Introduction
Esophageal carcinoma is the most common type of
cancer worldwide with ~482,300 novel cases and 406,800 mortalities
reported annually (1). In China, the
incidence of esophageal carcinoma accounts for 50% of cases
worldwide and esophageal carcinoma is the fourth leading cause of
malignant tumor-associated mortality, with 95% of esophageal cancer
cases diagnosed as squamous cell carcinoma (2). Surgery is the optimal treatment for
patients with esophageal carcinoma (3,4); however,
the majority of patients do not survive due to tumor recurrence, in
spite of radical resection and extended lymph node dissection
having been performed. A number of factors affect tumor recurrence
including age, sex, local tumor stage, tumor location, degree of
cell differentiation, lymph node metastases or vascular involvement
(5).
Aldehyde dehydrogenase 1 (ALDH1) is a detoxifying
enzyme which responds to the oxidation of intracellular aldehydes
(6,7).
The function of ALDH1 is to oxidize intracellular aldehydes and
therefore confer resistance to alkylating agents (8). As a modulator of cell viability, ALDH1
converts retinol into retinoic acid, which serves an important
function in the early differentiation of stem cells (9). Murine, human hematopoietic, neural stem
and progenitor cells have been identified to exhibit increased
ALDH1 activity, and ALDH1 activity is a commonly used marker for
healthy and malignant stem cells (10,11).
Previous immunohistochemistry results have demonstrated that ALDH1
expression was limited in healthy tissue, but was markedly
increased in malignant tissue, including breast, lung and
colorectal cancer (12–14). However, whether the expression of
ALDH1 is associated with the differentiation of tumor cells in
esophageal carcinoma remains unknown. Therefore, the present study
aimed at identifying whether ALDH1 expression exhibited an
association with patients with esophageal cancer using fluorescent
immunostaining.
In 1994, Grimason et al (15) used the fluorogen DAPI to interact with
the nuclei of sporulated oocysts in conjunction with a fluorescein
isothiocyanate-conjugated anti-cryptosporidium monoclonal antibody
and used fluorescence microscopy to visualize the oocyst nuclei,
which enabled improved observation. DAPI is a non-cytotoxic dye
that does not affect cell viability (16). DAPI is able to be combined with
cellular DNA, permeate through the membrane of cell, rapidly enter
the nucleus of living cells and bind with DNA to form a DAPI-DNA
complex. The wavelengths of the complex for excitation and emission
are 360 and 460 nm, respectively. Under the excitation of an
ultraviolet ray, DAPI exhibits blue fluorescence, therefore under a
fluorescence microscope a blue nucleus may be observed. The formula
of DAPI is C16H15N5 and the
molecular mass is 277,324 Da (17).
In the present study, DAPI was used to
non-specifically stain the nuclei of the tumor cells. Subsequently,
ALDH1-specific fluorescent staining was used on the cancer stem
cell cytoplasm and a merged image was developed. The aim of the
present study was to use indirect fluorescent immunostaining to
identify whether the expression of ALDH1 may be a notable
clinicopathological prognostic factor for human esophageal
carcinoma.
Materials and methods
Patients and tissues
Specimens of human esophageal squamous cell
carcinomas were obtained from the Department of Pathology, The
First Hospital of Qiqihar (Qiqihar, China) between January 2010 and
January 2014. Prior to surgery no patients had received any
therapy, including chemotherapy or radiation. Of the 50 specimens,
10 cases were well-differentiated, 20 were moderately
differentiated and 20 cases were poorly differentiated squamous
tumor cells. In addition, healthy esophageal tissues were obtained
from the same cohort of patients, but these were obtained from a
distant location from the esophageal carcinoma (≥5 cm). All tissues
(thickness, 4 µm) were fixed in formalin, embedded in paraffin.
When required, the tissues were deparaffinized and dehydrated in
10% formalin at 27°C for 5 min. The present study was approved by
the Ethical Committee of the First Hospital of Qiqihar.
Additionally, written informed consent was obtained from all
participating patients. All tissues were evaluated by two
pathologists individually.
Indirect fluorescent
immunostaining
Indirect fluorescent immunostaining was performed as
described previously (18). First,
DAPI (dilution, 1:500; cat no. D9564; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) was used to non-specifically stain the nuclei
of cancer cells (15). The cells were
then incubated at 37°C with fluorophore I-labeled IgG (dilution,
1:20; catalog no. HZ3387121; excitation, 360 nm; emission, 460 nm;
EarthOx Life Sciences, Millbrae, CA, USA) for 30 min. Sections were
rinsed with TBS-Tween-20 (TBST) three times and incubated at 4°C
with an antibody against ALDH1 (dilution, 1:400; cat no. HZ3487111;
EarthOx Life Sciences) overnight. Subsequently, sections were
incubated at 37°C with fluorophore II-labeled IgG (dilution, 1:50;
cat no. HZ3387125; excitation, 490 nm; emission, 520 nm; EarthOx
Life Sciences) for 30 min. Sections were rinsed with TBST three
times and coverslips were placed on the slides. Finally,
fluorescence microscopy (magnification, ×200; Nikon Eclipse 80i;
Nikon Corporation, Tokyo, Japan) was used to observe the
results.
Evaluation of labeling
Evaluation of the expression of ALDH1 was performed
by two pathologists independently. Imaging analysis of ALDH1
expression was performed in one selected area per case. Cases
exhibiting ≥20% positive cells were classified as significant and
the remaining cases were classified as negative ALDH1 expression
(19).
Statistical analysis
All data were analyzed using SPSS software (version
12.0; SPSS, Inc., Chicago, IL, USA). The association between the
expression of ALDH1 and the clinicopathological parameters was
evaluated using the χ2 test. In addition, the expression
of ALDH1 between esophageal squamous cell carcinoma and healthy
esophageal tissues were analyzed using the χ2 test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Patient characteristics
In the present study, a total of 50 patients were
included. A total of 33 were male (66%) and 17 were female (34%), a
ratio of 1.94:1. The patient median age was 52.3 years (range,
35–70 years), with 27 (54%) and 23 (46%) patients aged ≥60 and
<60 years, respectively. All patients were diagnosed with human
esophageal squamous cell carcinoma, and classified as exhibiting
well-, moderately or poorly differentiated tumor cells. A total of
10 (20%), 20 (40%) and 20 (40%) of patients exhibited well-,
moderately and poorly differentiated tumor cells, respectively. In
addition, patients were staged according to the
tumor-node-metastasis (TNM) classification (20). A total of 23 (46%) patients were at
TNM stages I/II and 27 (54%) were at stages III/IV. Lymphatic and
vein invasion was observed in 28 (56%) and 26 (52%) patients,
respectively (Table I).
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Clinical data | n (%) |
|---|
| Total | 50 |
| Male | 33 (66) |
| Female | 17 (34) |
| Age, years |
|
| ≥60 | 27 (54) |
|
<60 | 23 (46) |
|
Median | 52.3 (35–70) |
| Differentiation |
|
| Well | 10 (20) |
|
Moderate | 20 (40) |
| Poor | 20 (40) |
| TNM stage |
|
| I/II | 23 (46) |
|
III/IV | 27 (54) |
| Lymph node
metastasis |
|
|
Positive | 28 (56) |
|
Negative | 22 (44) |
| Vein invasion |
|
|
Positive | 26 (52) |
|
Negative | 24 (48) |
Expression of ALDH1 in healthy
esophageal tissues and esophageal carcinoma tissues
Expression of ALDH1 was identified in the cytoplasm
of esophageal carcinoma tissues and a limited number of healthy
esophageal tissues. Human esophageal carcinoma tissues exhibited
markedly increased expression levels of ALDH1 protein, compared
with that of healthy esophageal tissues. In addition, compared with
healthy esophageal tissues, human esophageal carcinoma tissues
exhibited significantly increased expression levels of ALDH1
protein (χ2=5.259; P<0.05). Of the 50 healthy
controls, ALDH1 activity was identified in ~16% of esophageal
cells. However, in the 50 esophageal cancer tissues, positive ALDH1
incidence was 46%. The results are presented in Table II.
 | Table II.Comparison between ALDH1-positive
expression in esophageal cancer and healthy esophageal tissues. |
Table II.
Comparison between ALDH1-positive
expression in esophageal cancer and healthy esophageal tissues.
| Group | Total | ALDH1-positive,
% | χ2 | P-value |
|---|
| Esophageal cancer
tissue | 50 | 23 (46) |
|
|
| Healthy esophageal
tissue | 50 | 8 (16) | 5.259 | <0.05 |
Association between the expression of
ALDH1 and the clinicopathological features of esophageal
carcinoma
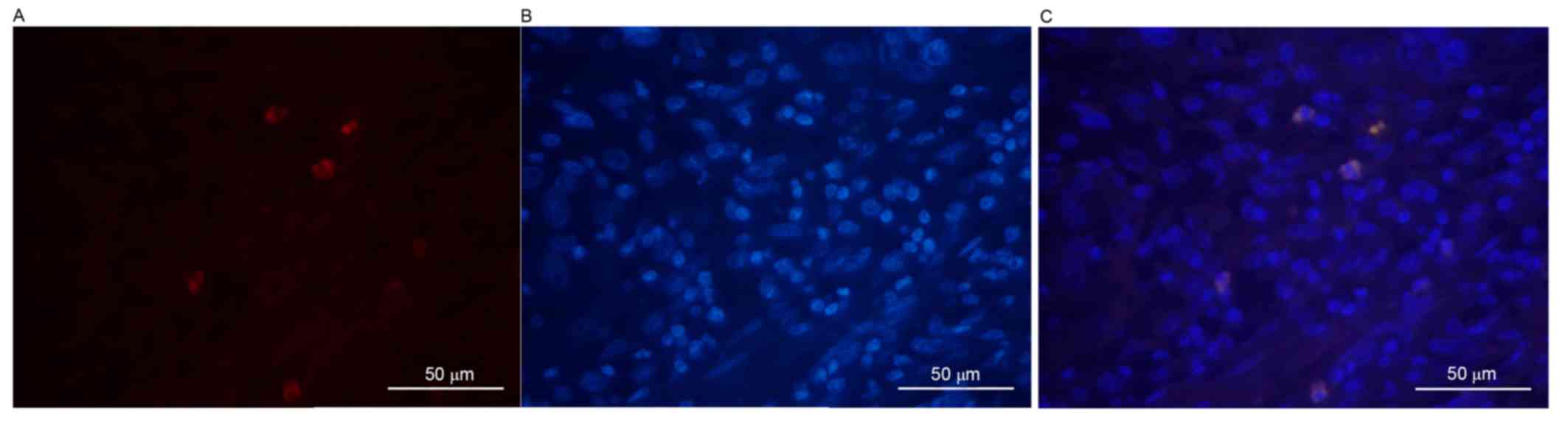
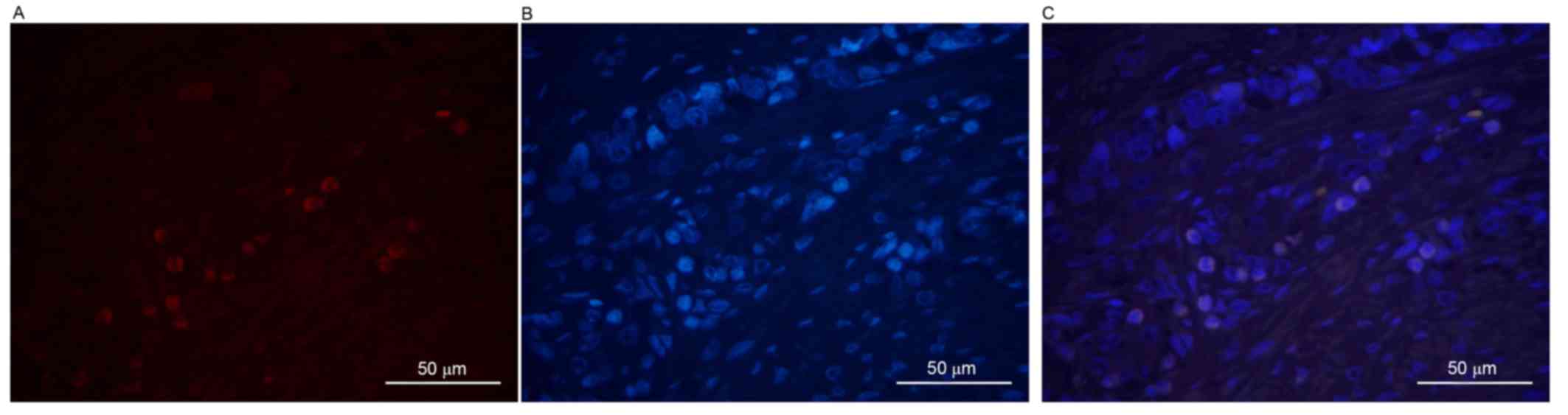
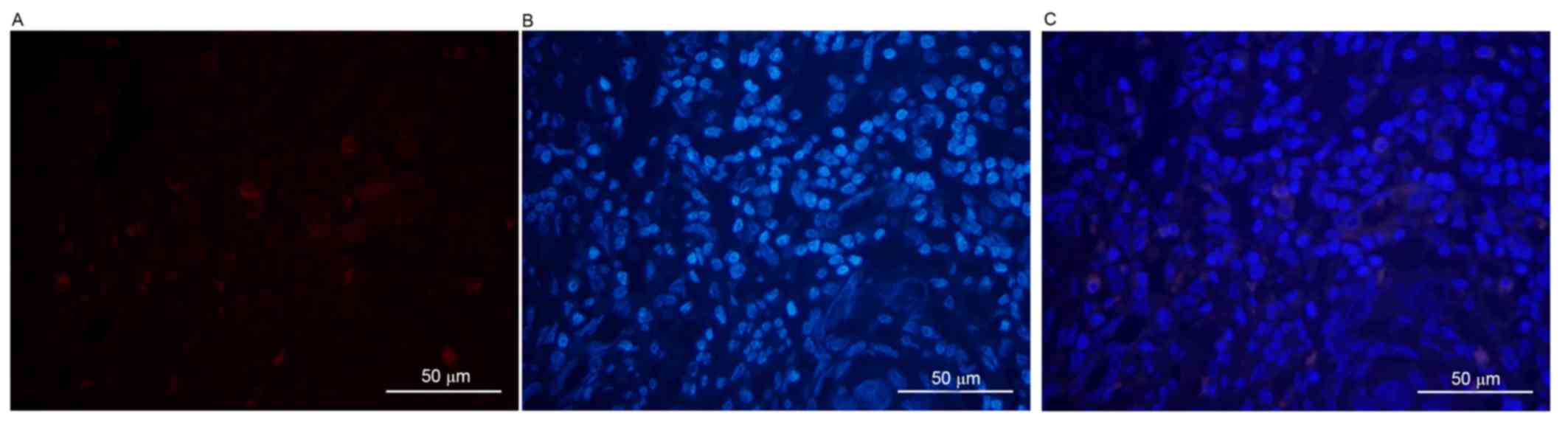
First, DAPI was used to stain the cancer cell
nuclei, which excluded non-tumor cells. The association between
ALDH1 protein expression and the clinicopathological features of
human esophageal squamous cell carcinoma are summarized in Table III. No significant difference was
identified between the expression of ALDH1 and sex, age or vein
invasion. However, ALDH1 expression was identified to be associated
with the level of differentiation of the tumor cells. It was
revealed that, as the level of differentiation of tumor cells
decreased, the positive rate of ALDH1 expression increased.
Compared with well- and moderately differentiated tumor cells
(Figs. 1 and 2), the ALDH1 intensity was markedly
increased in poorly differentiated malignant tumor cells (Fig. 3). All images were stained individually
and finally merged. A positive association was identified between
the differentiation of tumor cells and the positive expression of
ALDH1 (χ2=11.554; P<0.05). ALDH1-positive expression
was only observed in 2/10 (20%), 6/20 (30%) and 15/20 (75%) of the
well-, moderately and poorly differentiated cases, respectively. In
addition, patients of stages III/IV esophageal carcinoma exhibited
an increased expression rate of ALDH1 (63.0%), compared with those
of stages I/II (26.1%) (χ2=6.789; P<0.05).
Furthermore, in the cases of lymphatic invasion, the positive rate
of ALDH1 expression (64.3%) was increased, compared with that of
the cases without lymphatic invasion (22.7%; χ2=8.567;
P<0.05).
 | Table III.Association between ALDH1-positive
expression and the clinicopathological features of human esophageal
carcinoma. |
Table III.
Association between ALDH1-positive
expression and the clinicopathological features of human esophageal
carcinoma.
| Clinicopathological
feature | n | Positive | Negative | Positive rate, % | χ2 | P-value |
|---|
| Sex |
|
|
|
|
| >0.05 |
| Male | 33 | 13 | 20 | 39.4 | 1.705 |
|
|
Female | 17 | 10 | 7 | 58.8 |
|
|
| Age, years |
|
|
|
|
| >0.05 |
| ≥60 | 27 | 13 | 14 | 48.1 | 0.109 |
|
|
<60 | 23 | 10 | 13 | 43.5 |
|
|
| Histological
grade |
|
|
|
|
| <0.05 |
| Well | 10 | 2 | 8 | 20 | 11.554 |
|
|
Moderate | 20 | 6 | 14 | 30 |
|
|
| Poor | 20 | 15 | 5 | 75 |
|
|
| TNM stage |
|
|
|
|
| <0.05 |
| I/II | 23 | 6 | 7 | 26.1 |
|
|
|
III/IV | 27 | 17 | 10 | 63.0 | 6.798 |
|
| Lymphatic
invasion |
|
|
|
|
| <0.05 |
|
Positive | 28 | 18 | 10 | 64.3 | 8.567 |
|
|
Negative | 22 | 5 | 17 | 22.7 |
|
|
| Vein invasion |
|
|
|
|
| >0.05 |
|
Positive | 26 | 11 | 15 | 42.3 | 0.290 |
|
|
Negative | 24 | 12 | 12 | 50.0 |
|
|
Discussion
Human esophageal cancer is a life-threatening
disease worldwide. Although radical surgery may be performed, the
5-year survival rate rarely exceeds 30%. A number of patients with
the early-stage disease exhibit an increased risk of disease
recurrence following treatment (21).
Esophageal carcinomas are divided into the adenocarcinoma and
squamous cell carcinoma histological subtypes; the latter accounts
for the majority of esophageal carcinoma cases. Increased incidence
of esophageal cancer is due to obesity, smoking, consumption of hot
beverages and red meat, increased alcohol intake and a decreased
intake of fresh vegetables or fruit (22). The morbidity of squamous cell
carcinoma is increasing in developing countries, particularly in
China (23).
According to the cancer stem cell theory, malignant
tumors develop from a cancer stem cell, which has the capability of
pluripotency and self-renewal (24).
ALDH1, a detoxifying enzyme responsible for the oxidation of
intracellular aldehydes, is a marker of cancer stem cells (10). The results of the present study
demonstrated that, compared with healthy esophageal tissues,
esophageal cancer tissues expressed an increased positive rate of
ALDH1 (χ2=5.259; P<0.05). In addition, an association
between the differentiation degree of tumor cells and the
expression of ALDH1 was identified. Using indirect
immunofluorescence staining, it was demonstrated that an increased
degree of differentiation of the tumor cells was associated with
decreased expression of ALDH1 (χ2=11.554; P<0.05).
Furthermore, TNM stages III/IV exhibited an increased positive
expression of ALDH1, compared with that of TNM stages I/II
(χ2=6.789; P<0.05). Additionally, it was identified
that positive rates of ALDH1 expression were increased in cases of
lymphatic invasion, compared with that in the tissues without
lymphatic invasion (χ2=8.567; P<0.05). In the present
study, DAPI was used to non-specifically stain the nuclei of tumor
cells to exclude the impact of non-tumor cells.
The expression of ALDH1 was associated with the
clinicopathological characteristics of human esophageal squamous
cell carcinoma. ALDH1 may serve an important function in the
process of human esophageal cancer. However, whether ALDH1 may be
used alone to identify cancer stem cells and whether the prognosis
of patients may be predicted on the basis of ALDH1 expression
requires additional studies.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Guo M, Zhao YD, Yang HJ and Yan XF:
Analysis of clinicopathological characteristics for 5406 cases of
esophageal neoplasm. Chin J Cancer Prev Treat. 15:54–56. 2008.
|
|
3
|
Debevec L, Jerič T, Kovač V, Bitenc M and
Sok M: Is there any progress in routine management of lung cancer
patients? A comparative analysis of an institution in 1996 and
2006. Radiol Oncol. 43:47–53. 2009. View Article : Google Scholar
|
|
4
|
Kovac V, Zwitter M and Zagar T: Improved
survival after introduction of chemotherapy for malignant pleural
mesothelioma in Slovenia: Population-based survey of 444 patients.
Radiol Oncol. 46:136–144. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shi HY, Zhu SC, Shen WB and Liu ML:
Pathological characteristics of esophageal cancer. Oncol Lett.
8:533–538. 2014.PubMed/NCBI
|
|
6
|
Magni M, Shammah S, Schiró R, Mellado W,
Dalla-Favera R and Gianni AM: Induction of
cyclophosphamide-resistance by aldehyde-dehydrogenase gene
transfer. Blood. 87:1097–1103. 1996.PubMed/NCBI
|
|
7
|
Sophos NA and Vasiliou V: Aldehyde
dehydrogenase gene superfamily: The 2002 update. Chem Biol
Interact. 143–144:5–22. 2003. View Article : Google Scholar
|
|
8
|
Dylla SJ, Beviglia L, Park IK, Chartier C,
Raval J, Ngan L, Pickell K, Aguilar J, Lazetic S, Smith-Berdan S,
et al: Colorectal cancer stem cells are enriched in xenogeneic
tumors following chemotherapy. PLoS One. 3:e24282008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chute JP, Muramoto GG, Whitesides J,
Colvin M, Safi R, Chao NJ and McDonnell DP: Inhibition of aldehyde
dehydrogenase and retinoid signaling induces the expansion of human
hematopoietic stem cells. Proc Natl Acad Sci USA. 103:11707–11712.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Armstrong L, Stojkovic M, Dimmick I, Ahmad
S, Stojkovic P, Hole N and Lako M: Phenotypic characterization of
murine primitive hematopoietic progenitor cells isolated on basis
of aldehyde dehydrogenase activity. Stem Cells. 22:1142–1151. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hess DA, Wirthlin L, Craft TP, Herrbrich
PE, Hohm SA, Lahey R, Eades WC, Creer MH and Nolta JA: Selection
based on CD133 and high aldehyde dehydrogenase activity isolates
long-term reconstituting human hematopoietic stem cells. Blood.
107:2162–2169. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nogami T, Shien T, Tanaka T, Nishiyama K,
Mizoo T, Iwamto T, Ikeda H, Taira N, Doihara H and Miyoshi S:
Expression of ALDH1 in axillary lymph node metastases is a
prognostic factor of poor clinical outcome in breast cancer
patients with 1–3 lymph node metastases. Breast Cancer. 21:58–65.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Patel M, Lu L, Zander DS, Sreerama L, Coco
D and Moreb JS: ALDH1A1 and ALDH3A1b expression in lung cancers:
Correlation with histologic type and potential precursors. Lung
Cancer. 59:340–349. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou F, Mu YD, Liang J, Liu ZX, Chen HS
and Zhang JF: Expression and prognostic values of tumor stem cell
markers ALDH1 and CD133 in colorectal carcinoma. Oncol Lett.
7:507–512. 2014.PubMed/NCBI
|
|
15
|
Grimason AM, Smith HV, Parker JFW, Bukhari
Z, Campbell AT and Robertson LJ: Application of DAPI and
immunofluorescence for enhanced identification of
Cryptosporidium spp oocysts in water samples. Water Res.
28:733–736. 1994. View Article : Google Scholar
|
|
16
|
Leiker M, Suzuki G, Iyer VS, Canty JM Jr
and Lee T: Assessment of a nuclear affinity labeling method for
tracking implanted mesenchymal stem cells. Cell Transplant.
17:911–922. 2008. View Article : Google Scholar
|
|
17
|
Ocarino NM, Bozzi A, Pereira RD, Breyner
NM, Silva VL, Castanheira P, Goes AM and Serakides R: Behavior of
mesenchymal stem cells stained with 4′, 6-diamidino-2-phenylindole
dihydrochloride (DAPI) in osteogenic and non osteogenic cultures.
Biocell. 32:175–183. 2008.PubMed/NCBI
|
|
18
|
Van Vlierberghe RL, Sandel MH, Prins FA,
van Iersel LB, van de Velde CJ, Tollenaar RA and Kuppen PJ:
Four-color staining combining fluorescence and brightfield
microscopy for simultaneous immune cell phenotyping and
localization in tumor tissue sections. Microsc Res Tech. 67:15–21.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang EH, Hynes MJ, Zhang T, Ginestier C,
Dontu G, Appelman H, Fields JZ, Wicha MS and Boman BM: Aldehyde
dehydrogenase 1 is a marker for normal and malignant human colonic
stem cells (SC) and tracks SC overpopulation during colon
tumorigenesis. Cancer Res. 69:3382–3389. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
National Comprehensive Cancer Network.
(NCCN) clinical practice guidelines in oncology. Esophageal and
esophagogastric junction cancers. Version 2. 2017.
|
|
21
|
Xu Y, Chen Q, Yu X, Zhou X, Zheng X and
Mao W: Factors influencing the risk of recurrence in patients with
esophageal carcinoma treated with surgery: A single institution
analysis consisting of 1002 cases. Oncol Lett. 5:185–190.
2013.PubMed/NCBI
|
|
22
|
Rubenstein JH and Chen JW: Epidemiology of
gastroesophageal reflux disease. Gastroenterol Clin North Am.
43:1–14. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li S, Jiang S, Jiang W, Zhou Y, Shen XY,
Luo T, Kong LP and Wang HQ: Anticancer effects of crocetin in human
esophageal squamous cell carcinoma KYSE-150 cells. Oncol Lett.
9:1254–1260. 2015.PubMed/NCBI
|
|
24
|
Boman BM and Wicha MS: Cancer stem cells:
A step toward the cure. J Clin Oncol. 26:2795–2799. 2008.
View Article : Google Scholar : PubMed/NCBI
|