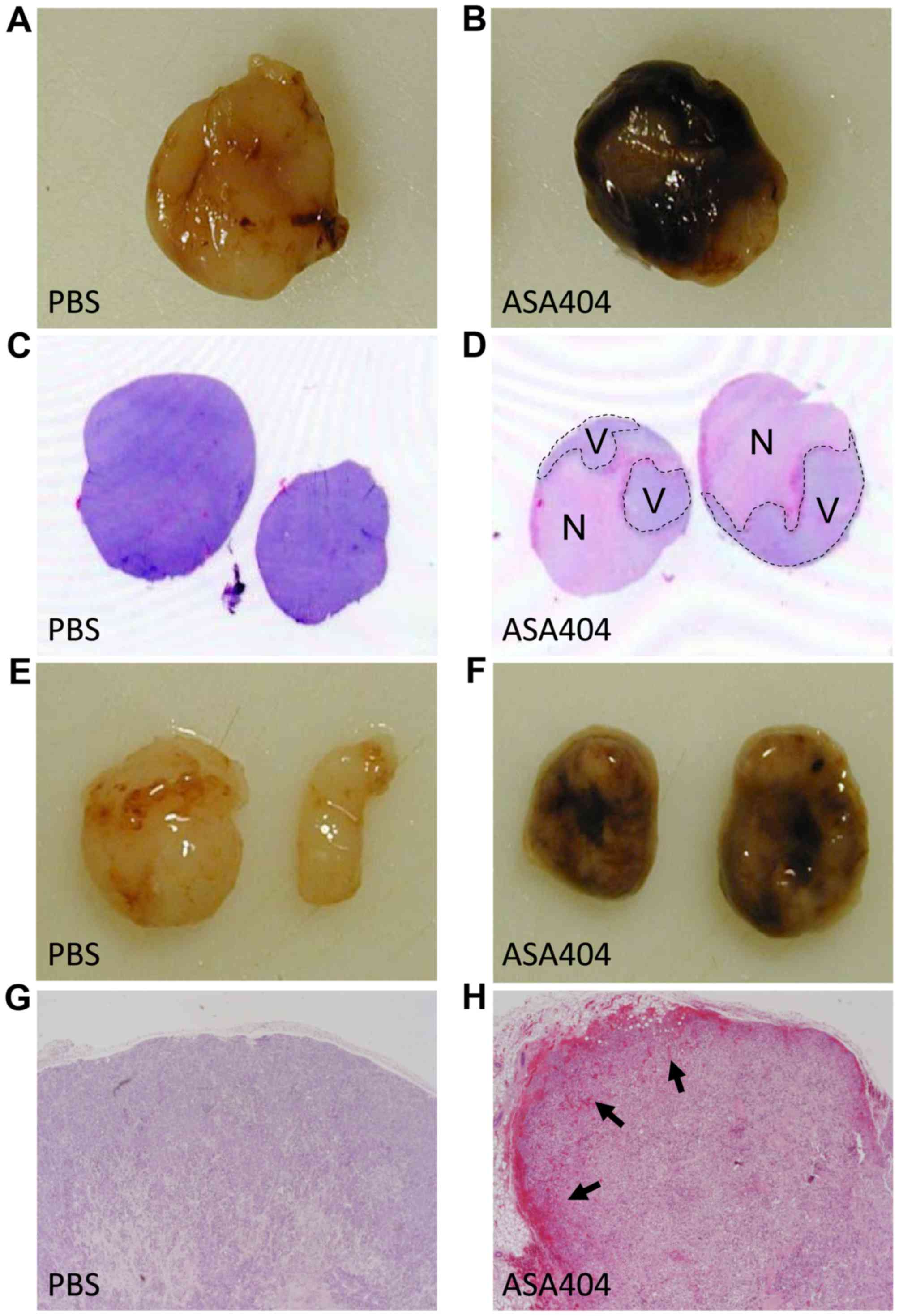
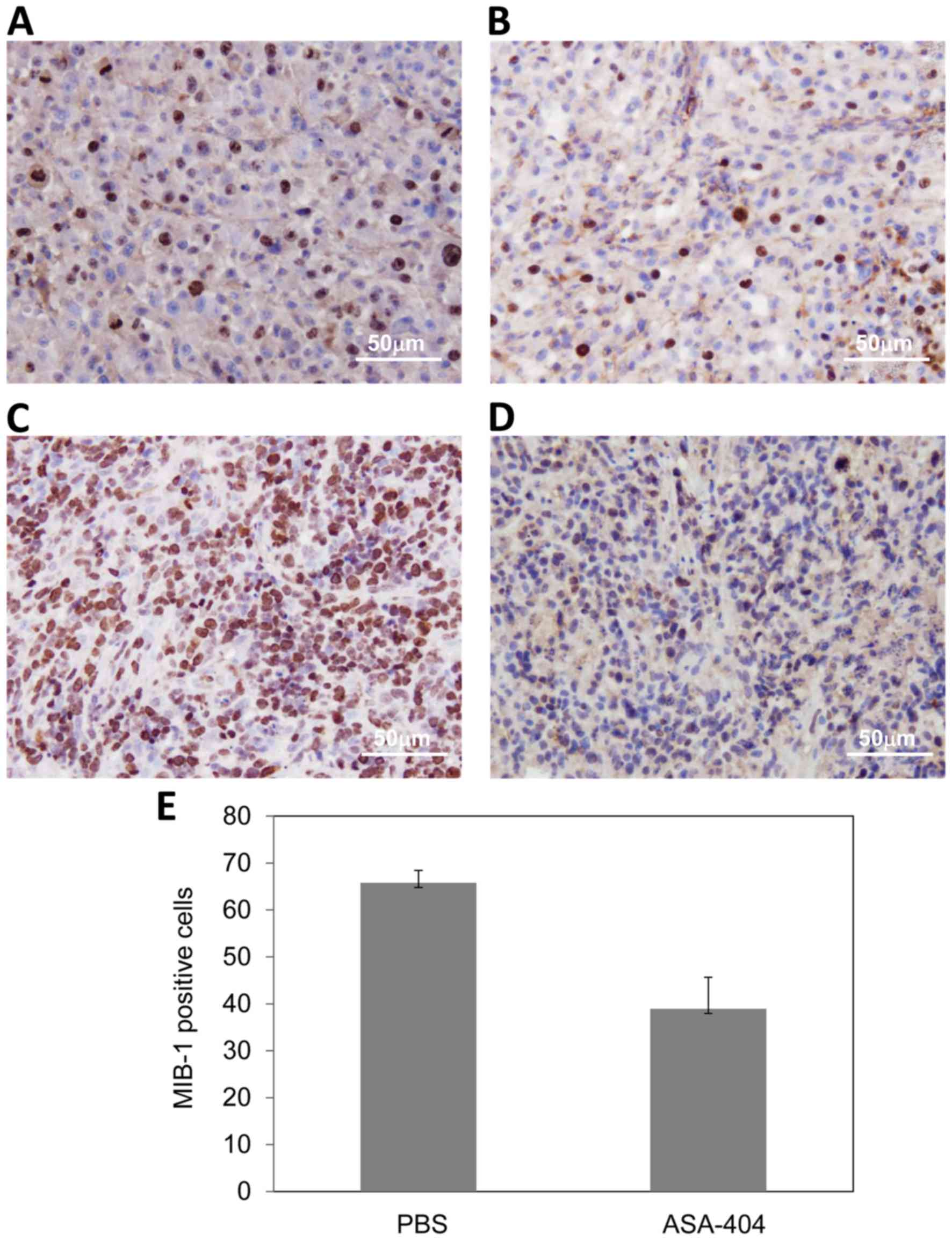
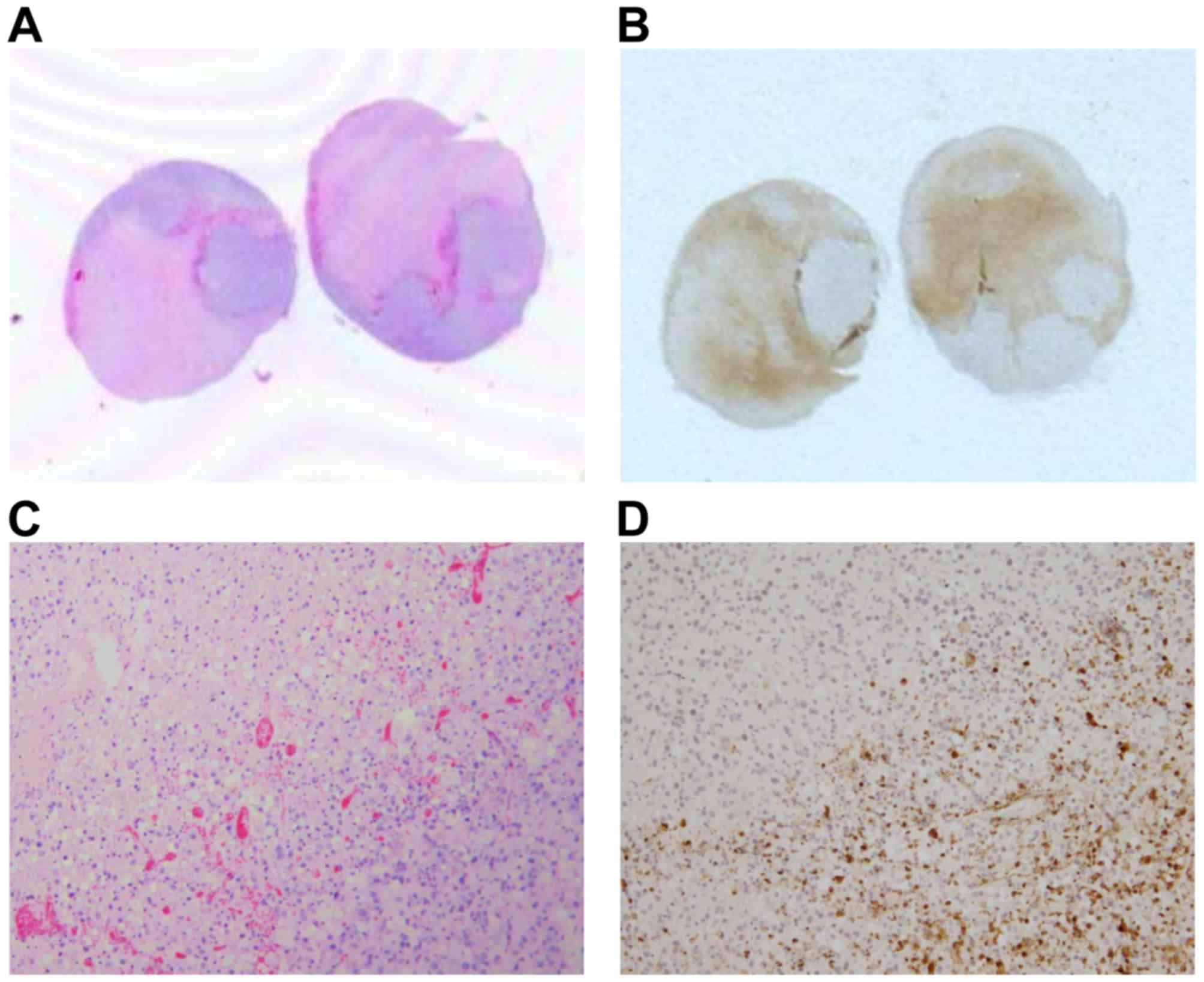
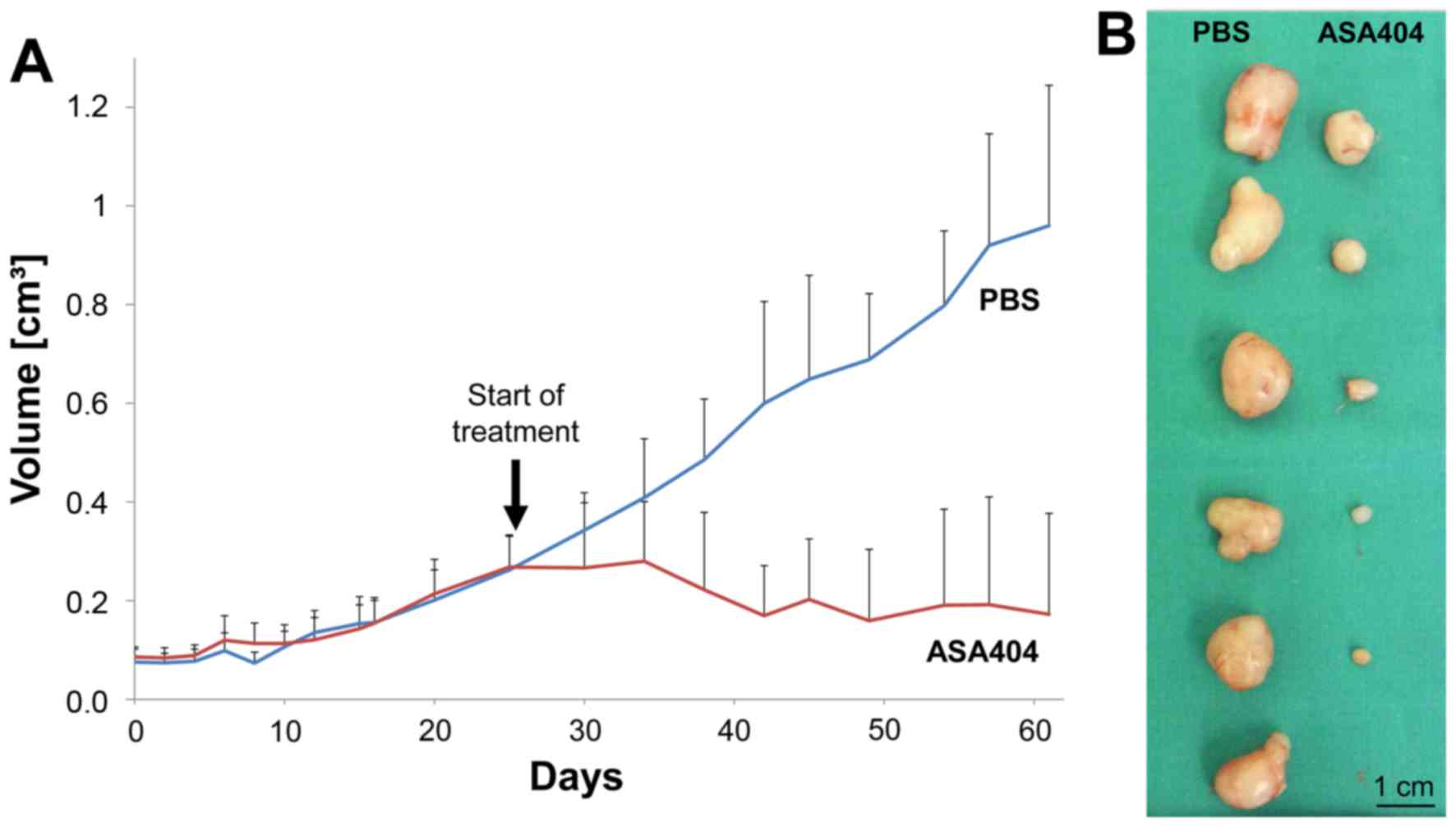
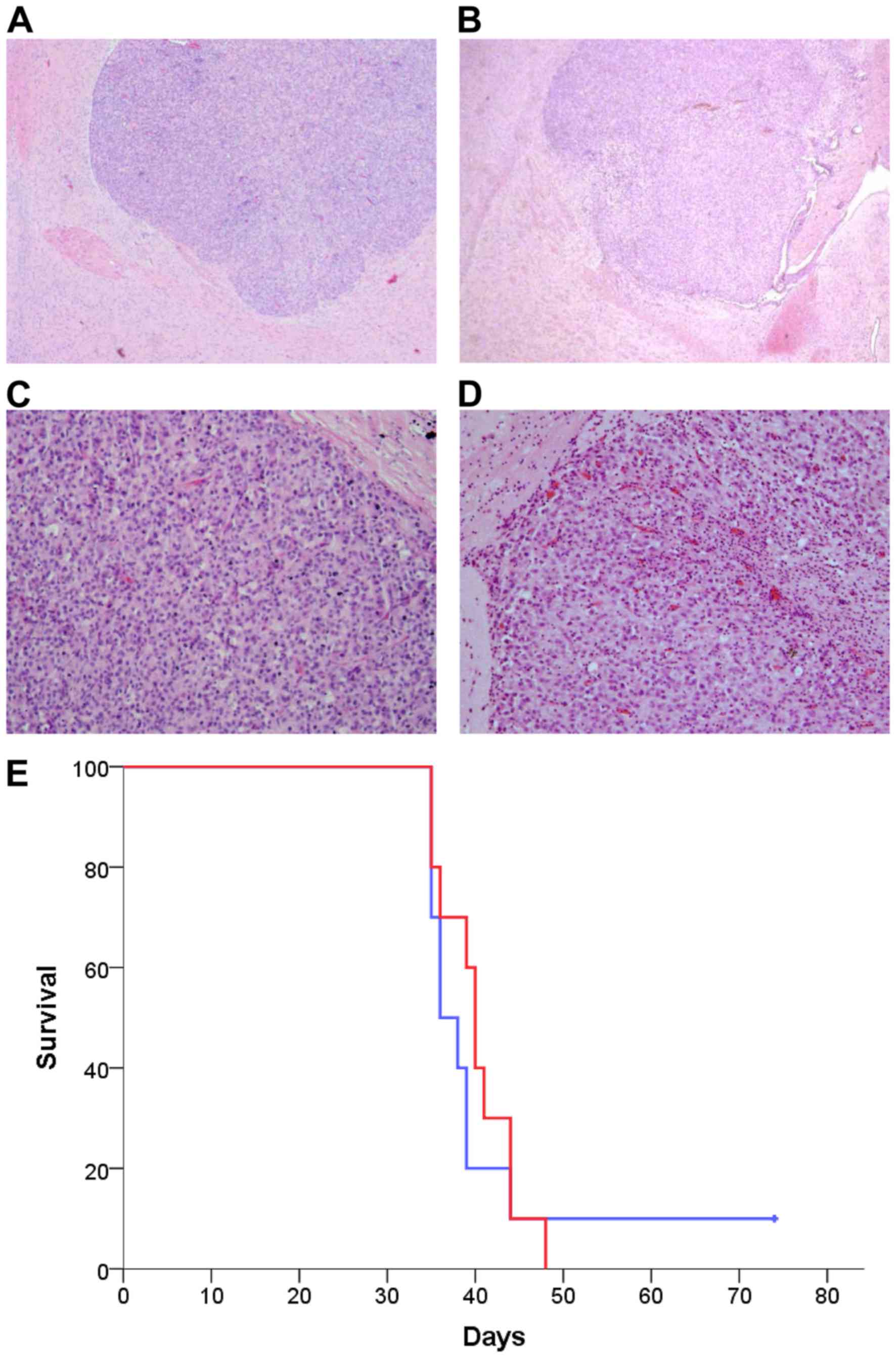
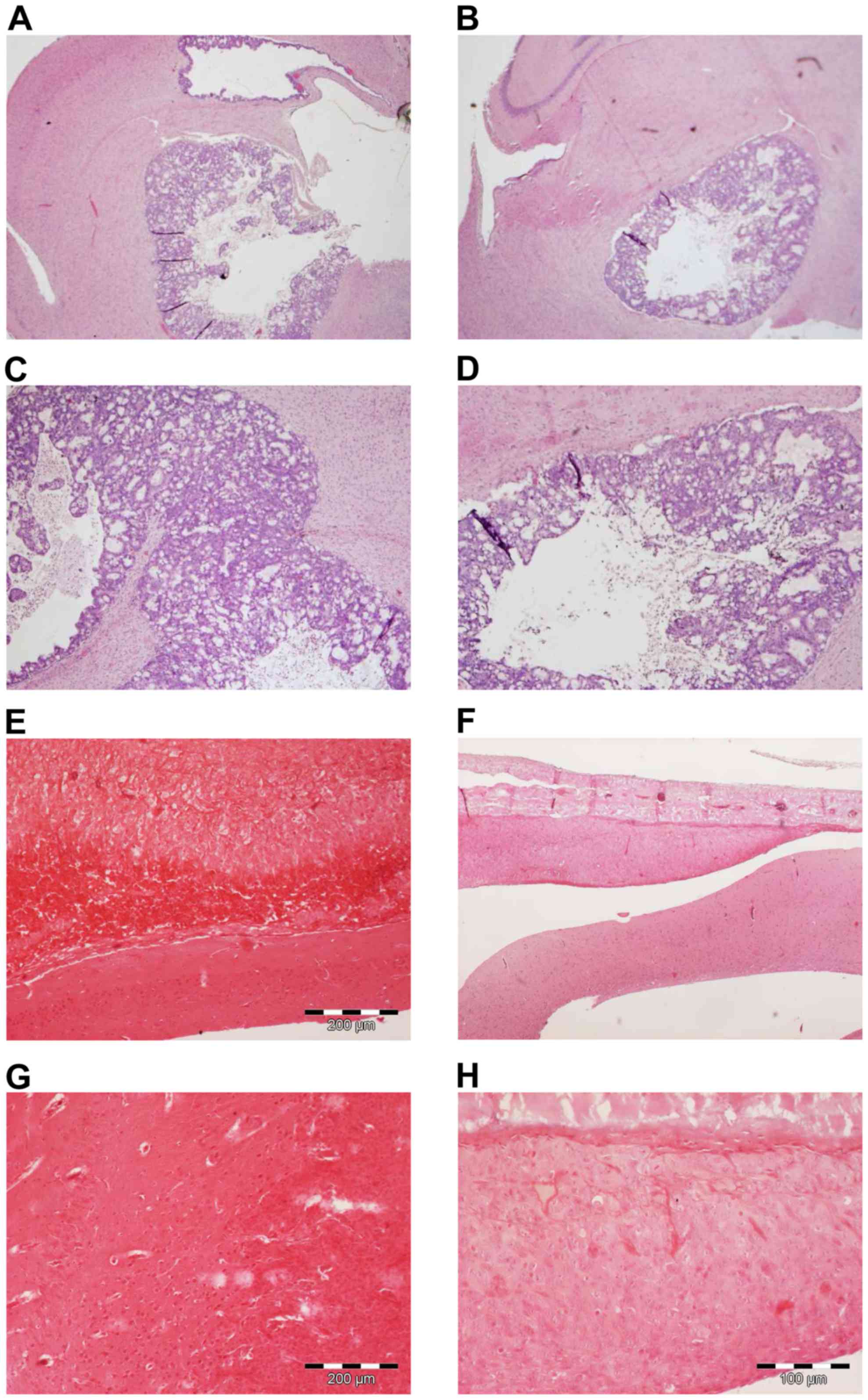
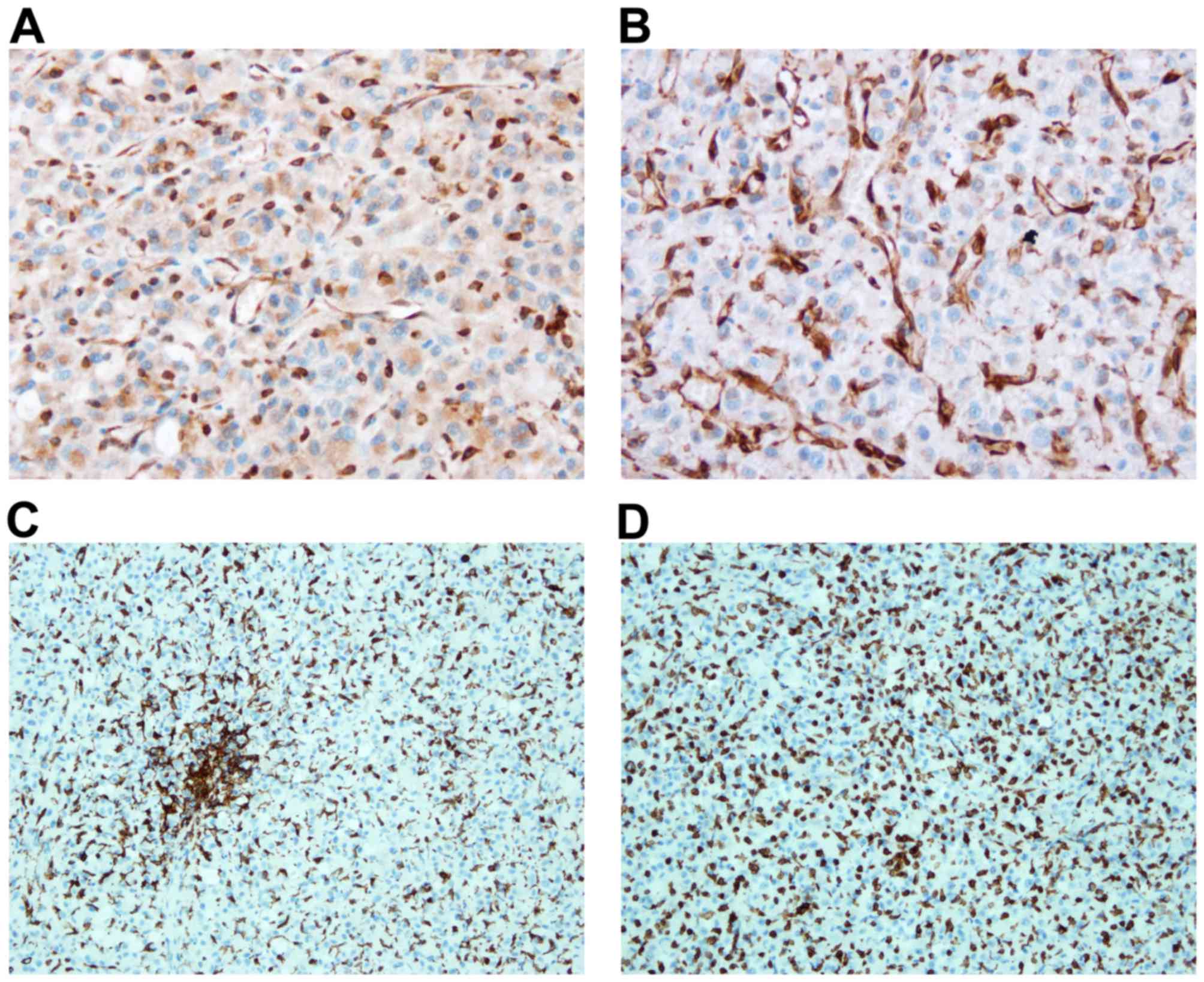
|
1
|
Carmeliet P and Jain RK: Molecular
mechanisms and clinical applications of angiogenesis. Nature.
473:298–307. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Louis DN, Perry A, Reifenberger G, von
Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD,
Kleihues P and Ellison DW: The 2016 world health organization
classification of tumors of the central nervous system: A summary.
Acta Neuropathol. 131:803–820. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Preusser M, Hassler M, Birner P, Rudas M,
Acker T, Plate KH, Widhalm G, Knosp E, Breitschopf H, Berger J and
Marosi C: Microvascularization and expression of VEGF and its
receptors in recurring meningiomas: Pathobiological data in favor
of anti-angiogenic therapy approaches. Clin Neuropathol.
31:352–360. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Takano S: Glioblastoma angiogenesis: VEGF
resistance solutions and new strategies based on molecular
mechanisms of tumor vessel formation. Brain Tumor Pathol. 29:73–86.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Batchelor TT, Reardon DA, de Groot JF,
Wick W and Weller M: Antiangiogenic therapy for glioblastoma:
Current status and future prospects. Clin Cancer Res. 20:5612–5619.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Friedman HS, Prados MD, Wen PY, Mikkelsen
T, Schiff D, Abrey LE, Yung WK, Paleologos N, Nicholas MK, Jensen
R, et al: Bevacizumab alone and in combination with irinotecan in
recurrent glioblastoma. J Clin Oncol. 27:4733–4740. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chinot OL, Wick W, Mason W, Henriksson R,
Saran F, Nishikawa R, Carpentier AF, Hoang-Xuan K, Kavan P, Cernea
D, et al: Bevacizumab plus radiotherapy-temozolomide for newly
diagnosed glioblastoma. N Engl J Med. 370:709–722. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gilbert MR, Dignam JJ, Armstrong TS, Wefel
JS, Blumenthal DT, Vogelbaum MA, Colman H, Chakravarti A, Pugh S,
Won M, et al: A randomized trial of bevacizumab for newly diagnosed
glioblastoma. N Engl J Med. 370:699–708. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Batchelor TT, Mulholland P, Neyns B,
Nabors LB, Campone M, Wick A, Mason W, Mikkelsen T, Phuphanich S,
Ashby LS, et al: Phase III randomized trial comparing the efficacy
of cediranib as monotherapy and in combination with lomustine,
versus lomustine alone in patients with recurrent glioblastoma. J
Clin Oncol. 31:3212–3218. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wick W, Puduvalli VK, Chamberlain MC, van
den Bent MJ, Carpentier AF, Cher LM, Mason W, Weller M, Hong S,
Musib L, et al: Phase III study of enzastaurin compared with
lomustine in the treatment of recurrent intracranial glioblastoma.
J Clin Oncol. 28:1168–1174. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wick W, Steinbach JP, Platten M, Hartmann
C, Wenz F, von Deimling A, Shei P, Moreau-Donnet V, Stoffregen C
and Combs SE: Enzastaurin before and concomitant with radiation
therapy, followed by enzastaurin maintenance therapy, in patients
with newly diagnosed glioblastoma without MGMT promoter
hypermethylation. Neuro Oncol. 15:1405–1412. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Stupp R, Hegi ME, Gorlia T, Erridge SC,
Perry J, Hong YK, Aldape KD, Lhermitte B, Pietsch T, Grujicic D, et
al: Cilengitide combined with standard treatment for patients with
newly diagnosed glioblastoma with methylated MGMT promoter (CENTRIC
EORTC 26071–22072 study): A multicentre, randomised, open-label,
phase 3 trial. Lancet Oncol. 15:1100–1108. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Baguley BC: Antivascular therapy of
cancer: DMXAA. Lancet Oncol. 4:141–148. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hinnen P and Eskens FA: Vascular
disrupting agents in clinical development. Br J Cancer.
96:1159–1165. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Thorpe PE: Vascular targeting agents as
cancer therapeutics. Clin Cancer Res. 10:415–427. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tozer GM, Kanthou C and Baguley BC:
Disrupting tumour blood vessels. Nat Rev Cancer. 5:423–435. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Folaron M, Kalmuk J, Lockwood J, Frangou
C, Vokes J, Turowski SG, Merzianu M, Rigual NR, Sullivan-Nasca M,
Kuriakose MA, et al: Vascular priming enhances chemotherapeutic
efficacy against head and neck cancer. Oral Oncol. 49:893–902.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kanwar JR, Kanwar RK, Pandey S, Ching LM
and Krissansen GW: Vascular attack by
5,6-dimethylxanthenone-4-acetic acid combined with B7.1
(CD80)-mediated immunotherapy overcomes immune resistance and leads
to the eradication of large tumors and multiple tumor foci. Cancer
Res. 61:1948–1956. 2001.PubMed/NCBI
|
|
19
|
Matthews KE, Hermans IF, Roberts JM, Ching
LM and Ronchese F: 5,6-Dimethylxanthenone-4-acetic acid treatment
of a non-immunogenic tumour does not synergize with active or
passive CD8+ T-cell immunotherapy. Immunol Cell Biol.
84:383–389. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Seshadri M and Ciesielski MJ: MRI-based
characterization of vascular disruption by
5,6-dimethylxanthenone-acetic acid in gliomas. J Cereb Blood Flow
Metab. 29:1373–1382. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ching LM, Zwain S and Baguley BC:
Relationship between tumour endothelial cell apoptosis and tumour
blood flow shutdown following treatment with the antivascular agent
DMXAA in mice. Br J Cancer. 90:906–910. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jassar AS, Suzuki E, Kapoor V, Sun J,
Silverberg MB, Cheung L, Burdick MD, Strieter RM, Ching LM, Kaiser
LR, et al: Activation of tumor-associated macrophages by the
vascular disrupting agent 5,6-dimethylxanthenone-4-acetic acid
induces an effective CD8+ T-cell-mediated antitumor
immune response in murine models of lung cancer and mesothelioma.
Cancer Res. 65:11752–11761. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Corrales L, Glickman LH, McWhirter SM,
Kanne DB, Sivick KE, Katibah GE, Woo SR, Lemmens E, Banda T, Leong
JJ, et al: Direct activation of STING in the tumor microenvironment
leads to potent and systemic tumor regression and immunity. Cell
Rep. 11:1018–1030. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Corrales L, McWhirter SM, Dubensky TW Jr
and Gajewski TF: The host STING pathway at the interface of cancer
and immunity. J Clin Invest. 126:2404–2411. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Roberts ZJ, Goutagny N, Perera PY, Kato H,
Kumar H, Kawai T, Akira S, Savan R, van Echo D, Fitzgerald KA, et
al: The chemotherapeutic agent DMXAA potently and specifically
activates the TBK1-IRF-3 signaling axis. J Exp Med. 204:1559–1569.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Curran E, Chen X, Corrales L, Kline DE,
Dubensky TW Jr, Duttagupta P, Kortylewski M and Kline J: STING
pathway activation stimulates potent immunity against acute myeloid
leukemia. Cell Rep. 15:2357–2366. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gao P, Ascano M, Zillinger T, Wang W, Dai
P, Serganov AA, Gaffney BL, Shuman S, Jones RA, Deng L, et al:
Structure-function analysis of STING activation by c[G (2′,5′)pA
(3′,5′)p] and targeting by antiviral DMXAA. Cell. 154:748–762.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ishikawa H and Barber GN: STING is an
endoplasmic reticulum adaptor that facilitates innate immune
signalling. Nature. 455:674–678. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Conlon J, Burdette DL, Sharma S, Bhat N,
Thompson M, Jiang Z, Rathinam VA, Monks B, Jin T, Xiao TS, et al:
Mouse, but not human STING, binds and signals in response to the
vascular disrupting agent 5,6-dimethylxanthenone-4-acetic acid. J
Immunol. 190:5216–5225. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Prantner D, Perkins DJ, Lai W, Williams
MS, Sharma S, Fitzgerald KA and Vogel SN:
5,6-Dimethylxanthenone-4-acetic acid (DMXAA) activates stimulator
of interferon gene (STING)-dependent innate immune pathways and is
regulated by mitochondrial membrane potential. J Biol Chem.
287:39776–39788. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
McKeage MJ, Von Pawel J, Reck M, Jameson
MB, Rosenthal MA, Sullivan R, Gibbs D, Mainwaring PN, Serke M,
Lafitte JJ, et al: Randomised phase II study of ASA404 combined
with carboplatin and paclitaxel in previously untreated advanced
non-small cell lung cancer. Br J Cancer. 99:2006–2012. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lara PN Jr, Douillard JY, Nakagawa K, von
Pawel J, McKeage MJ, Albert I, Losonczy G, Reck M, Heo DS, Fan X,
et al: Randomized phase III placebo-controlled trial of carboplatin
and paclitaxel with or without the vascular disrupting agent
vadimezan (ASA404) in advanced non-small-cell lung cancer. J Clin
Oncol. 29:2965–2971. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wallace A, LaRosa DF, Kapoor V, Sun J,
Cheng G, Jassar A, Blouin A, Ching LM and Albelda SM: The vascular
disrupting agent, DMXAA, directly activates dendritic cells through
a MyD88-independent mechanism and generates antitumor cytotoxic T
lymphocytes. Cancer Res. 67:7011–7019. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Smilowitz HM, Weissenberger J, Weis J,
Brown JD, O'Neill RJ and Laissue JA: Orthotopic transplantation of
v-src-expressing glioma cell lines into immunocompetent mice:
Establishment of a new transplantable in vivo model for malignant
glioma. J Neurosurg. 106:652–659. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tuchen M, Wilisch-Neumann A, Daniel EA,
Baldauf L, Pachow D, Scholz J, Angenstein F, Stork O, Kirches E and
Mawrin C: Receptor tyrosine kinase inhibition by
regorafenib/sorafenib inhibits growth and invasion of meningioma
cells. Eur J Cancer. 73:9–21. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pachow D, Andrae N, Kliese N, Angenstein
F, Stork O, Wilisch-Neumann A, Kirches E and Mawrin C: mTORC1
inhibitors suppress meningioma growth in mouse models. Clin Cancer
Res. 19:1180–1189. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
McCutcheon IE, Friend KE, Gerdes TM, Zhang
BM, Wildrick DM and Fuller GN: Intracranial injection of human
meningioma cells in athymic mice: An orthotopic model for
meningioma growth. J Neurosurg. 92:306–314. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lee WH: Characterization of a newly
established malignant meningioma cell line of the human brain:
IOMM-Lee. Neurosurgery. 27:389–396. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kliese N, Gobrecht P, Pachow D, Andrae N,
Wilisch-Neumann A, Kirches E, Riek-Burchardt M, Angenstein F,
Reifenberger G, Riemenschneider M, et al: miRNA-145 is
downregulated in atypical and anaplastic meningiomas and negatively
regulates motility and proliferation of meningioma cells. Oncogene.
32:4712–4720. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
RayChaudhury A, Frazier WA and D'Amore PA:
Comparison of normal and tumorigenic endothelial cells: Differences
in thrombospondin production and responses to transforming growth
factor-beta. J Cell Sci. 107:39–46. 1994.PubMed/NCBI
|
|
41
|
Muhlner U, Mohle-Steinlein U,
Wizigmann-Voos S, Christofori G, Risau W and Wagner EF: Formation
of transformed endothelial cells in the absence of VEGFR-2/Flk-1 by
Polyoma middle T oncogene. Oncogene. 18:4200–4210. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Montesano R, Pepper MS, Möhle-Steinlein U,
Risau W, Wagner EF and Orci L: Increased proteolytic activity is
responsible for the aberrant morphogenetic behavior of endothelial
cells expressing the middle T oncogene. Cell. 62:435–445. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Weller M, Rieger J, Grimmel C, Van Meir
EG, Tribolet N, De Krajewski S, Reed JC, von Deimling A and
Dichgans J: Predicting chemoresistance in human malignant glioma
cells: The role of molecular genetic analyses. Int J Cancer.
79:640–644. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yung R, Seyfoddin V, Guise C, Tijono S,
McGregor A, Connor B and Ching LM: Efficacy against subcutaneous or
intracranial murine GL261 gliomas in relation to the concentration
of the vascular-disrupting agent, 5,6-dimethylxanthenone-4-acetic
acid (DMXAA), in the brain and plasma. Cancer Chemother Pharmacol.
73:639–649. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Renner DN, Jin F, Litterman AJ, Balgeman
AJ, Hanson LM, Gamez JD, Chae M, Carlson BL, Sarkaria JN, Parney
IF, et al: Effective treatment of established GL261 murine gliomas
through picornavirus vaccination-enhanced tumor antigen-specific
CD8+ T cell responses. PLoS One. 10:e01255652015.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhao L, Ching LM, Kestell P and Baguley
BC: The antitumour activity of 5,6-dimethylxanthenone-4-acetic acid
(DMXAA) in TNF receptor-1 knockout mice. Br J Cancer. 87:465–470.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Conlon J, Burdette DL, Sharma S, Bhat N,
Thompson M, Jiang Z, Rathinam VA, Monks B, Jin T, Xiao TS, et al:
Mouse, but not human STING, binds and signals in response to the
vascular disrupting agent 5,6-dimethylxanthenone-4-acetic acid. J
Immunol. 190:5216–5225. 2013. View Article : Google Scholar : PubMed/NCBI
|