Introduction
Salivary adenoid cystic carcinoma (SACC) is a highly
malignant neoplasm, which accounts for ~10% of salivary gland
malignancies (1–3). In patients with SACC, distant metastasis
and perineural invasion are frequently observed, even during early
stages of the disease (4). These
features are associated with a poor prognosis and a 5-year survival
rate of <41.8% (5). Several
proteins, including the epidermal growth factor receptor,
transforming growth factor β-1 and the extracellular matrix (ECM)
component fibronectin (6–8), have been shown to affect the
aggressiveness of SACC. However, the precise mechanisms mediating
the aggressive invasiveness of SACC remain unclear. Therefore,
novel markers that predict prognosis in patients with SACC require
identification and novel treatments must be developed.
According to classical immunology, immunoglobulin
(Ig) molecules are only produced by differentiated B-lymphocytes
and plasma cells (9). However,
multiple studies have shown that numerous Igs, including IgA, IgM
and IgG, can also be produced and secreted by cells of other
lineages, including neurons, germ cells and epithelial cells.
Notably, non-B cell-derived Igs, particularly IgG, have been found
to be overexpressed in a number of types of cancer, including those
of the colon, breast, lung, stomach, liver, cervix, ovary, pancreas
and prostate gland (10–15). Furthermore, cancer cell-derived IgG
(cancer-IgG) has been shown to be involved in the progression and
survival of cancer cells. Our previous study found that IgG was
expressed in epithelial tumor cells in the oral cavity, including
in SACC cells (16), using commercial
human IgG antibodies. However, the role of cancer-IgG in the
progression of SACC remains unclear.
The present study aimed to elucidate the role of
cancer-IgG in SACC by using the monoclonal antibody RP215, which
specifically recognizes an unidentified glycosylated epitope of the
heavy chain of cancer-IgG (RP215-bound IgG) (17), to investigate the association between
cancer-IgG and the clinicopathological characteristics of patients
with SACC. Additionally, the effects of cancer-IgG on the
proliferation, migration and invasion of SACC cells were examined
to provide important insights into the role of cancer-IgG in
SACC.
Materials and methods
Patient samples
SACC tissues, including adjacent non-tumor salivary
gland tissues, were collected from 96 patients aged between 32 and
83 years (mean age, 54 years) at the Peking University School of
Stomatology (Beijing, China) between January 2004 and December 2009
(Table I). Tissue was fixed in
formalin, embedded in paraffin and sectioned (4-µm) for analysis.
Data regarding pathological and clinical characteristics and
outcomes were collected in a review of medical records. Ethical
approval for this study was granted by the Peking University Health
Service Trust Research Ethics Committee.
 | Table I.Association between cancer-IgG
expression and clinicopathological parameters in patients with
salivary adenoid cystic carcinoma. |
Table I.
Association between cancer-IgG
expression and clinicopathological parameters in patients with
salivary adenoid cystic carcinoma.
| Clinical
characteristics | Cases (n) | Low cancer-IgG
expression (n, %) | High cancer-IgG
expression (n, %) | χ2
P-value |
|---|
| Age (years) |
|
|
|
|
|
<50 | 44 | 23 (52.3) | 21 (47.7) | 0.550 |
| ≥50 | 52 | 24 (46.2) | 28 (53.8) |
|
| Sex |
|
|
|
|
| Male | 36 | 16 (44.4) | 20 (55.6) | 0.345 |
|
Female | 60 | 11 (18.3) | 49 (81.7) |
|
| Tumor size |
|
|
|
|
| <30
mm | 41 | 24(58.5) | 17(41.5) | 0.674 |
| ≥30
mm | 55 | 35(63.6) | 20(36.4) |
|
| Histological
subtype |
|
|
|
|
|
Solid | 31 | 9
(29.0) | 22 (71.0) | 0.767 |
|
Cribriform/tubular | 65 | 17 (26.2) | 48 (73.8) |
|
| Metastasis |
|
|
|
|
|
Positive | 42 | 14 (33.3) | 28 (66.7) |
0.019a |
|
Negative | 54 | 31 (57.4) | 23 (42.6) |
|
| Nerve invasion |
|
|
|
|
| Yes | 35 | 11 (31.4) | 24 (68.6) |
0.009b |
| No | 61 | 36 (59.0) | 25 (41.0) |
|
| Recurrence |
|
|
|
|
| Yes | 60 | 18 (30.0) | 42 (70.0) |
0.013a |
| No | 36 | 20 (55.6) | 16 (44.4) |
|
Cell culture
Human salivary adenoid cystic carcinoma SACC-83
cells (18) were obtained from
Professor Shengling Li (Peking University School of Stomatology,
Beijing, China) and cultured in RPMI-1640 medium (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10%
fetal bovine serum (FBS; HyClone Laboratories, Logan, UT, USA) at
37°C with 5% CO2.
Immunohistochemistry (IHC)
The RP215 monoclonal antibody was a gift from
Professor Gregory Lee (University of British Columbia, Vancouver,
BC, Canada). Primary tumor tissues were sectioned into 4-µm thick
slices, deparaffinized and rehydrated using a graded ethanol
series. Antigen retrieval was conducted by boiling the samples in
Tris-EDTA buffer (pH 9.0) for 2 min. All tissues were incubated
with the RP215 primary antibody (1:200 dilution) at 4°C overnight
or 37°C for 1 h. Tissues were then washed thoroughly with PBS,
incubated with horseradish peroxidase (HRP)-conjugated goat
anti-mouse polyclonal antibodies (1:200, cat. no. ab97022; Abcam,
Cambridge, UK) at 37°C for 20 min. The immunocomplexes were
detected using diaminobenzidine (Beijing Zhongshan Golden Bridge
Biotechnology Co., Ltd., Beijing, China). The number of positive
cells and the intensity of staining were determined in five
randomly chosen fields at ×200 magnification. The percentage of
stained cells per field was graded as follows: 0, Negative; 1,
1–25% of cells; 2, 26–50% of cells; and 3, 51–100% of cells. The
staining intensity was graded as follows: 0, Absence of signal; 1,
light brown for low-intensity signal; 2, brown for a
moderate-intensity signal; and 3, dark brown for a high-intensity
signal. The score for each field was obtained by multiplying the
frequency grade and the intensity grade; the final score for each
section was an average of all five fields. The scores for RP215
staining were described as negative (−) if the field scored 0–1,
low expression for a score of 2–3 (+), and high expression for
scores of 4–6 and 7–9 (++ and +++, respectively). All evaluations
were conducted using a Leica DM4000B/M microscope (Leica
Microsystems, Inc., Buffalo Grove, IL, USA).
Western blot analysis
Western blot analysis was performed using standard
procedures. Cells were lysed using RIPA buffer with protease
inhibitor cocktail (Roche Diagnostics, Basel, Switzerland).
Proteins (50 µg) were transferred to nitrocellulose membranes
following separation by 12.5% SDS-PAGE, and the membranes were then
probed with the following antibodies overnight at 4°C: RP215 (1
µg/ml), anti-E-cadherin (cat. no. ab184633, dilution 1:1,000) and
anti-β-actin (cat. no. ab8226, 1 µg/ml) (both from Abcam).
HRP-conjugated secondary antibodies (1:2,000, cat. no. ab97040;
Abcam) were used to stain the membranes at room temperature for 1
h. Immunocomplexes were then detected with the eECL Western Blot
kit (cat. no. CW00495; Beijing ComWin Biotech Co., Ltd., Beijing,
China).
RNA extraction, reverse
transcription-polymerase chain reaction (PCR)
Total RNA was isolated from cells using TRIzol
Reagent (Thermo Fisher Scientific, Inc.) and then reverse
transcribed into cDNA using a SuperScript First-Strand Synthesis
system (Thermo Fisher Scientific, Inc.). Quantitative PCR was
conducted as previously described (6). The primers used for gene expression are
listed in Table II.
 | Table II.Primers used for quantitative
polymerase chain reaction. |
Table II.
Primers used for quantitative
polymerase chain reaction.
| Primers | Sequence (5′-3′) | Gene ID |
|---|
| E-cadherin | Forward:
TGCCCCCAATACCCCAGCGT | NM_004360.3 |
|
| Reverse:
TCCCTGTCCAGCTCAGCCCG |
|
| Vimentin | Forward:
AAGGCGAGGAGAGCAGGATT | NM_003380.3 |
|
| Reverse:
GGTCATCGTGATGCTGAGAAG |
|
| MMP9 | Forward:
GAGCCACGGCCTCCAACCAC | NM_004994.2 |
|
| Reverse:
GAGTCCAGCTTGCGGGGCAG |
|
| MMP2 | Forward:
GACCATGCGGAAGCCACGCT | NM_001127891.2 |
|
| Reverse:
CACCAGTGCCTGGGGCGAAG |
|
| Slug | Forward:
GAGCATTTGCAGACAGGTCA | NM_005985.3 |
|
| Reverse:
CCTCATGTTTGTGCAGGAGA |
|
| Twist | Forward:
TCTCGGTCTGGAGGATGGAG | NM_000474.3 |
|
| Reverse:
GTTATCCAGCTCCAGAGTCT |
|
| ZEB1/2 | Forward:
AGCAGTGAAAGAGAAGGGAATGC | NM_001174094.1 |
|
| Reverse:
GGTCCTCTTCAGGTGCCTCAG |
|
Short interfering RNA (siRNA)
synthesis and IgG-knockdown assay
siRNAs (Table III)
targeting the constant region of the heavy chain in non-B
cell-derived IgG were synthesized by Shanghai GenePharma Co., Ltd.
(Shanghai, China). SACC-83 cells were seeded in 6-well culture
plates at a density of 1×106 cells/well. After 24 h,
when the SACC-83 cells had grown to 50–70% confluence, the siRNAs
and a non-targeting control (NC) were transfected into the SACC-83
cells using Lipofectamine 2000 (Thermo Fisher Scientific, Inc.) in
RPMI-1640 without FBS for 6 h. The medium was then replaced with
complete medium without penicillin and streptomycin according to
the manufacturer's instructions. The knockdown efficiency was
verified by western blot analysis.
 | Table III.siRNA sequences used for
knockdown. |
Table III.
siRNA sequences used for
knockdown.
| Name | Sequence
(5′-3′) |
|---|
| siRNA NC |
UUCUCCGAACGUGUCACGU |
| siRNA1 |
GGUGGACAAGACAGUUGAG |
| siRNA2 |
AGUGCAAGGUCUCCAACAA |
Colony formation and population
doubling time (PDT) assays
The number of colony forming units (CFU) and the
PDTs were assayed as described in our previous study (6). In brief, cells were seeded in 100-mm
dishes, and aggregates of >50 cells were defined as a CFU after
14 days of incubation. For PDT assays, the cells were seeded in
96-well plates and counted daily.
Wound healing and invasion assays
As described in our previous study (6), IgG-knockdown and control cells were
seeded into 6-well plates, grown to confluence and a scratch was
made using a 300–400-µm pipette tip. The average linear speed of
movement of the wound edges was quantified over 24 h. Cell invasion
assays were performed using Transwell chambers coated with 20 µg
ECM gel (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). After 20
h, the membranes were stained with 1% crystal violet and cells on
the upper surface of the membrane were removed using a cotton swab.
Cells that had invaded through the membrane were then counted in at
least 5 randomized fields under a light microscope. The results
were obtained from at least three individual experiments.
Statistical analysis
Correlations between non-B cell-derived IgG
expression and clinicopathological features, including age, sex,
local invasion, recurrence and metastasis, were assessed using
χ2 tests (for positive rates) and Mann-Whitney tests
(for staining scores). Kaplan-Meier survival curves were used to
estimate survival rates in patients with SACC. Comparisons of
experimental data were performed to determine differences between
knockdown and control SACC cells. Experiments were performed in
triplicate. P<0.05 was considered to indicate statistical
significance. All analyses were performed using SPSS 19 software
(IBM SPSS, Armonk, NY, USA).
Results
Non-B cell-derived IgG expression in
SACC
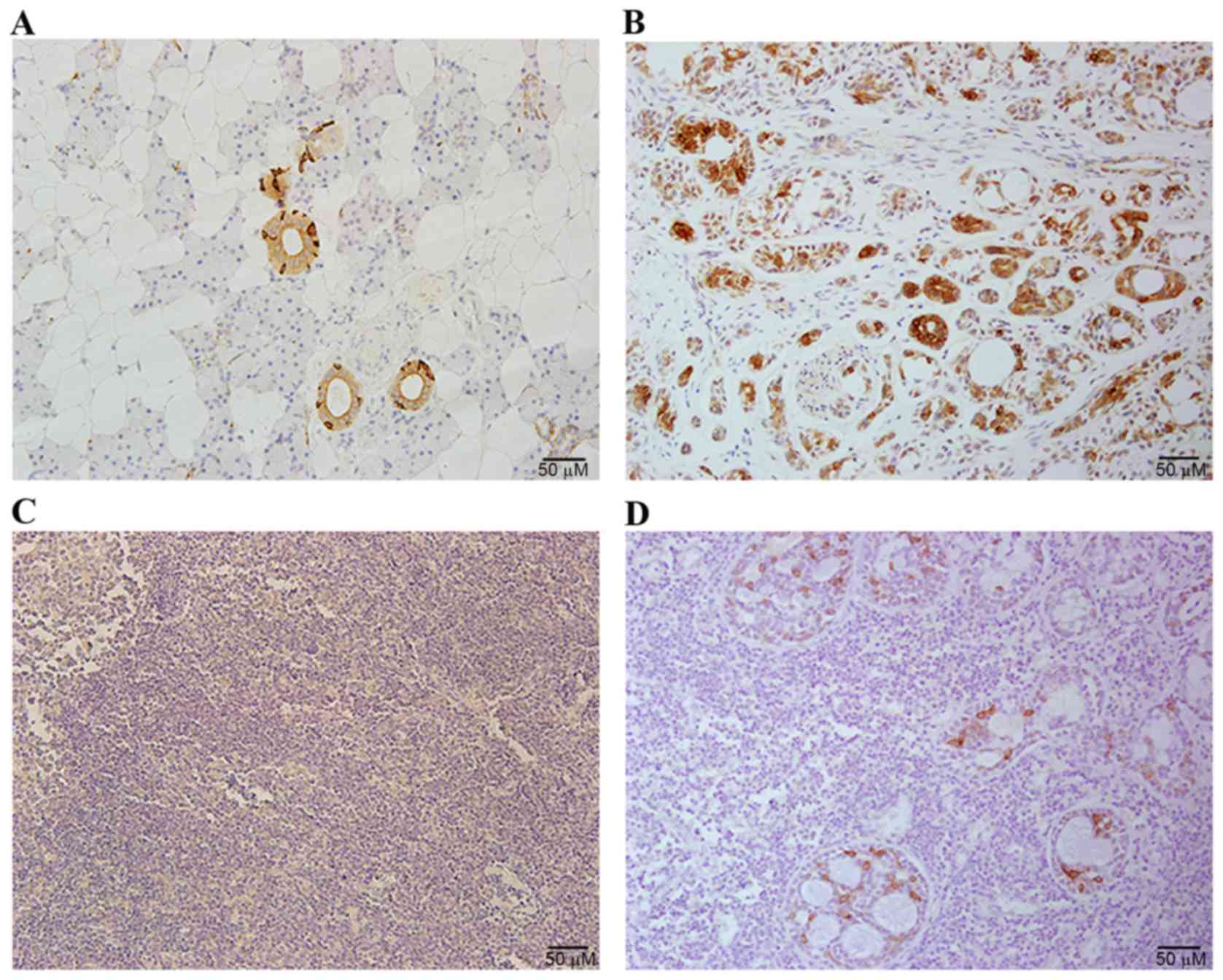
IgG expression was investigated in SACC samples by
immunohistochemical staining with RP215. Positive RP215 signals
were primarily detected in the cytoplasm and plasma membrane of the
cancer cells, whereas no signal was detected in mesenchymal cells
or healthy salivary gland tissue. However, certain cells in the
normal glandular ducts exhibited strong staining with RP215
(Fig. 1).
Next, whether IgG expression was correlated with
various clinicopathological parameters in patients with SACC was
investigated using IHC. Although there was no significant
association between positivity of RP215 staining and sex, age
(Table I), tumor size (data not
shown), histological subtype, the level of RP215 staining was
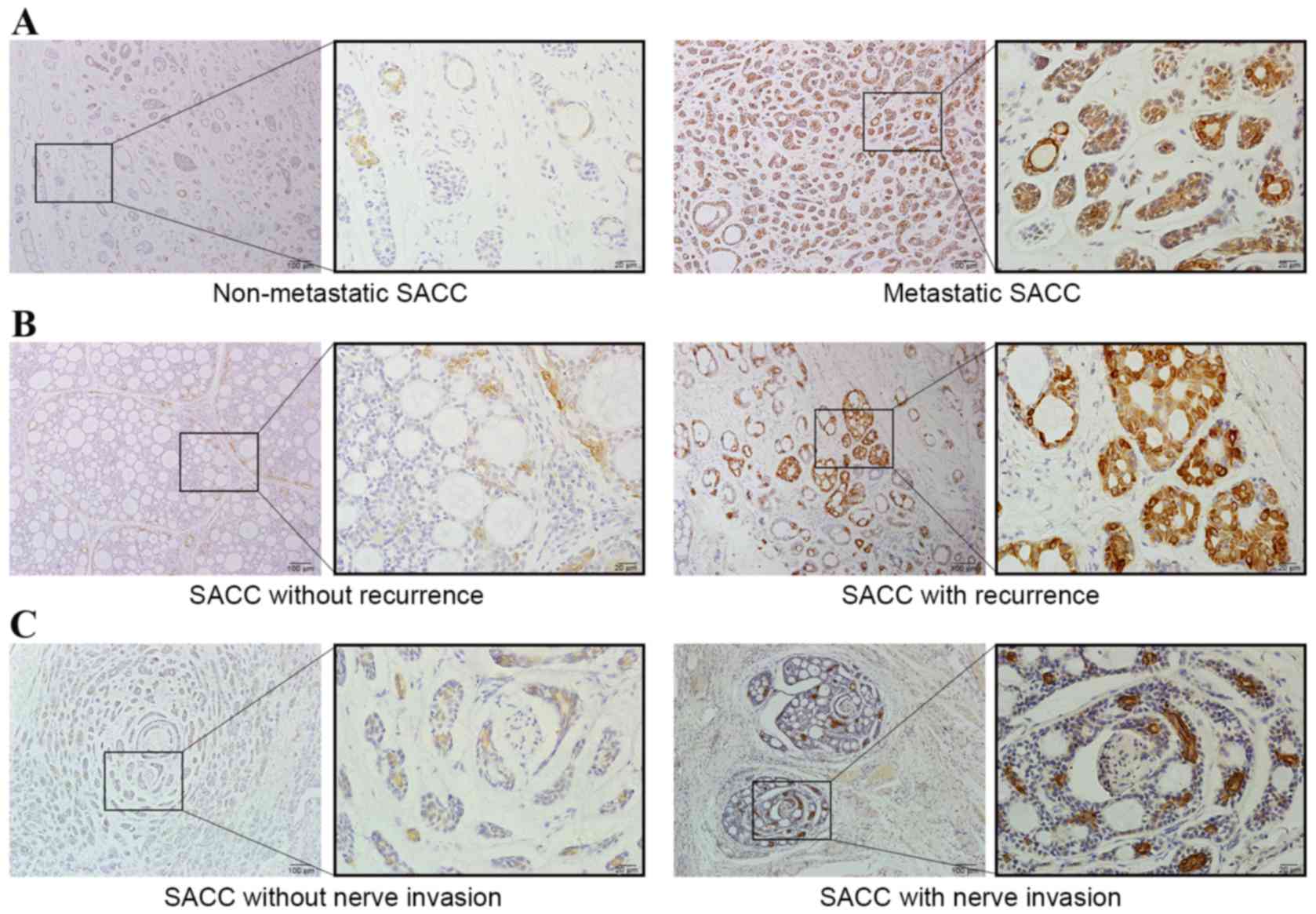
significantly associated with metastasis (P=0.019), recurrence
(P=0.013) and nerve invasion (P=0.009) (Table I). Indeed, the rate of metastasis
(66.7%), recurrence (70.0%) and nerve invasion (68.6%) was higher
in patients with high expression of non-B cell-derived IgG than in
those with low expression of IgG (33.3, 30 and 31.4%,
respectively). The discrepancy between high and low RP215 staining
in SACC tissues is shown in Fig. 2;
strong positive staining was observed in cases with metastasis,
recurrence and nerve invasion.
High cancer-IgG expression is
associated with poor overall survival in patients with SACC
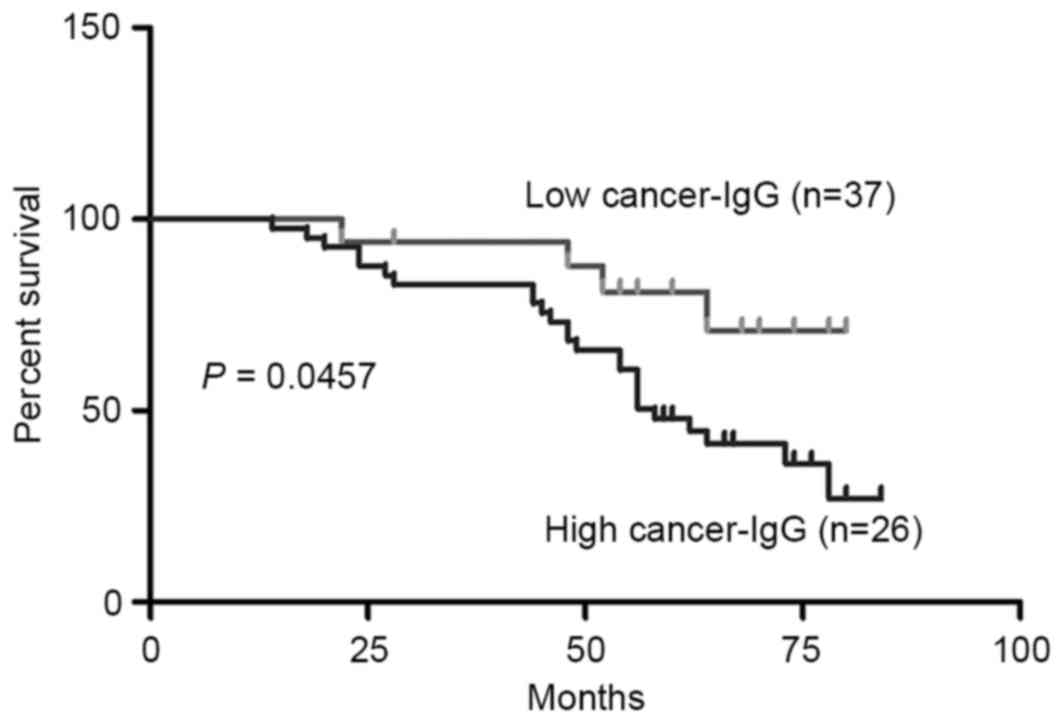
With the score for RP215 staining of cancer-IgG
expression (T/N) chosen as the cut-off point, the patients were
divided into high- and low-expression groups. The patients with
high cancer-IgG expression exhibited significantly shorter survival
times than those with low cancer-IgG expression (Fig. 3). The results therefore showed that
low cancer-IgG expression in SACC was associated with a poor
prognosis.
Proliferation and motility of cancer
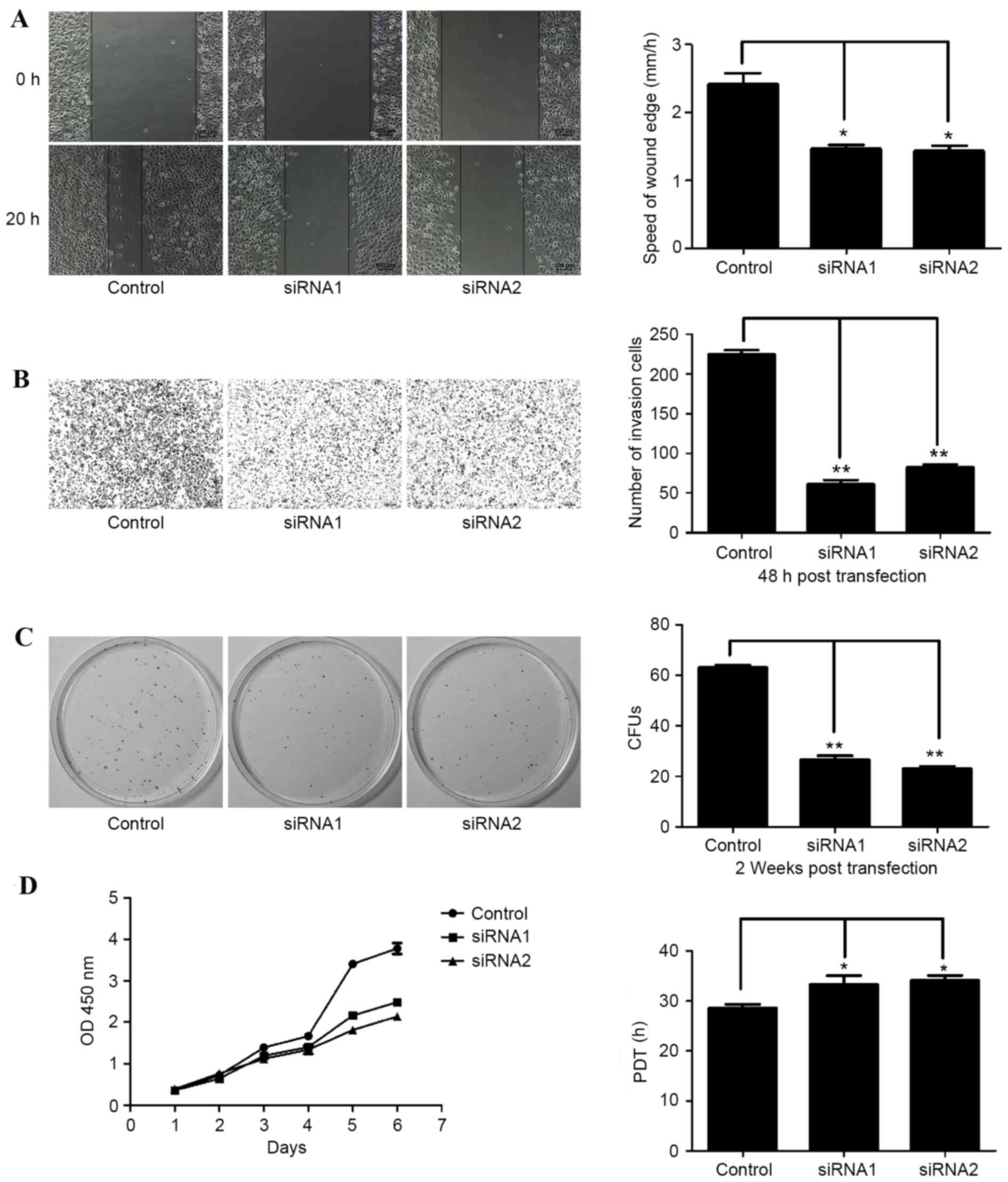
cells is suppressed by knockdown of non-B cell-derived IgG
Two siRNAs that target the constant region of the
heavy chain of cancer-IgG were used to knockdown cancer-IgG
expression in SACC-83 cells. Consistent with the analysis of
clinicopathological characteristics, downregulation of cancer-IgG
resulted in reduced cellular motility as evidenced by wound healing
assays, in which the healing speed of the IgG-knockdown groups was
significantly lower in SACC tissues than in control tissues
(Fig. 4A). Moreover,
cancer-IgG-knockdown decreased the invasion of SACC cells (Fig. 4B). Reduced IgG also suppressed colony
formation (Fig. 4C) and increased PDT
(Fig. 4D). The results of assays
analyzing CFUs, PDT, speed and cell numbers after knockdown of IgG
in SACC are shown in Table IV.
 | Table IV.CFU, PDT, cell speed and cell number
following IgG-knockdown. |
Table IV.
CFU, PDT, cell speed and cell number
following IgG-knockdown.
| Assays |
Controla | siRNA1a | siRNA2a | P1b | P2c |
|---|
| CFUs (n) | 61.33±4.51 | 32.33±2.52 | 36.33±2.08 | 0.015 | 0.021 |
| PDT (h) | 49.67±3.79 | 62.33±5.51 | 63.67±6.66 | 0.113 | 0.044 |
| Speed (µm/h) | 18.58±0.52 | 12.17±1.51 | 12.58±0.80 | 0.033 | 0.016 |
| Cell no. | 107.33±5.87 | 33.67±4.51 | 36.33±1.53 | 0.006 | 0.003 |
Knockdown of cancer-IgG suppresses the
epithelial-mesenchymal transition (EMT) in SACC cells
To examine whether cancer-IgG was involved in the
EMT, changes in the expression of EMT-associated molecules were
evaluated. Knockdown of cancer-IgG significantly enhanced the
expression of E-cadherin (Fig. 5A),
which is negatively correlated with the EMT. By contrast, the
expression levels of Twist, Slug and ZEB1/2, which are positively
correlated with the EMT, were clearly decreased. In addition,
IgG-knockdown caused the downregulation of matrix
metalloproteinases 2 and 9 (MMP2 and MMP9), whose expression is
associated with the invasion and metastasis of cancer cells
(although the downregulation of MMP2 was not significant) (Fig. 5B). Furthermore, transfection with IgG
siRNA altered the morphology of the SACC cells, resulting in a
polygonal shape and a reduction in membrane ruffles, filopodia,
lamellipodia and extensions when compared with control cells, in
which F-actin tended to be arranged as filaments and cells
exhibited a spindle shape (Fig.
5C).
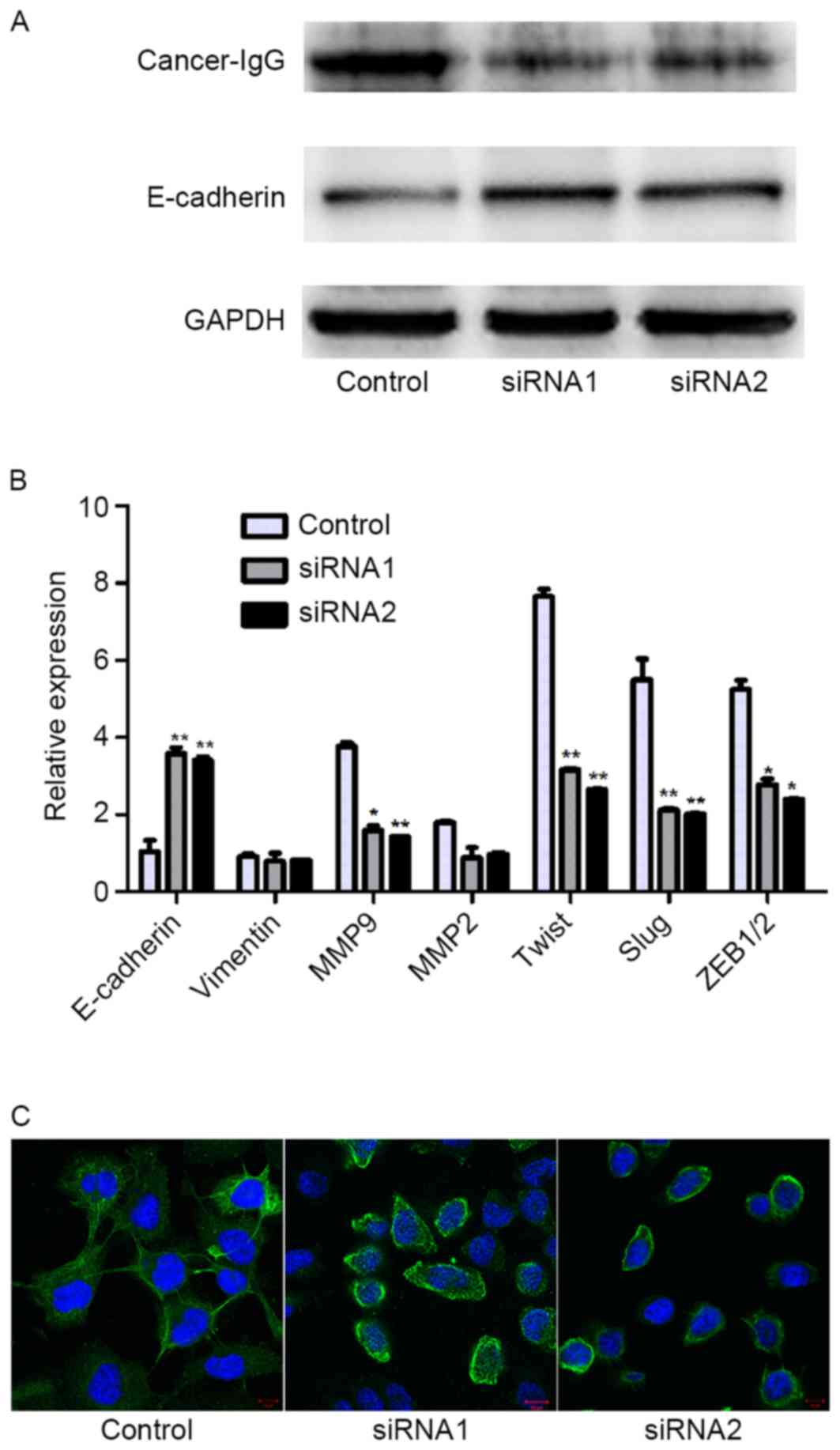 | Figure 5.Knockdown of cancer-IgG suppressed the
EMT in salivary adenoid cystic carcinoma cells. (A) The knockdown
of IgG altered the expression of E-cadherin, as demonstrated by
western blot analysis. (B) The mRNA levels of genes regulating the
EMT were different to those of controls. (C) Immunofluorescence
staining showed that F-actin filaments were concentrated in
IgG-knockdown cells, whereas control cells exhibited an increased
number of F-actin filaments and ruffles. Original magnification,
×400; scale bar, 20 µm. Cancer-IgG, cancer cell-derived
immunoglobulin G; EMT, epithelial-mesenchymal transition; GAPDH,
glyceraldehyde-3 phosphate dehydrogenase; siRNA, short interfering
RNA; MMP, matrix metalloproteinase 9; ZEB1/2, zinc finger E-box
binding homeobox 1/2. *P<0.05, **P<0.01. |
Discussion
In the present study, IgG was found to be highly
expressed in SACC tissues; this expression was found to be
associated with metastasis, recurrence and nerve invasion in SACC.
Additionally, IgG expression was involved in the proliferation,
migration and invasion of SACC cell lines in vitro. These
results provided important insights into the role of cancer-IgG and
could facilitate the identification of biomarkers for SACC and the
development of novel therapies for the treatment of the
disease.
Studies have demonstrated that IgG is expressed in B
cells and non-B cells. Additionally, IgG is overexpressed in
various cancer types, including lung, colorectal and breast cancer
(11,13,19). In
our previous study, the expression of IgG was detected in SACC
tissues using commercial antibodies; however, recent studies have
suggested that a monoclonal antibody, RP215, which mainly
recognizes cancer-IgG, can be used to discriminate the origin of
IgG between B cells and epithelial cancer cells (20,21). In
the present study, this antibody was used to detect cancer-IgG; it
was found that IgG was significantly expressed in primary SACC
tissues and an SACC cell line. In addition, adjacent normal
salivary gland tissues, particularly gland tubular epithelial
cells, also expressed cancer-IgG. However, further studies are
required to determine the functions of cancer-IgG expressed by
SACC.
In the present study, analysis of tissue from
patients with SACC and corresponding clinicopathological
characteristics showed that cases without metastasis, recurrence
and nerve invasion exhibited a relatively low staining intensity
compared with those cases in which metastasis, recurrence and nerve
invasion occurred. Moreover, patients with high cancer-IgG
expression (indicated by strong RP215 staining) exhibited a marked
increase in the rates of metastasis, recurrence and nerve invasion
compared with those having low cancer-IgG expression. High
cancer-IgG expression was also associated with shorter survival
times. Similar results have been reported in patients with lung
adenoid carcinoma, further indicating that relatively high
cancer-IgG expression may be associated with a poor prognosis
(21). These results suggested that
cancer-IgG was positively associated with the progression of SACC
and may be a prognostic biomarker for the prediction of outcomes in
patients with SACC. On the basis of the immunochemistry results,
cancer-IgG may play an important role in the metastasis and
invasion of SACC. However, further studies are required to confirm
these initial findings.
To date, IgG secreted from cancer cells has been
hypothesized to function primarily as an antibody. However, studies
have also shown that cancer-IgG has growth factor-like activity and
is involved in tumorigenesis, promoting the invasion and survival
of cancer cells (20,21). In the present study, it was found that
SACC cell-derived IgG could promote cell proliferation in SACC-83
cells and that SACC cell-derived IgG significantly promoted cell
invasion and migration in SACC-83 cells. Furthermore,
cancer-IgG-knockdown suppressed the EMT, as evidenced by the
increased expression levels of E-cadherin and decreased levels of
Twist, Slug and ZEB1/2, as well as the reduced numbers of
lamellipodia and disruption of F-actin filament arrangement.
Moreover, MMP9 expression was reduced following
cancer-IgG-knockdown, further supporting the fact that the invasive
capacity of the tumor cells was impaired. It is possible that
cancer-IgG may be associated with the invasion and migration of
SACC through induction of the EMT.
In conclusion, the findings of the present study
demonstrated that high expression of cancer-IgG was strongly
correlated with metastasis, recurrence, nerve invasion and poor
prognosis in patients with SACC. In addition, cancer-IgG also
promoted cellular motility, aggressiveness and proliferation of
SACC cells in vitro. These findings suggest that cancer-IgG
can serve as a novel biomarker for predicting the prognosis of SACC
and as a potential target for novel cancer therapies.
Acknowledgements
This study was supported by the National Nature
Science Foundation of China (grant nos. 81072214, 81171006 and
81272237).
References
|
1
|
van der Wal JE, Becking AG, Snow GB and
van der Waal I: Distant metastases of adenoid cystic carcinoma of
the salivary glands and the value of diagnostic examinations during
follow-up. Head Neck. 24:779–783. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tian Z, Li L, Wang L, Hu Y and Li J:
Salivary gland neoplasms in oral and maxillofacial regions: A
23-year retrospective study of 6982 cases in an eastern Chinese
population. Int J Oral Maxillofac Surg. 39:235–242. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang YY, Chen WL, Huang ZQ, Yang ZH, Zhang
B, Wang JG, Li HG and Li JS: Expression of the
membrane-cytoskeletal linker Ezrin in salivary gland adenoid cystic
carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod.
112:96–1042. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kokemueller H, Eckardt A, Brachvogel P and
Hausamen JE: Adenoid cystic carcinoma of the head and neck - a 20
years experience. Int J Oral Maxillofac Surg. 33:25–31. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liang LZ, Ma B, Liang YJ, Liu HC, Zheng
GS, Zhang TH, Chu M, Xu PP, Su YX and Liao GQ: High expression of
the autophagy gene Beclin-1 is associated with favorable prognosis
for salivary gland adenoid cystic carcinoma. J Oral Pathol Med.
41:621–629. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang HC, Yang Y, Xu SY, Peng J, Jiang JH
and Li CY: The CRISPR/Cas system inhibited the pro-oncogenic
effects of alternatively spliced fibronectin extra domain A via
editing the genome in salivary adenoid cystic carcinoma cells. Oral
Dis. 21:608–618. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ge MH, Ling ZQ, Tan Z, Chen C, Xu JJ and
Yu JL: Expression of epidermal growth factor receptor in salivary
adenoid cystic carcinoma and its role in cancer invasion. Zhonghua
Zhong Liu Za Zhi. 34:278–280. 2012.(In Chinese). PubMed/NCBI
|
|
8
|
Dong L, Wang YX, Li SL, Yu GY, Gan YH, Li
D and Wang CY: TGF-beta1 promotes migration and invasion of
salivary adenoid cystic carcinoma. J Dent Res. 90:804–809. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Arnold A, Cossman J, Bakhshi A, Jaffe ES,
Waldmann TA and Korsmeyer SJ: Immunoglobulin-gene rearrangements as
unique clonal markers in human lymphoid neoplasms. New Engl J Med.
309:1593–1599. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Qiu X, Zhu X, Zhang L, Mao Y, Zhang J, Hao
P, Li G, Lv P, Li Z, Sun X, et al: Human epithelial cancers secrete
immunoglobulin g with unidentified specificity to promote growth
and survival of tumor cells. Cancer Res. 63:6488–6495.
2003.PubMed/NCBI
|
|
11
|
Zheng S, Cao J and Geng L: Structure and
expression of colorectal cancer related Immunoglobulin novel gene
SNC73. Zhonghua Yi Xue Za Zhi. 81:485–488. 2001.(In Chinese).
PubMed/NCBI
|
|
12
|
Chen Z and Gu J: Immunoglobulin G
expression in carcinomas and cancer cell lines. FASEB J.
21:2931–2938. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jiang C, Huang T, Wang Y, Huang G, Wan X
and Gu J: Immunoglobulin G expression in lung cancer and its
effects on metastasis. PLoS One. 9:e973592014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu Y, Chen Z, Niu N, Chang Q, Deng R,
Korteweg C and Gu J: IgG gene expression and its possible
significance in prostate cancers. Prostate. 72:690–701. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kimoto Y: Expression of heavy-chain
constant region of immunoglobulin and T-cell receptor gene
transcripts in human non-hematopoietic tumor cell lines. Genes
Chromosomes Cancer. 22:83–86. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhu X, Li C, Sun X, Mao Y, Li G, Liu X,
Zhang Y and Qiu X: Immunoglobulin mRNA and protein expression in
human oral epithelial tumor cells. Appl Immunohistochem Mol
Morphol. 16:232–238. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee G and Ge B: Cancer cell expressions of
immunoglobulin heavy chains with unique carbohydrate-associated
biomarker. Cancer Biomark. 5:177–188. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li SL: Establishment of a human cancer
cell line from adenoid cystic carcinoma of the minor salivary
gland. Zhonghua Kou Qiang Yi Xue Za Zhi. 25:29–31. 1990.(In
Chinese). PubMed/NCBI
|
|
19
|
Babbage G, Ottensmeier CH, Blaydes J,
Stevenson FK and Sahota SS: Immunoglobulin heavy chain locus events
and expression of activation-induced cytidine deaminase in
epithelial breast cancer cell lines. Cancer Res. 66:3996–4000.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu Y, Liu D, Wang C, Liao Q, Huang J,
Jiang D, Shao W, Yin CC, Zhang Y, Lee G and Qiu X: Binding of the
monoclonal antibody RP215 to immunoglobulin G in metastatic lung
adenocarcinomas is correlated with poor prognosis. Histopathology.
67:645–653. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liao Q, Liu W, Liu Y, Wang F, Wang C,
Zhang J, Chu M, Jiang D, Xiao L, Shao W, et al: Aberrant high
expression of immunoglobulin G in epithelial stem/progenitor-like
cells contributes to tumor initiation and metastasis. Oncotarget.
6:40081–40094. 2015. View Article : Google Scholar : PubMed/NCBI
|