Introduction
FK506-binding proteins (FKBPs), known as such as
they bind to the immunosuppressive drug FK506, were initially found
to be intracellular receptors of immunosuppressive drugs (1). FKBPs are highly conserved proteins,
owing to their peptidylprolyl isomerase (PPIase) domains, which
catalyze the isomerization of peptidylprolyl imide bonds (from
cis to trans) in protein substrates (2). In view of their isomerase activity and
the capability to interact with other proteins, more attention has
been focused on their modulatory function in several signal
transduction pathways in the cell. A previous study supported the
concept that certain FKBPs serve a role in cancer-associated
pathways (3).
The FK506-binding protein 52 gene (FKBP52), an FKBP,
is located at chromosome 12 (12p13.33) (4) and contains 10 exons and 9 introns
spanning ~10 kb of genomic DNA. The FKBP52 protein contains a
PPIase domain and a C-terminal tetratricopeptide repeat (TPR)
domain. In addition to promoting binding to the immunosuppressive
drug FK506, the PPIase domain is vital for correct protein folding.
Through the TPR domain, FKBP52 binds the 90-kDa heat shock protein
(Hsp90), and forms an Hsp90 co-chaperone. This complex regulates
steroid receptor signaling, including regulation of receptor
maturation, hormone binding and nuclear translocation, and is
involved in a wide variety of endocrine-associated diseases
(5,6).
Through its functional domains, FKBP52 also interacts with other
proteins (7). The most widely
discussed role of FKBP52 is its regulation of steroid hormone
receptor (SHR) activity in hormone-dependent cancer.
FKBP52 is proposed to inhibit the nuclear movement
of the tumor suppressor protein p53 by forming
p53-hsp90-immunophilin-dynein complexes, resulting in the
inactivation of p53 (8). This
indicates that FKBP52 may be able to promote cancer initiation and
progression. In addition, an elevated level of FKBP52 was observed
in several types of cancer, including prostate cancer,
hepatocellular carcinoma and breast cancer (9–13); notably
though, the majority of these studies focused on cancer cell lines.
Furthermore, previous studies on the role of FKBP52 in breast
cancer do not form a consensus of results. Certain studies reported
that FKBP52 was expressed at a higher level in breast cancer and
precancerous lesions (14,15), but another (16) reported that the expression of FKBP52
decreased in breast cancer cell line-formed mammospheres,
suggesting that FKBP52 could elicit a tumor suppressor function.
Therefore, the current study investigated the association of FKBP52
with clinical features and the outcome in patients with breast
cancer.
Patients and methods
Patients and clinicopathological
features
Archived paraffin-embedded pathological specimens,
complete clinicopathological features and follow-up data were
retrieved for 145 breast cancer patients (median age, 51 years; age
range, 18–84 years) diagnosed in the Cancer Hospital of Shantou
University Medical College (Shantou, China) between October 2001
and November 2011. All participating patients, initially diagnosed
with invasive breast cancer, underwent surgery without radiation,
chemo- or endocrine therapy. If patients were suffering from a
different type of cancer, they were excluded from research group.
The unmatched adjacent normal tissues of 66 patients were also
obtained from surgical resections. Some patients were excluded from
analysis due to lack of pathological data. Clinical
Tumor-Node-Metastasis stage was grouped in accordance with the
American Joint Committee on Cancer 6th Edition Cancer Staging
Manual (2002) (17,18). In the present study, stage III and V
disease were designated as being advanced stages, while stages I
and II were designated as early stages (19). A previous study identified that the
histological grade of breast cancer exhibited an effect on the
prognosis (20). The most common
method used to grade breast tumors is the Bloom Richardson grading
system (also known as the Nottingham Grading System), which
classifies the following groups: Grade 1 (G1), well-differentiated
slow-growing; grade 2 (G2), moderately differentiated; and grade 3
(G3), poorly-differentiated highly proliferative (21–23).
Well-differentiated G1 tumors are close to the cell of origin, and
exhibit a low malignant grade. In comparison, poorly differentiated
G3 tumors exhibit a poorer differentiation, increased degree of
malignancy, and poor prognosis. G2 tumors are in between G2 and G3
(21–23). As certain samples were not stained for
Ki-67, no further division of the luminal breast cancer specimens
into luminal A or luminal B subtypes could be performed.
Consequently, the individual breast subgroups were divided into
three subgroups: Luminal, human epidermal growth factor receptor 2
(HER-2)-enriched and triple-negative breast cancer (TNBC). The
observation period ranged between 7 and 151 months (median, 40
months). Informed consent for the use of their samples was obtained
from all the patients. This study was approved by the Medical
Ethics Committee of the Cancer Hospital of Shantou University
Medical College.
Immunohistochemistry (IHC) assay
IHC for FKBP52 was performed using a standard
Envision complex method (24).
Briefly, sections (4 µm) were fixed in 10% buffered formalin at
room temperature for 24 h and embedded in paraffin. The sections
were deparaffinized by xylene for 1 h at room temperature,
rehydrated using decreasing concentrations of ethanol (100, 95, 90,
80 and 70%, 5 min each) and washed in PBS. Endogenous peroxidase
activity was blocked with 0.3% hydrogen peroxide at room
temperature for 30 min. Next, tissue sections were autoclaved at
121°C in citrate buffer (pH 6.0) for 10 min, and incubated with
rabbit anti-FKBP52 monoclonal antibody (dilution, 1:100; catalog
no. ab54991; Abcam, Cambridge, UK) at 4°C overnight. Slides were
subsequently washed in PBS and incubated with biotinylated
secondary antibody (GTVision™ I Detection System kit;
Anti Mouse/Rabbit Detection System; Gene Tech Co., Ltd., Hong Kong,
China; used as supplied) for 30 min at 37°C. Staining was performed
using 3, 3-diaminobenzidine (DAB-0031/1031; Fuzhou Maixin Biotech
Co., Ltd., Fuzhou, China; used as supplied) at room temperature for
2 or 3 min, and counterstained with hematoxylin (PT001; Shanghai
Bogoo Biotechnology Co., Ltd., Shanghai, China; used as supplied)
at room temperature for 2 min. A negative control was obtained by
replacing the primary antibody with normal goat serum (used as
provided, AR0009; Wuhan Boster Biological Technology, Ltd., Wuhan,
China) at 4°C overnight.
IHC staining for FKBP52 was scored as previously
described (25), by a combination of
intensity (0, no staining; 1, weak staining; 2, moderate staining;
and 3, strong staining) and proportion (0, <5% of tumor cells
stained; 1, 5–25% cells stained; 2, 26–50% cells stained; 3, 51–75%
cells stained; 4, >75% cells stained) scores. If the product of
multiplication between staining intensity and the proportion of
positive cells was >2 (the upper quartiles), expression was
defined as FKBP52-positive (+), but if the score was ≤2, the sample
was designated as FKBP52-negative (−). Two pathologists
independently assessed the cellular location and intensity of
immunostaining in each section, in a blinded manner.
Gene expression data
The microarray datasets employed in this study are
publicly available from ArrayExpress (http://www.ebi.ac.uk/arrayexpress/) of the European
Bioinformatics Institute, and include 4 independent cohorts of
breast cancer [accession numbers: E-GEOD-42568 (26), E-GEOD-15852 (27), E-GEOD-21422 (28), E-GEOD-29044]. The CEL files containing
the raw data from each experiment were directly downloaded from the
ArrayExpress website. Details of these datasets are summarized in
Table I.
 | Table I.Independent datasets from
ArrayExpress. |
Table I.
Independent datasets from
ArrayExpress.
|
|
| Sample size, n |
|
|
|---|
|
|
|
|
|
|
|---|
| Accession number | Array | Control | Breast cancer | Log-2 FC
(cases/controls)a | P-value |
|---|
| E-GEOD-42568 | HG-U133_Plus_2 | 17 | 104 | 0.777 | <0.001 |
| E-GEOD-21422 | HG-U133A | 5 | 14 | 0.626 | 0.044 |
| E-GEOD-15852 | HG-U133A | 43 | 43 | 0.138 | <0.001 |
| E-GEOD-29044 | HG-U133_Plus_2 | 49 | 43 | 0.343 | 0.001 |
In the present study, KM Plotter (http://kmplot.com/analysis/), a tool for
themeta-analysis-based biomarker assessment that includes gene
expression and survival data of more than 4,000 breast cancer
patients was used (29). The tool was
used to perform Kaplan Meier survival analysis to further assess
the association between FKBP52 mRNA expression and OS. Patients
with breast cancer were split by the median expression of FKBP52
into two groups, namely patients with high or low expression of
FKBP52.
Statistical analysis
Data are expressed as the mean ± standard deviation.
Statistical analyses were performed using software SPSS (version
13.0) (SPSS, Inc., Chicago, IL, USA) and R (version 3.0.2;
www.r-project.org). The difference in FKBP52
expression between tumors and non-cancerous tissues was detected by
Mann-Whitney U test, and the difference in FKBP52 mRNA expression
retrieved from online datasets between breast cancer cases and
healthy controls included in this study was detected by an unpaired
Student's t-test. Associations between FKBP52 expression and
clinicopathological features were analyzed using the χ2
test. Survival curves were calculated using the Kaplan-Meier method
with log-rank test. Cox regression analysis was used to study the
effects of FKBP52 expression on overall survival (OS) time. OS was
defined as the time from surgery to the date of last contact or
mortality from any cause. The three-year survival period or
five-year survival period is a professional term for evaluating the
survival of a tumor patient (30,31).
Breast cancer patients with an OS time of >3 or 5 years were
defined as the better prognostic group, while those with an OS time
of ≤3 or 5 years were classified into the poor prognostic group.
Student's t-test was used for differential expression analysis of
FKBP52 between control and better/poor prognosis-tumor samples. For
gene expression microarray analyses, data were normalized using
Robust Multi-array Analysis with R-package ‘affy’ (32). The normalized expression values (on a
log-2 scale) of probes representing the same gene were averaged. A
two-tailed P-value of <0.05 was considered to indicate a
statistically significant difference.
Results
Difference in FKBP52 expression
between breast tumors and normal tissues
FKBP52 expression was evaluated in 145 breast cancer
patient samples and 66 unmatched breast non-cancerous tissues by
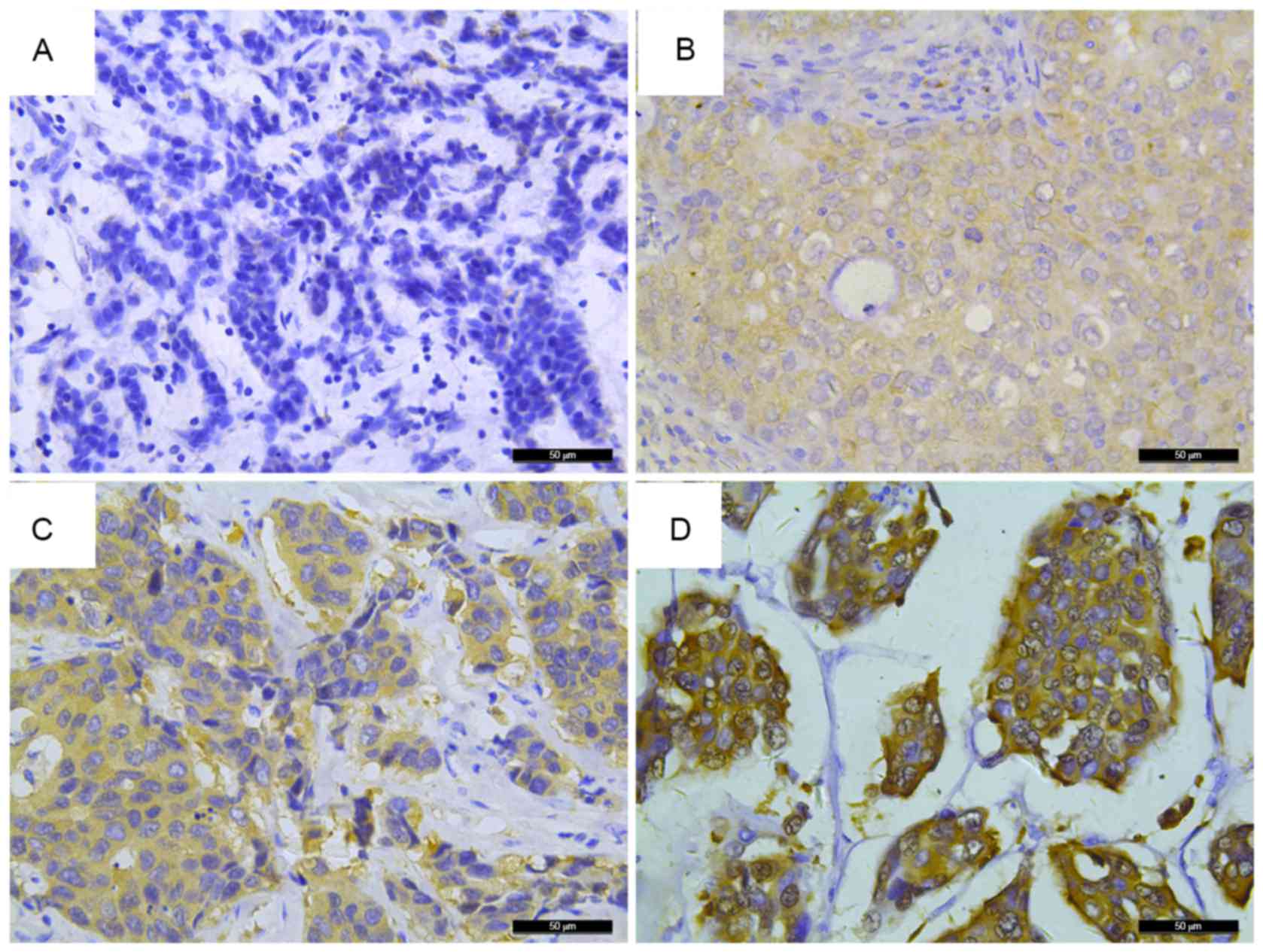
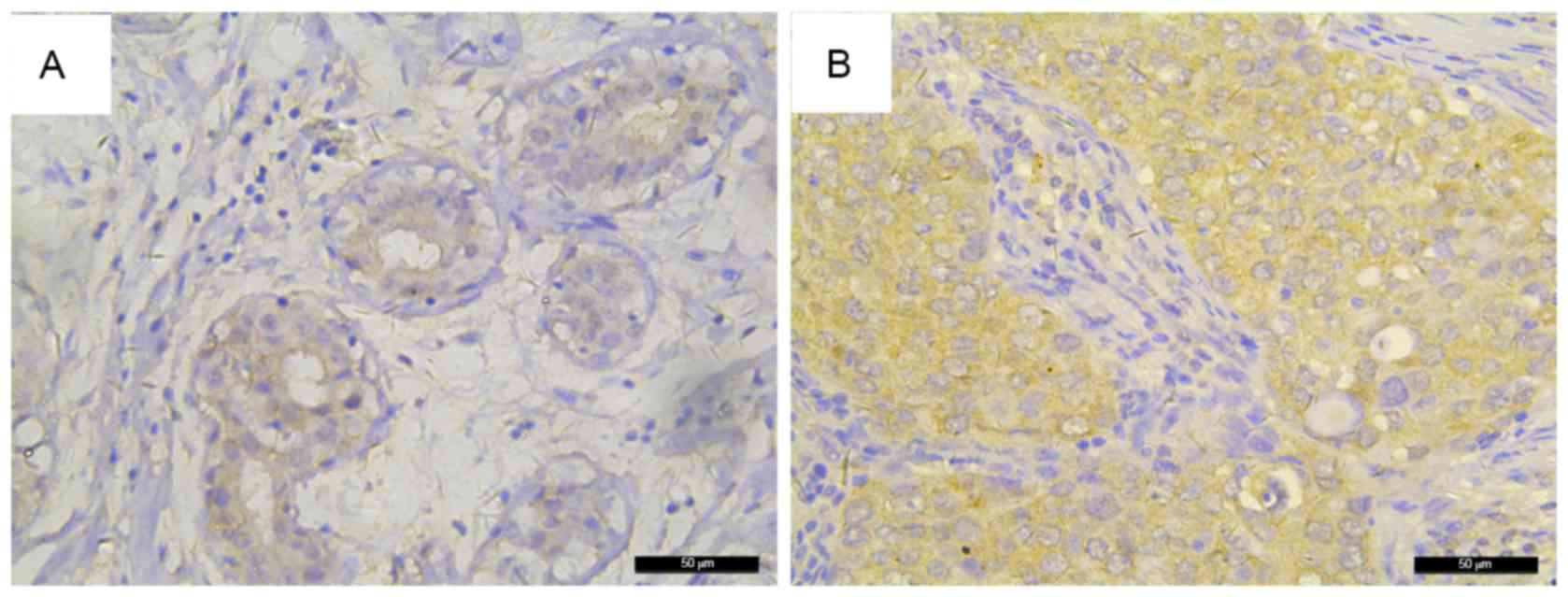
IHC. As shown in Fig. 1, different
staining intensities were observed; positive staining of FKBP52 was
mainly observed in the cytoplasm of the majority of tumor cells.
FKBP52 expression was slightly higher in the tumors than that in
the non-cancerous tissues (Fig. 2),
but this difference was not significant (Table II; P=0.176). Conversely, different
independent datasets from public databases demonstrated that a
significant upregulation of FKBP52 mRNA was found in breast tumors
compared with that in the corresponding controls (Table I).
 | Table II.Differential expression of 52-kDa
FK506-binding protein in 66 unmatched breast non-cancerous samples
and 145 breast tumor tissues. |
Table II.
Differential expression of 52-kDa
FK506-binding protein in 66 unmatched breast non-cancerous samples
and 145 breast tumor tissues.
| Term | Expression
levela | Median (P25,
P75) | Mann-Whitney
U-value | P-value |
|---|
| Healthy tissue | 3.94±2.992 | 4.00 (1.75,
6.00) | 4234.5 | 0.176 |
| Tumor tissue | 4.75±3.605 | 4.00 (2.00,
8.00) |
|
|
Association of FKBP52 with
clinicopathological features in a cohort of 145 breast cancer
patients
To evaluate the association of FKBP52 expression
with clinicopathological features, tumor sections from 145 primary
breast cancer patients were subsequently divided into two groups
according to their IHC scores: 50 (34.5%) tumors exhibited low
expression of FKBP52 [FKBP52(−) group] and 95 (65.5%) tumors
exhibited high expression of FKBP52 [FKBP52(+) group]. As shown in
Table III, FKBP52(+) was detected
in 36 patients with N1 stage disease (36/66, 54.5%) and 50 patients
with N2-N3 stage disease (50/67, 74.6%), which demonstrated that
the FKBP52 (+) rate was significantly higher in patients with more
affected lymph nodes (P=0.015). Similarly, elevated FKBP52
expression was associated with advanced TNM stage (57/78, 73.1% vs.
26/50, 52.0%; P=0.015). Additionally, FKBP52(+) rate was positively
associated with patients with a histological grade of G3 (P=0.047).
Patients with G3 tumors had the highest FKBP52 (+) rate (54/74,
73.0%), successively followed by those with G2 (21/36, 58.3%) and
G1 tumors (8/18, 44.4%). For molecular receptors, FKBP52 was
negatively associated with estrogen receptor (ER) expression
(47/82, 57.3% vs. 42/56, 75.0%; P=0.033), but positively associated
with HER-2 expression (42/56, 75% vs. 47/82, 57.3%; P=0.033).
Similarly, there was a statistically significant difference among
the three breast cancer subgroups (P=0.036): FKBP52 expression was
the highest in the HER-2-enriched subtype of breast cancer (23/27,
85.2%), second highest in the luminal-subtype breast cancer (17/27,
63.0%) and third highest in TNBC (48/83, 57.8%). By contrast, no
significant differences were found between FKBP52 expression and
other clinicopathological features assessed in this study,
including age, tumor size and PR (Table
III).
 | Table III.Association between FKBP52 expression
and clinicopathological features. |
Table III.
Association between FKBP52 expression
and clinicopathological features.
|
|
| FKBP52 expression,
n (%) |
|
|
|---|
|
|
|
|
|
|
|---|
| Clinicopathological
feature | Patients, n
(%) | Negative
(n=50) | Positive
(n=95) | χ2 | P-value |
|---|
| Age at diagnosis,
years |
|
|
|
|
|
|
≤50 | 69 (49.3) | 28 (40.6) | 41 (59.4) | 1.862 | 0.172 |
|
>50 | 71 (50.7) | 21 (29.6) | 50 (70.4) |
|
|
| Primary tumor
stage |
|
|
|
|
|
|
T1-T2 | 88 (66.2) | 32 (36.4) | 56 (63.6) | 0.120 | 0.729 |
|
T3-T4 | 45 (33.8) | 15 (33.3) | 30 (66.7) |
|
|
| Regional lymph node
stage |
|
|
|
|
|
|
N0-N1 | 66 (49.6) | 30 (45.5) | 36 (54.5) | 5.868 | 0.015 |
|
N2-N3 | 67 (50.4) | 17 (25.4) | 50 (74.6) |
|
|
| TNM stage |
|
|
|
|
|
|
1–2 | 50 (39.1) | 24 (48.0) | 26 (52.0) | 5.937 | 0.015 |
|
3–4 | 78 (60.9) | 21 (26.9) | 57 (73.1) |
|
|
| Histological
grade |
|
|
|
|
|
| G1 | 18 (14.1) | 10 (55.6) | 8 (44.4) | 6.1 | 0.047 |
| G2 | 36 (28.1) | 15 (41.7) | 21 (58.3) |
|
|
| G3 | 74 (57.8) | 20 (27.0) | 54 (73.0) |
|
|
| Estrogen
receptor |
|
|
|
|
|
|
Negative | 56 (40.6) | 14 (25.0) | 42 (75.0) | 4.544 | 0.033 |
|
Positive | 82 (59.4) | 35 (42.7) | 47 (57.3) |
|
|
| Progesterone
receptor |
|
|
|
|
|
|
Negative | 71 (51.4) | 21 (29.6) | 50 (70.4) | 2.245 | 0.134 |
|
Positive | 67 (48.6) | 28 (41.8) | 39 (58.2) |
|
|
| HER-2 |
|
|
|
|
|
|
Negative | 82 (59.4) | 35 (42.7) | 47 (57.3) | 4.544 | 0.033 |
|
Positive | 56 (40.6) | 14 (25.0) | 42 (75.0) |
|
|
| Molecular
subtypes |
|
|
|
|
|
|
Luminal | 83 (60.6) | 35 (42.2) | 48 (57.8) |
|
|
| HER-2
enriched | 27 (19.7) | 4 (14.8) | 23 (85.2) |
|
|
|
TNBC | 27 (19.7) | 10 (37.0) | 17 (63.0) | 6.659 | 0.036 |
Effect of FKBP52 expression on the OS
of breast cancer patients
To examine whether the expression status of FKBP52
has any prognostic value for breast cancer, univariate and
multivariate analyses using the Kaplan-Meier method and Cox
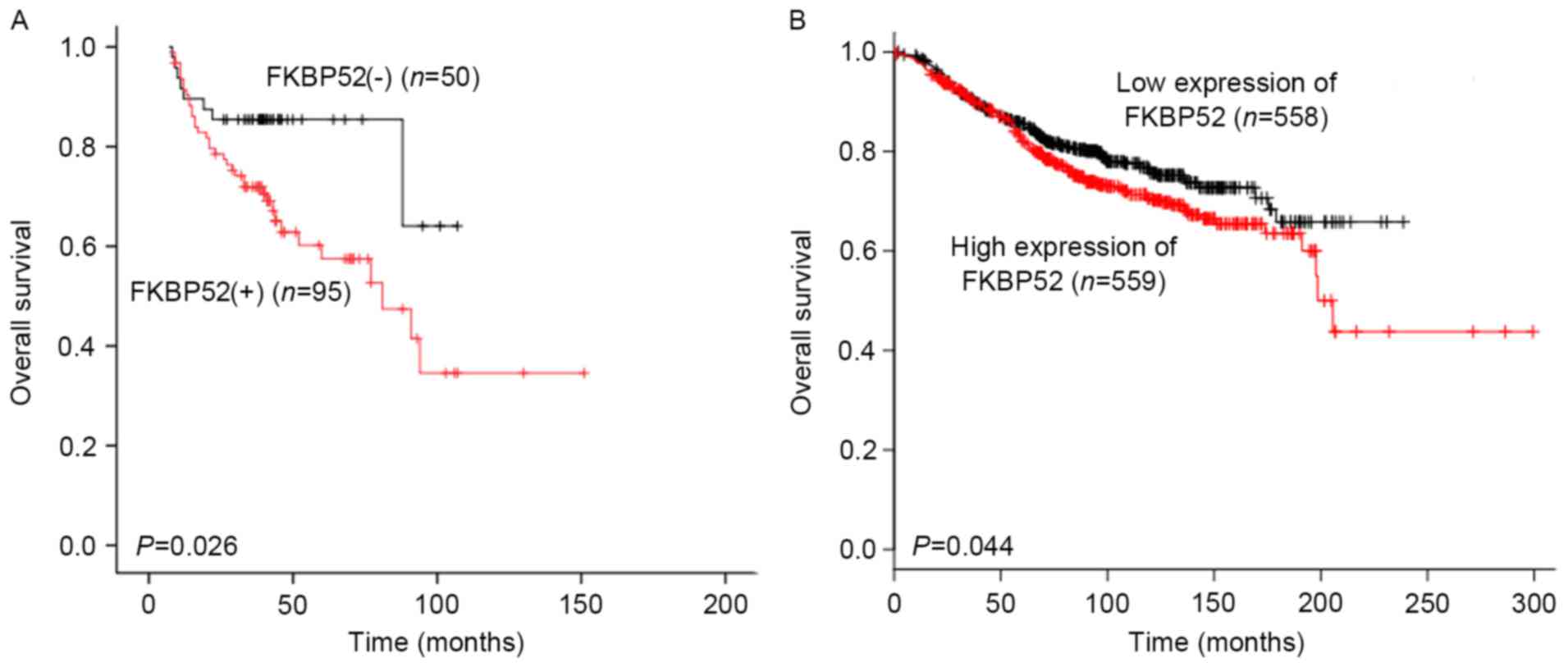
regression analysis were performed. As shown in Fig. 3A, the Kaplan-Meier survival curve
revealed that for the 145 breast cancer patients, the OS rate in
the FKBP52(−) group was significantly higher than that in the
FKBP52(+) group (P=0.026). The KM Plotter tool was used to further
assess the association between the mRNA expression of FKBP52 and
the OS of the breast cancer patients. As shown in Fig. 3B, low expression of FKBP52 predicted a
significantly better OS rate in the breast cancer patients
(P=0.044). In addition to FKBP52 (HR, 2.315; 95% CI, 1.077–4.975;
P=0.032), TNM stage was another adverse predictor for breast cancer
patients (HR: 2.148; 95% CI: 1.011–4.566, P=0.047) upon univariate
analysis. However, multivariate analysis demonstrated that TNM
stage, but not FKBP52, was an independent prognostic factor (HR,
2.721; 95% CI, 1.169–6.335; P=0.020) (Table IV).
 | Table IV.Cox proportional hazard regression
model analysis of overall survival in patients with breast
cancer. |
Table IV.
Cox proportional hazard regression
model analysis of overall survival in patients with breast
cancer.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Terms | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age (>50
years) | 1.011
(0.562–1.818) | 0.972 |
|
|
| Tumor size
(T3-T4) | 1.630
(0.852–3.117) | 0.140 |
|
|
| Nodal status
(N2-N3) | 1.594
(0.819–3.102) | 0.170 |
|
|
| TNM stage (3–4) | 2.148
(1.011–4.566) | 0.047 | 2.721
(1.169–6.335) | 0.020 |
| Histological
grade |
| 0.427 |
|
|
| G2 | 0.627
(0.290–1.356) | 0.236 |
|
|
| G3 | 0.672
(0.254–1.778) | 0.423 |
|
|
| FKBP52
(positive) | 2.315
(1.077–4.975) | 0.032 | 2.343
(0.956–5.742) | 0.063 |
| Estrogen receptor
(positive) | 0.926
(0.503–1.707) | 0.806 |
|
|
| Progesterone
receptor (positive) | 0.664
(0.358–1.230) | 0.193 |
|
|
| HER-2
(positive) | 1.266
(0.689–2.327) | 0.447 |
|
|
Association between controls and
better/poor prognosis-tumor samples
Breast cancer patients were divided into two groups
according to their OS: Better-prognosis group (OS >3 or 5 years)
and poor-prognosis group (OS ≤3 or 5 years). As shown in Table V, FKBP52 expression in the patients
with an OS ≤3-years (5.39±3.409; P=0.042) and an OS ≤5-years
(5.88±3.473; P=0.005) was significantly higher than that in the
controls (3.94±2.992). However, no statistical significance was
determined for the comparison between the controls and the
better-prognosis group (OS >3 years: 4.84±3.769, P=0.109; OS
>5 years: 5.32±3.372, P=0.090).
 | Table V.Difference between controls and
poor/better prognosis tumor patients. |
Table V.
Difference between controls and
poor/better prognosis tumor patients.
| Group | Patients, n | Survival
timea |
P-valueb |
|---|
| Controls | 66 | 3.94±2.992 |
|
| Patients by OS
time, yearsc |
|
|
|
| ≤3 | 33 | 5.39±3.409 | 0.042 |
|
>3 | 93 | 4.84±3.769 | 0.109 |
| ≤5 | 40 | 5.88±3.473 | 0.005 |
|
>5 | 28 | 5.32±3.372 | 0.090 |
Discussion
As a component of the Hsp90 co-chaperones, FKBP52
potentiates the gene activation by glucocorticoid (5), androgen (33,34) and
progesterone (35) receptors through
its involvement in nuclear receptor maturation. Our understanding
of the mechanisms by which Hsp90 co-chaperones regulate SHR
signaling and the role that they play in endocrine-associated
physiological processes has progressed, although certain previous
studies controversially suggested that FKBP52 had a potential role
in breast cancer (14–16). In the present study, IHC was used to
assess the difference in FKBP52 expression at the protein level
between breast cancer and non-cancerous tissues. FKBP52 was
slightly upregulated in breast tumor samples, but this did not
reach statistical significance. However, by utilizing publicly
available gene expression data, FKBP52 expression was found to be
higher in breast tumors (Table I).
The limited sample size in the current study may have led to the
inconsistency in protein and mRNA expression levels observed
between the samples analyzed and those obtained from previously
generated datasets. Further study of FKBP52 expression with a
larger sample size is therefore required to confirm the results of
the present study.
In addition to the fact that FKBP52 was observed to
be upregulated in breast cancer cell lines, previous reports
further demonstrated that FKBP52 was predominantly expressed in
ER-positive cells (14,36). However, IHC analysis in the present
study revealed that the expression of FKBP52 was negatively
associated with ER expression, but positively associated with HER-2
expression. These inconsistencies may reflect the complex
association between FKBP52 and molecular receptors in breast
cancer. The difference between cancer cells and organisms could
also result from the cytoplasmic retention of ER by
Hsp90-cochaperons; only nuclear staining of ER is assessed
clinically. The difference in FKBP52 expression between luminal,
HER-2-enriched and triple-negative-subtype breast cancer also
indicated that higher FKBP52 expression was associated with
negative ER or positive HER-2 expression. Considering its clinical
significance FKBP52 may be an adverse prognostic factor, although
further studies are required to confirm this. The current study
also indicated that elevated FKBP52 expression tended to be
observed in breast cancer patients with lymph node metastasis, poor
cell differentiation and advanced TNM stage, suggesting that FKBP52
may be a useful biomarker for the evaluation of differentiation and
metastasis in human breast carcinoma. FKBP52 could also serve as a
novel therapeutic target for breast cancer patients. Through IHC
analysis using tissue microarrays, Liu et al (11) found that FKBP52 regulation was
relevant to hepatocellular carcinoma staging, with relatively high
expression at stages I and II, but a marked decline at stage III,
demonstrating that FKBP52 could be used for early HCC diagnosis.
When considering that it was highly expressed in breast cancer
patients with advanced TNM stage, FKBP52 was hypothesized to be
expressed at different levels depending on the tissue types, as
proposed by previous studies (10,37,38).
In the present study, FKBP52 protein expression was
an adverse predictor for OS in breast cancer patients. However,
multivariate survival analysis demonstrated that FKBP52 was not an
independent factor, partially due to its association with TNM
stage. Analysis using a publically available online tool, KM
Plotter, revealed a valid association between FKBP52 mRNA
expression and OS. This association may be caused by
FKBP52-associated breast cancer resistance to chemotherapies
(39). Thus, FKBP52 could be used as
an objective biological marker to estimate the outcome of breast
cancer. There was no statistically significant association between
lymph node metastasis and OS, which may be a result of the
insufficient sample size in the current study. A cohort with a
larger sample size would be required for a further study.
The present study revealed that FKBP52 may serve a
role in promoting breast cell growth, and could be one of the key
factors affecting the prognosis of breast cancer patients. However,
it cannot be excluded that FKBP52 is a biomarker for increased
breast cancer risk. A comprehensive functional study should
therefore be performed to elucidate this association.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (grant nos. 81272931
and 81572588), the Guangdong Provincial Key Laboratory, Guangdong
Provincial Natural Science Foundation (grant no. S2013010015969)
and a Youth Research Grant from Shantou University Medical College
Cancer Hospital (grant no. 2014/10).
References
|
1
|
Siekierka JJ, Hung SH, Poe M, Lin CS and
Sigal NH: A cytosolic binding protein for the immunosuppressant
FK506 has peptidyl-prolyl isomerase activity but is distinct from
cyclophilin. Nature. 341:755–757. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Theuerkorn M, Fischer G and
Schiene-Fischer C: Prolyl cis/trans isomerase signalling pathways
in cancer. Curr Opin Pharmacol. 11:281–287. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Romano MF: FKBPs: Opportunistic modifiers
or active players in cancer? Curr Opin Pharmacol. 11:279–280. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cioffi DL, Hubler TR and Scammell JG:
Organization and function of the FKBP52 and FKBP51 genes. Curr Opin
Pharmacol. 11:308–313. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Riggs DL, Roberts PJ, Chirillo SC,
Cheung-Flynn J, Prapapanich V, Ratajczak T, Gaber R, Picard D and
Smith DF: The Hsp90-binding peptidylprolyl isomerase FKBP52
potentiates glucocorticoid signaling in vivo. EMBO J. 22:1158–1167.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pirkl F and Buchner J: Functional analysis
of the Hsp90-associated human peptidyl prolyl cis/trans isomerases
FKBP51, FKBP52 and Cyp40. J Mol Biol. 308:795–806. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Davies TH and Sánchez ER: FKBP52. Int J
Biochem Cell Biol. 37:42–47. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Galigniana MD, Harrell JM, O'Hagen HM,
Ljungman M and Pratt WB: Hsp90-binding immunophilins link p53 to
dynein during p53 transport to the nucleus. J Biol Chem.
279:22483–22489. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Teiten MH, Gaigneaux A, Chateauvieux S,
Billing AM, Planchon S, Fack F, Renaut J, Mack F, Muller CP, Dicato
M and Diederich M: Identification of differentially expressed
proteins in curcumin-treated prostate cancer cell lines. OMICS.
16:289–300. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lin JF, Xu J, Tian HY, Gao X, Chen QX, Gu
Q, Xu GJ, Song JD and Zhao FK: Identification of candidate prostate
cancer biomarkers in prostate needle biopsy specimens using
proteomic analysis. Int J Cancer. 121:2596–2605. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu Y, Li C, Xing Z, Yuan X, Wu Y, Xu M,
Tu K, Li Q, Wu C, Zhao M and Zeng R: Proteomic mining in the
dysplastic liver of WHV/c-myc mice-insights and indicators for
early hepatocarcinogenesis. FEBS J. 277:4039–4053. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Guerrero-Preston R, Hadar T, Ostrow KL,
Soudry E, Echenique M, Ili-Gangas C, Pérez G, Perez J,
Brebi-Mieville P, Deschamps J, et al: Differential promoter
methylation of kinesin family member 1a in plasma is associated
with breast cancer and DNA repair capacity. Oncol Rep. 32:505–512.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ostrow KL, Park HL, Hoque MO, Kim MS, Liu
J, Argani P, Westra W, Van Criekinge W and Sidransky D:
Pharmacologic unmasking of epigenetically silenced genes in breast
cancer. Clin Cancer Res. 15:1184–1191. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ward BK, Mark PJ, Ingram DM, Minchin RF
and Ratajczak T: Expression of the estrogen receptor-associated
immunophilins, cyclophilin 40 and FKBP52, in breast cancer. Breast
Cancer Res Treat. 58:267–280. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Desmetz C, Bascoul-Mollevi C, Rochaix P,
Lamy PJ, Kramar A, Rouanet P, Maudelonde T, Mangé A and Solassol J:
Identification of a new panel of serum autoantibodies associated
with the presence of in situ carcinoma of the breast in younger
women. Clin Cancer Res. 15:4733–4741. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li G, Zhao F and Cui Y: Proteomics using
mammospheres as a model system to identify proteins deregulated in
breast cancer stem cells. Curr Mol Med. 13:459–463. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Connolly JL: Changes and problematic areas
in interpretation of the AJCC cancer staging manual, 6th edition,
for breast cancer. Arch Pathol Lab Med. 130:287–291.
2006.PubMed/NCBI
|
|
18
|
Singletary SE, Allred C, Ashley P, Bassett
LW, Berry D, Bland KI, Borgen PI, Clark G, Edge SB, Hayes DF, et
al: Revision of the American Joint Committee on Cancer staging
system for breast cancer. J Clin Oncol. 20:3628–3636. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hwang SB, Bae JW, Lee HY and Kim HY:
Circulating tumor cells detected by RT-PCR for CK-20 before surgery
indicate worse prognostic impact in triple-negative and HER2
subtype breast cancer. J Breast Cancer. 15:34–42. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Miller DV, Leontovich AA, Lingle WL, Suman
VJ, Mertens ML, Lillie J, Ingalls KA, Perez EA, Ingle JN, Couch FJ
and Visscher DW: Utilizing nottingham prognostic index in
microarray gene expression profiling of breast carcinomas. Mod
Pathol. 17:756–764. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Elston CW and Ellis IO: Pathological
prognostic factors in breast cancer. I. The value of histological
grade in breast cancer: Experience from a large study with
long-term follow-up. Histopathology. 19:403–410. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pereira H, Pinder SE, Sibbering DM, Galea
MH, Elston CW, Blamey RW, Robertson JF and Ellis IO: Pathological
prognostic factors in breast cancer. IV: Should you be a typer or a
grader ? A comparative study of two histological prognostic
features in operable breast carcinoma. Histopathology. 27:219–226.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Parham DM: Mitotic activity and
histological grading of breast cancer. Pahtol Annu. 30:189–207.
1995.
|
|
24
|
Patel RM and Folpe AL:
Immunohistochemistry for human telomerase reverse transcriptase
catalytic subunit (hTERT): A study of 143 benign and malignant soft
tissue and bone tumours. Pathology. 41:527–532. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Han YP, Ma CK, Wang SQ, Enomoto A, Zhao Y,
Takahashi M and Ma J: Evaluation of osteopontin as a potential
biomarker for central nervous system embryonal tumors. J
Neurooncol. 119:343–351. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Clarke C, Madden SF, Doolan P, Aherne ST,
Joyce H, O'Driscoll L, Gallagher WM, Hennessy BT, Moriarty M, Crown
J, et al: Correlating transcriptional networks to breast cancer
survival: A large-scale coexpression analysis. Carcinogenesis.
34:2300–2308. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ni Pau IB, Zakaria Z, Muhammad R, Abdullah
N, Ibrahim N, Emran Aina N, Abdullah Hisham N and Hussain Syed SN:
Gene expression patterns distinguish breast carcinomas from normal
breast tissues: The Malaysian context. Pathol Res Pract.
206:223–228. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kretschmer C, Sterner-Kock A, Siedentopf
F, Schoenegg W, Schlag PM and Kemmner W: Identification of early
molecular markers for breast cancer. Mol Cancer. 10:152011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Győrffy B, Surowiak P, Budczies J and
Lánczky A: Online survival analysis software to assess the
prognostic value of biomarkers using transcriptomic data in
non-small-cell lung cancer. PLoS One. 8:e822412013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li XP, Cao GW, Sun Q, Yang C, Yan B, Zhang
MY, Fu YF and Yang LM: Cancer incidence and patient survival rates
among the residents in the Pudong New Area of Shanghai between 2002
and 2006. Chin J Cancer. 32:512–519. 2013.PubMed/NCBI
|
|
31
|
Zeng H, Zheng R, Guo Y, Zhang S, Zou X,
Wang N, Zhang L, Tang J, Chen J, Wei K, et al: Cancer suevival in
China, 2003–2005: A population-based study. Int J Cancer.
136:1921–1930. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gautier L, Cope L, Bolstad BM and Irizarry
RA: affy-analysis of Affymetrix GeneChip data at the probe level.
Bioinformatics. 20:307–315. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
De Leon JT, Iwai A, Feau C, Garcia Y,
Balsiger HA, Storer CL, Suro RM, Garza KM, Lee S, Kim YS, et al:
Targeting the regulation of androgen receptor signaling by the heat
shock protein 90 cochaperone FKBP52 in prostate cancer cells. Proc
Natl Acad Sci USA. 108:11878–11883. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cheung-Flynn J, Prapapanich V, Cox MB,
Riggs DL, Suarez-Quian C and Smith DF: Physiological role for the
cochaperone FKBP52 in androgen receptor signaling. Mol Endocrinol.
19:1654–1666. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hirota Y, Tranguch S, Daikoku T, Hasegawa
A, Osuga Y, Taketani Y and Dey SK: Deficiency of immunophilin
FKBP52 promotes endometriosis. Am J Pathol. 173:1747–1757. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kumar P, Mark PJ, Ward BK, Minchin RF and
Ratajczak T: Estradiol-regulated expression of the immunophilins
cyclophilin 40 and FKBP52 in MCF-7 breast cancer cells. Biochem
Biophys Res Commun. 284:219–225. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Solassol J, Mange A and Maudelonde T: FKBP
family proteins as promising new biomarkers for cancer. Curr Opin
Pharmacol. 11:320–325. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ott M, Litzenburger UM, Rauschenbach KJ,
Bunse L, Ochs K, Sahm F, Pusch S, Opitz CA, Blaes J, von Deimling
A, et al: Suppression of TDO-mediated tryptophan catabolism in
glioblastoma cells by a steroid-responsive FKBP52-dependent
pathway. Glia. 63:78–90. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yang WS, Moon HG, Kim HS, Choi EJ, Yu MH,
Noh DY and Lee C: Proteomic approach reveals FKBP4 and S100A9 as
potential prediction markers of therapeutic response to neoadjuvant
chemotherapy in patients with breast cancer. J Proteome Res.
11:1078–1088. 2012. View Article : Google Scholar : PubMed/NCBI
|