have been few studies
investigating CCND2 promoter hypomethylation in colon cancer
(46). These studies explain that
CCND2 overexpression was an early event noted in colon polyps
(47), and possibly the
overexpression of CCND2, but not of CCND1 or CCND3, was associated
with metastatic tumors (46). As
patients with diffuse gastric cancer exhibit poorer prognoses and
higher incidences of metastasis compared with those of patients
with intestinal type tumors (48,49), it is
possible that CCND2 overexpression in diffuse gastric
adenocarcinoma is also associated with promoter hypomethylation in
the early stage, and reveals a potential metastatic role for
CCND2.
The Kaplan-Meier analysis of the present study
identified correlations between CCND1, CCND2 and
CCND3 gene expression and clinical outcomes. An elevated
expression of CCND1 was significantly associated with poor
differentiation and poorer PFS. Clinically, the major histological
types of metastatic gastric cancer include poorly differentiated
and signet ring adenocarcinomas (50,51).
CCND1 overexpression is associated with shorter patient
survival in mantle cell lymphoma and head and neck squamous cell
carcinoma (11,12). In addition, CCND1
overexpression is often associated with increased metastasis, which
is consistent with the ability of CCND1 to enhance migration
and invasion (12,52). The expression and potential roles of
CCND1 in gastric cancer have been investigated, and previous
studies have demonstrated the manner in which it contributes to
carcinogenesis (53–58). For example, direct evidence that CCND1
serves an essential role in the cell proliferation of gastric
cancer cell lines has been presented (54). Evidence has also demonstrated that
increased CCND1 expression was associated with decreased OS
in patients with resected gastric adenocarcinoma (59).
The majority of tumor markers are produced at much
higher levels in cancer cells than in normal cells, and may be
identified in the blood, urine or tumor tissues of patients with
cancer (60–62). Thus, tumor marker analysis may reflect
the various stages of the cancer and may assist clinicians in the
planning and monitoring of cancer treatment. The present study
attempted to identify CCND1 as a prognostic biomarker of poorly
differentiated gastric cancer. In a previous study, inhibition of
CCND1 using specific targeting was presented as a novel gastric
cancer therapy (56). Amplification
of CCND1 causes resistance to certain cytotoxic drugs and targeted
therapies, including gefitinib and tamoxifen (63,64), and
is a potential predictor of resistance to cancer therapy in breast
cancer (65). In addition,
HER2 is overexpressed and/or gene-amplified in gastric
cancer, although numerous studies have yielded inconsistent data
regarding the prognostic relevance of HER2 (66). Whereas certain studies demonstrated
that HER2 positivity was associated with significantly poor
prognosis (66), other studies
identified no association between HER2 status and prognosis
(66). In a previous study, 7–17% of
patients with gastric cancer were HER2-positive and, thus, suitable
candidates for trastuzumab therapy (67). In addition, amplification of CCND1 was
revealed in 17.4% of gastric cancers (60). Clinically, intra-tumor heterogeneity
often results in failure of gene therapy and targeted therapy in
gastric adenocarcinoma (60). In the
present study, the heterogeneity of the potential target genes,
CCND1, CCND2 and CCND3, was systematically
analyzed. To identify the most prevalent molecular targets in
poorly differentiated gastric cancer, the patient outcomes relative
to CCND1 overexpression and HER2 status were determined. The
results imply that CCND1 overexpression of poorly differentiated
gastric cancer causes resistance to certain cytotoxic drugs and
targeted therapies.
In conclusion, to the best of our knowledge, the
present study is the first to suggest that overexpression of CCND1
protein is correlated with lower PFS in poorly differentiated
gastric cancer. These results demonstrate that CCND1, but not CCND2
or CCND3, is overexpressed in human gastric carcinoma, and that the
expression of this protein is correlated with tumor
differentiation. Thus, CCND1 expression is a valuable prognostic
indicator for gastric cancer.
Acknowledgements
The present study was supported by grants from the
Ministry of Science and Technology of Taiwan (grant nos. MOST
102-2311-B041-001 and MOST 103-2311-B-041-001). The authors would
like to thank the Human Biobank, the Research Center of Clinical
Medicine and the National Cheng Kung University Hospital for their
support.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lauren P: The two histological main types
of gastric carcinoma: Diffuse and so-called intestinal-type
carcinoma. An attempt at a histo-clinical classification. Acta
Pathol Microbiol Scand. 64:31–49. 1965. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Correa P: Human gastric carcinogenesis: A
multistep and multifactorial process-first American cancer society
award lecture on cancer epidemiology and prevention. Cancer Res.
52:6735–6740. 1992.PubMed/NCBI
|
|
4
|
Bang YJ, Van Cutsem E, Feyereislova A,
Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T,
et al: Trastuzumab in combination with chemotherapy versus
chemotherapy alone for treatment of HER2-positive advanced gastric
or gastro-oesophageal junction cancer (ToGA): A phase 3,
open-label, randomised controlled trial. Lancet. 376:687–697. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nagini S: Carcinoma of the stomach: A
review of epidemiology, pathogenesis, molecular genetics and
chemoprevention. World J Gastrointest Oncol. 4:156–169. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang W, Raufi A and Klempner SJ: Targeted
therapy for gastric cancer: Molecular pathways and ongoing
investigations. Biochim Biophys Acta. 1846:232–237. 2014.PubMed/NCBI
|
|
7
|
Matsuoka T and Yashiro M: The role of
PI3K/Akt/mTOR signaling in gastric carcinoma. Cancers. 6:1441–1463.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Matsushime H, Ewen ME, Strom DK, Kato JY,
Hanks SK, Roussel MF and Sherr CJ: Identification and properties of
an atypical catalytic subunit (p34PSK-J3/cdk4) for mammalian D type
G1 cyclins. Cell. 71:323–334. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Büschges R, Weber RG, Actor B, Lichter P,
Collins VP and Reifenberger G: Amplification and expression of
cyclin D genes (CCND1, CCND2 and CCND3) in human malignant gliomas.
Brain Pathol. 9:435–433. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Musgrove EA, Caldon CE, Barraclough J,
Stone A and Sutherland RL: Cyclin D as a therapeutic target in
cancer. Nat Rev Cancer. 7:558–572. 2011. View Article : Google Scholar
|
|
11
|
Jares P, Colomer D and Campo E: Genetic
and molecular pathogenesis of mantle cell lymphoma: Perspectives
for new targeted therapeutics. Nat Rev Cancer. 7:750–762. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Thomas GR, Nadiminti H and Regalado J:
Molecular predictors of clinical outcome in patients with head and
neck squamous cell carcinoma. Int J Exp Pathol. 86:347–363. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
van Diest PJ, Michalides RJ, Jannink L,
van der Valk P, Peterse HL, de Jong JS, Meijer CJ and Baak JP:
Cyclin D1 expression in invasive breast cancer. Correlations and
prognostic value. Am J Pathol. 150:705–711. 1997.PubMed/NCBI
|
|
14
|
Jin ML, Inoue S, Umemura T, Moriya J,
Arakawa M, Nagashima K and Kato H: Cyclin D1, p16 and
retinoblastoma gene product expression as a predictor for prognosis
in non-small cell lung cancer at stages I and II. Lung Cancer.
34:207–218. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yamanouchi H, Furihata M, Fujita J,
Murakami H, Yoshinouchi T, Takahara J and Ohtsuki Y: Expression of
cyclin E and cyclin D1 in non-small cell lung cancers. Lung Cancer.
31:3–8. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ikeguchi M, Sakatani T, Ueta T and Kaibara
N: Cyclin D1 expression and retinoblastoma gene protein (pRB)
expression in esophageal squamous cell carcinoma. J Cancer Res Clin
Oncol. 127:531–536. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Izzo JG, Papadimitrakopoulou VA, Li XQ,
Ibarguen H, Lee JS, Ro JY, El-Naggar A, Hong WK and Hittelman WN:
Dysregulated cyclin D1 expression early in head and neck
tumorigenesis: In vivo evidence for an association with subsequent
gene amplification. Oncogene. 17:2313–2322. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bartkova J, Lukas J, Müller H, Strauss M,
Gusterson B and Bartek J: Abnormal patterns of D-type cyclin
expression and G1 regulation in human head and neck cancer. Cancer
Res. 55:949–956. 1995.PubMed/NCBI
|
|
19
|
Gansauge S, Gansauge F, Ramadani M, Stobbe
H, Rau B, Harada N and Beger HG: Overexpression of cyclin D1 in
human pancreatic carcinoma is associated with poor prognosis.
Cancer Res. 57:1634–1637. 1997.PubMed/NCBI
|
|
20
|
Carthon BC, Neumann CA, Das M, Pawlyk B,
Li T, Geng Y and Sicinski P: Genetic replacement of cyclin D1
function in mouse development by cyclin D2. Mol Cell Biol.
25:1081–1088. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Choi D, Yoon S, Lee E, Hwang S, Yoon B and
Lee J: The expression of pseudogene cyclin D2 mRNA in the human
ovary may be a novel marker for decreased ovarian function
associated with the aging process. J Assist Reprod Genet.
18:110–113. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bartkova J, Rajpert-De Meyts E, Skakkebaek
NE and Bartek J: D-type cyclins in adult human testis and
testicular cancer: Relation to cell type, proliferation,
differentiation, and malignancy. J Pathol. 187:573–581. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Takano Y, Kato Y, Masuda M, Ohshima Y and
Okayasu I: Cyclin D2, but not cyclin D1, overexpression closely
correlates with gastric cancer progression and prognosis. J Pathol.
189:194–200. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yu J, Leung WK, Ng EK, To KF, Ebert MP, Go
MY, Chan WY, Chan FK, Chung SC, Malfertheiner P, et al: Effect of
helicobacter pylori eradication on expression of cyclin D2
and p27 in gastric intestinal metaplasia. Aliment Pharmacol Ther.
15:1505–1511. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bartkova J, Lukas J, Strauss M and Bartek
J: Cyclin D3: Requirement for G1/S transition and high abundance in
quiescent tissues suggest a dual role in proliferation and
differentiation. Oncogene. 17:1027–1037. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Flørenes VA, Faye RS, Maelandsmo GM,
Nesland JM and Holm R: Levels of cyclin D1 and D3 in malignant
melanoma: Deregulated cyclin D3 expression is associated with poor
clinical outcome in superficial melanoma. Clin Cancer Res.
6:3614–3620. 2000.PubMed/NCBI
|
|
27
|
Ito Y, Takeda T, Wakasa K, Tsujimoto M and
Matsuura N: Expression and possible role of cyclin D3 in human
pancreatic adenocarcinoma. Anticancer Res. 21:1043–1048.
2001.PubMed/NCBI
|
|
28
|
Wong SC, Chan JK, Lee KC and Hsiao WL:
Differential expression of p16/p21/p27 and cyclin D1/D3 and their
relationships to cell proliferation, apoptosis and tumour
progression in invasive ductal carcinoma of the breast. J Pathol.
194:35–42. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rhodes DR, Kalyana-Sundaram S, Mahavisno
V, Varambally R, Yu J, Briggs BB, Barrette TR, Anstet MJ,
Kincead-Beal C, Kulkarni P, et al: Oncomine 3.0: Genes, pathways,
and networks in a collection of 18,000 cancer gene expression
profiles. Neoplasia. 9:166–180. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shan YS, Hsu HP, Lai MD, Yen MC, Luo YP
and Chen YL: Increased expression of argininosuccinate synthetase
protein predicts poor prognosis in human gastric cancer. Oncol Rep.
33:49–57. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Edge SB and Compton CC: The American joint
committee on cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shan YS, Fang JH, Lai MD, Yen MC, Lin PW,
Hsu HP, Lin CY and Chen YL: Establishment of an orthotopic
transplantable gastric cancer animal model for studying the
immunological effects of new cancer therapeutic modules. Mol
Carcinog. 50:739–750. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shan YS, Hsu HP, Lai MD, Yen MC, Chen WC,
Fang JH, Weng TY and Chen YL: Argininosuccinate synthetase 1
suppression and arginine restriction inhibit cell migration in
gastric cancer cell lines. Sci Rep. 5:97832015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rhodes DR, Yu J, Shanker K, Deshpande N,
Varambally R, Ghosh D, Barrette T, Pandey A and Chinnaiyan AM:
ONCOMINE: A cancer microarray database and integrated data-mining
platform. Neoplasia. 6:1–6. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Győrffy B, Surowiak P, Budczies J and
Lànczky A: Online survival analysis software to assess the
prognostic value of biomarkers using transcriptomic data in
non-small-cell lung cancer. PLoS one. 8:e822412013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Szász AM, Lánczky A, Nagy Á, Förster S,
Hark K, Green JE, Boussioutas A, Busuttil R, Szabó A and Győrffy B:
Cross-validation of survival associated biomarkers in gastric
cancer using transcriptomic data of 1,065 patients. Oncotarget.
7:49322–49333. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Győrffy B, Benke Z, Lánczky A, Balázs B,
Szállási Z, Timár J and Schäfer R: RecurrenceOnline: An online
analysis tool to determine breast cancer recurrence and hormone
receptor status using microarray data. Breast Cancer Res Treat.
132:1025–1034. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Takano Y, Kato Y, van Diest PJ, Masuda M,
Mitomi H and Okayasu I: Cyclin D2 overexpression and lack of p27
correlate positively and cyclin E inversely with a poor prognosis
in gastric cancer cases. Am J Pathol. 156:585–594. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cho JY, Lim JY, Cheong JH, Park YY, Yoon
SL, Kim SM, Kim SB, Kim H, Hong SW, Park YN, et al: Gene expression
signature-based prognostic risk score in gastric cancer. Clin
Cancer Res. 17:1850–1857. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
D'Errico M, de Rinaldis E, Blasi MF, Viti
V, Falchetti M, Calcagnile A, Sera F, Saieva C, Ottini L, Palli D,
et al: Genome-wide expression profile of sporadic gastric cancers
with microsatellite instability. Eur J Cancer. 45:461–469. 2009.
View Article : Google Scholar
|
|
41
|
Kaptain S, Tan LK and Chen B: Her-2/neu
and breast cancer. Diagn Mol Pathol. 10:139–152. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gravalos C and Jimeno A: HER2 in gastric
cancer: A new prognostic factor and a novel therapeutic target. Ann
Oncol. 19:1523–1529. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yu J, Leung WK, Ebert MP, Leong RW, Tse
PC, Chan MW, Bai AH, To KF, Malfertheiner P and Sung JJ: Absence of
cyclin D2 expression is associated with promoter hypermethylation
in gastric cancer. Br J Cancer. 88:1560–1565. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Evron E, Umbricht CB, Korz D, Raman V,
Loeb DM, Niranjan B, Buluwela L, Weitzman SA, Marks J and Sukumar
S: Loss of cyclin D2 expression in the majority of breast cancers
is associated with promoter hypermethylation. Cancer Res.
61:2782–2787. 2001.PubMed/NCBI
|
|
45
|
Padar A, Sathyanarayana UG, Suzuki M,
Maruyama R, Hsieh JT, Frenkel EP, Minna JD and Gazdar AF:
Inactivation of cyclin D2 gene in prostate cancers by aberrant
promoter methylation. Clin Cancer Res. 9:4730–4734. 2003.PubMed/NCBI
|
|
46
|
Mermelshtein A, Gerson A, Walfisch S,
Delgado B, Shechter-Maor G, Delgado J, Fich A and Gheber L:
Expression of D-type cyclins in colon cancer and in cell lines from
colon carcinomas. Br J Cancer. 93:338–345. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Bartkova J, Thullberg M, Slezak P,
Jaramillo E, Rubio C, Thomassen LH and Bartek J: Aberrant
expression of G1-phase cell cycle regulators in flat and exophytic
adenomas of the human colon. Gastroenterology. 120:1680–1688. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Cunningham D, Allum WH, Stenning SP,
Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ,
Falk SJ, Iveson TJ, et al: Perioperative chemotherapy versus
surgery alone for resectable gastroesophageal cancer. N Engl J Med.
355:11–20. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lim L, Michael M, Mann GB and Leong T:
Adjuvant therapy in gastric cancer. J Clin Oncol. 23:6220–6232.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Matsuura Y, Saito R, Kawagoe T, Toki N,
Sugihara K and Kashimura M: Cytologic analysis of primary stomach
adenocarcinoma metastatic to the uterine cervix. Acta Cytol.
41:291–294. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Pèrez-Montiel D, Serrano-Olvera A, Salazar
LC, Cetina-Pèrez L, Candelaria M, Coronel J, Montalvo LA and de
Leon DC: Adenocarcinoma metastatic to the uterine cervix: A case
series. J Obstet Gynaecol Res. 38:541–549. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Jares P, Colomer D and Campo E: Genetic
and molecular pathogenesis of mantle cell lymphoma: Perspectives
for new targeted therapeutics. Nat Rev Cancer. 7:750–762. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Nakagawa H, Zukerberg L, Togawa K, Meltzer
SJ, Nishihara T and Rustgi AK: Human cyclin D1 oncogene and
esophageal squamous cell carcinoma. Cancer. 76:541–549. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Chen B, Zhang XY, Zhang YJ, Zhou P, Gu Y
and Fan DM: Antisense to cyclin D1 reverses the transformed
phenotype of human gastric cancer cells. World J Gastroenterol.
5:18–21. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zhou P, Jiang W, Zhang YJ, Kahn SM,
Schieren I, Santella RM and Weinstein IB: Antisense to cyclin D1
inhibits growth and reverses the transformed phenotype of human
esophageal cancer cells. Oncogene. 11:571–580. 1995.PubMed/NCBI
|
|
56
|
Seo JH, Jeong ES and Choi YK: Therapeutic
effects of lentivirus-mediated shRNA targeting of cyclin D1 in
human gastric cancer. BMC cancer. 14:1752014. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Jiang W, Kahn SM, Zhou P, Zhang YJ, Cacace
AM, Infante AS, Doi S, Santella RM and Weinstein IB: Overexpression
of cyclin D1 in rat fibroblasts causes abnormalities in growth
control, cell cycle progression and gene expression. Oncogene.
8:3447–3457. 1993.PubMed/NCBI
|
|
58
|
Baldin V, Lukas J, Marcote MJ, Pagano M
and Draetta G: Cyclin D1 is a nuclear protein required for cell
cycle progression in G1. Genes Dev. 7:812–821. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Ma L, Wang X, Lan F, Yu Y, Ouyang X, Liu
W, Xie F and Huang Q: Prognostic value of differential CCND1
expression in patients with resected gastric adenocarcinoma. Med
Oncol. 32:3382015. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Stahl P, Seeschaaf C, Lebok P, Kutup A,
Bockhorn M, Izbicki JR, Bokemeyer C, Simon R, Sauter G and Marx AH:
Heterogeneity of amplification of HER2, EGFR, CCND1 and MYC in
gastric cancer. BMC Gastroenterol. 15:72015. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Liu SC, Bassi DE, Zhang SY, Holoran D,
Conti CJ and Klein-Szanto AJ: Overexpression of cyclin D2 is
associated with increased in vivo invasiveness of human squamous
carcinoma cells. Mol Carcinog. 34:131–139. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Wang W, Zhao LJ, Tan YX, Ren H and Qi ZT:
MiR-138 induces cell cycle arrest by targeting cyclin D3 in
hepatocellular carcinoma. Carcinogenesis. 33:1113–1120. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Kalish LH, Kwong RA, Cole IE, Gallagher
RM, Sutherland RL and Musgrove EA: Deregulated cyclin D1 expression
is associated with decreased efficacy of the selective epidermal
growth factor receptor tyrosine kinase inhibitor gefitinib in head
and neck squamous cell carcinoma cell lines. Clin Cancer Res.
10:7764–7774. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Stendahl M, Kronblad A, Rydén L, Emdin S,
Bengtsson NO and Landberg G: Cyclin D1 overexpression is a negative
predictive factor for tamoxifen response in postmenopausal breast
cancer patients. Br J Cancer. 90:1942–1948. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Lundgren K, Brown M, Pineda S, Cuzick J,
Salter J, Zabaglo L, Howell A, Dowsett M and Landberg G: TransATAC
investigators: Effects of cyclin D1 gene amplification and protein
expression on time to recurrence in postmenopausal breast cancer
patients treated with anastrozole or tamoxifen: A TransATAC study.
Breast Cancer Res. 14:R572012. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Boku N: HER2-positive gastric cancer.
Gastric Cancer. 17:1–12. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Deng NT, Goh LK, Wang HN, Das K, Tao J,
Tan IB, Zhang SL, Lee MH, Wu JN, Lim KH, et al: A comprehensive
survey of genomic alterations in gastric cancer reveals systematic
patterns of molecular exclusivity and co-occurrence among distinct
therapeutic targets. Gut. 61:673–684. 2012. View Article : Google Scholar : PubMed/NCBI
|