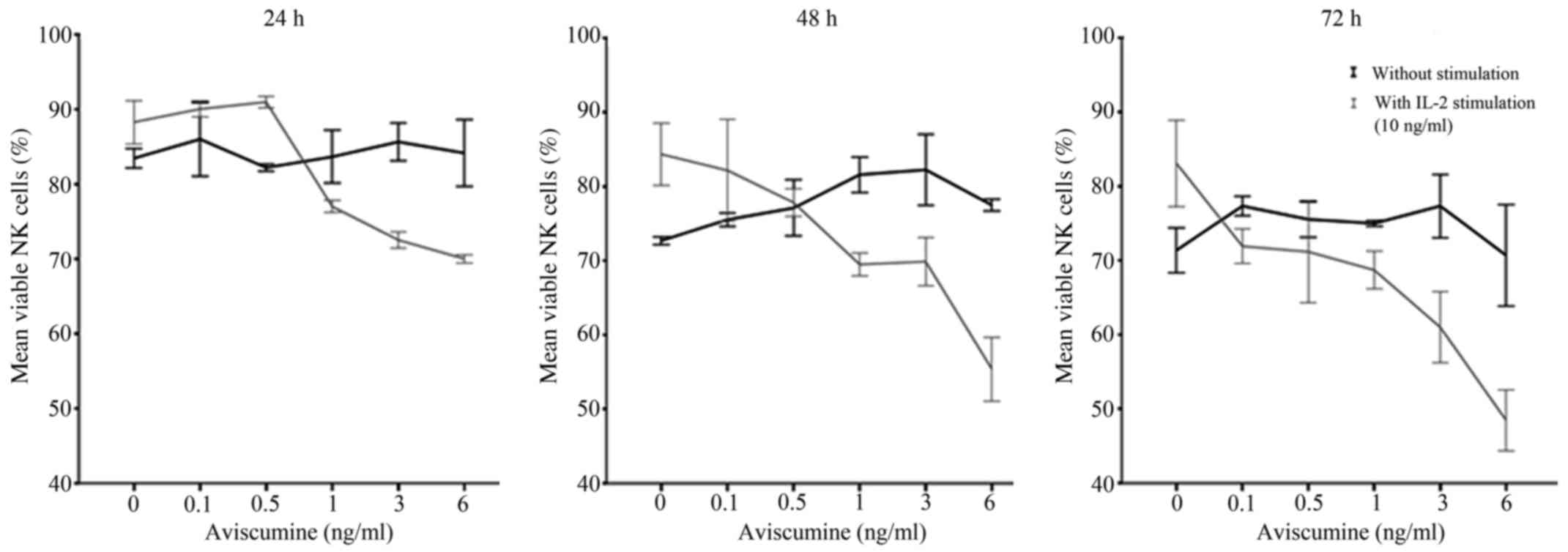
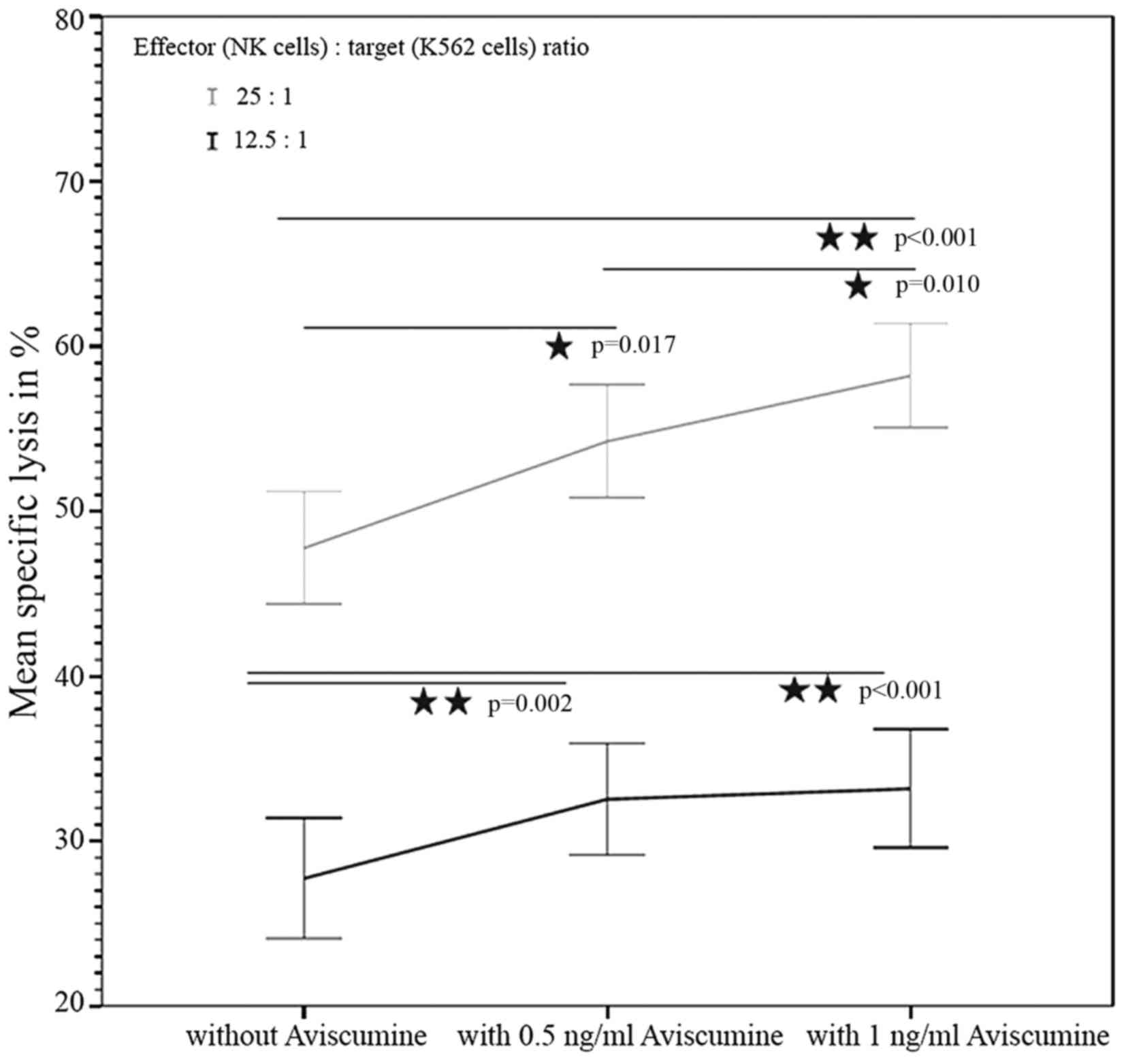
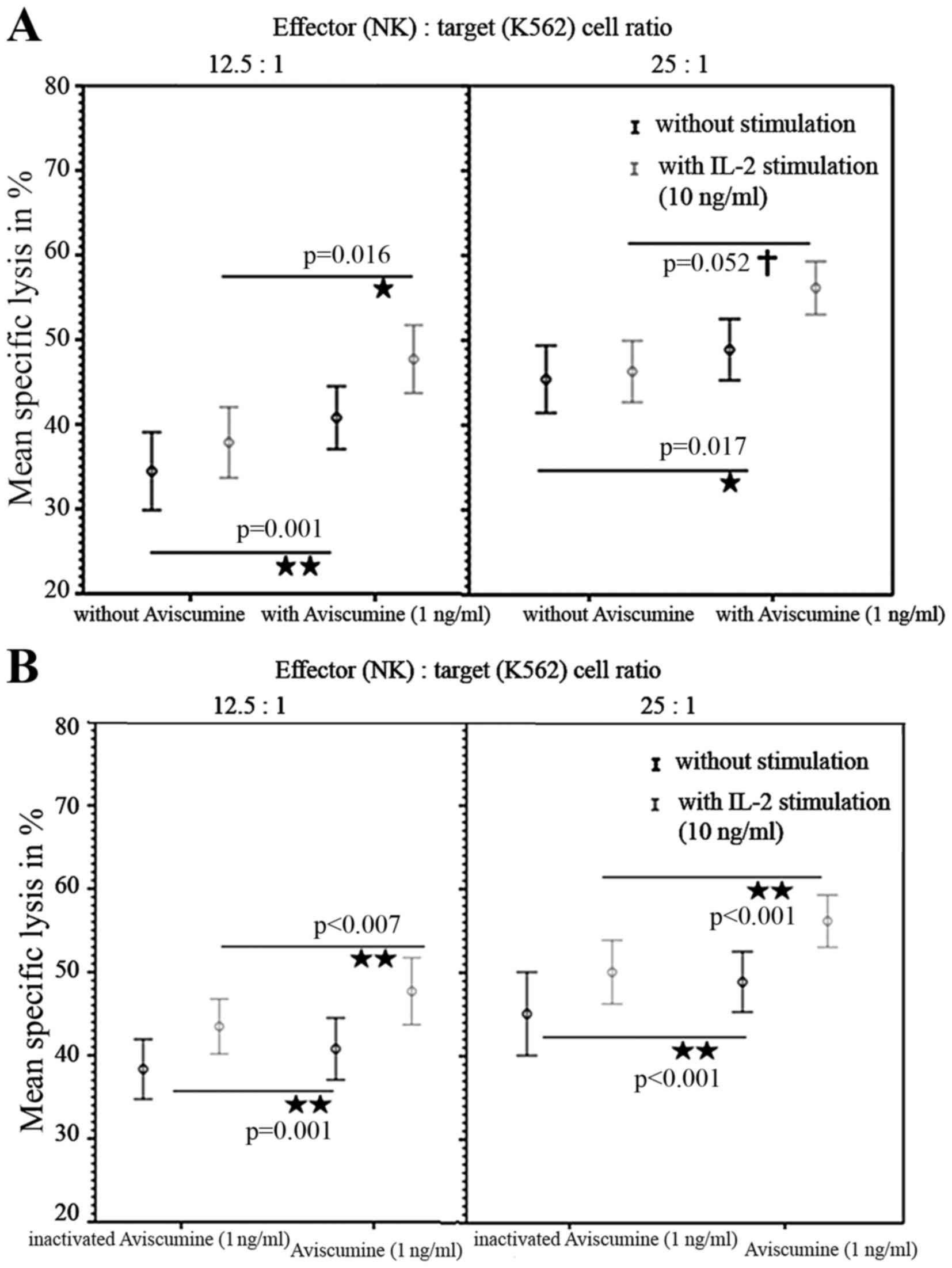
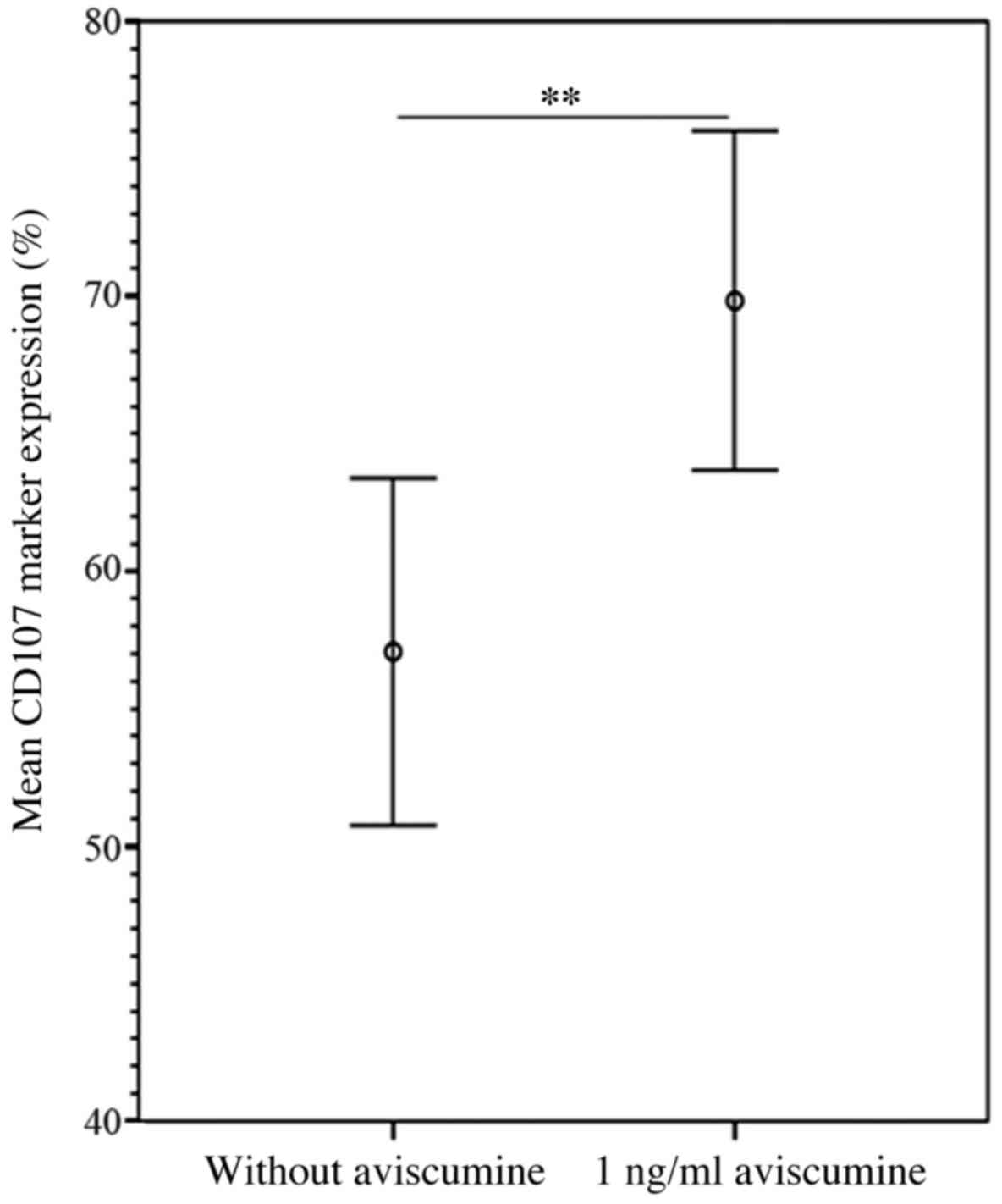
|
1
|
Brower V: Checkpoint blockade
immunotherapy for cancer comes of age. J Natl Cancer Inst. 107:pii:
djv069. 2015. View Article : Google Scholar
|
|
2
|
Postow MA, Callahan MK and Wolchok JD:
Immune checkpoint blockade in cancer therapy. J Clin Oncol.
33:1974–1982. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Perez-Gracia JL, Labiano S, Rodriguez-Ruiz
ME, Sanmamed MF and Melero I: Orchestrating immune check-point
blockade for cancer immunotherapy in combinations. Curr Opin
Immunol. 27:89–97. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gardner TA, Elzey BD and Hahn NM:
Sipuleucel-T (Provenge) autologous vaccine approved for treatment
of men with asymptomatic or minimally symptomatic
castrate-resistant metastatic prostate cancer. Hum Vaccin
Immunother. 8:534–539. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jiang QL, Zhang S, Tian M, Zhang SY, Xie
T, Chen DY, Chen YJ, He J, Liu J, Ouyang L and Jiang X: Plant
lectins, from ancient sugar-binding proteins to emerging
anti-cancer drugs in apoptosis and autophagy. Cell Prolif.
48:17–28. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vanneman M and Dranoff G: Combining
immunotherapy and targeted therapies in cancer treatment. Nat Rev
Cancer. 12:237–251. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Eck J, Langer M, Möckel B, Baur A, Rothe
M, Zinke H and Lentzen H: Cloning of the mistletoe lectin gene and
characterization of the recombinant A-chain. Eur J Biochem.
264:775–784. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Eck J, Langer M, Möckel B, Witthohn K,
Zinke H and Lentzen H: Characterization of recombinant and
plant-derived mistletoe lectin and their B-chains. Eur J Biochem.
265:788–797. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Langer M, Möckel B, Eck J, Zinke H and
Lentzen H: Site-specific mutagenesis of mistletoe lectin: The role
of RIP activity in apoptosis. Biochem Biophys Res Commun.
264:944–948. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Möckel B, Burger A, Schultz RJ,
Wilhelm-Ogunbiyi K, Langer M, Zinke H, Fiebig HH and Lentzen H:
Assessing the cancerostatic potency of rViscumin towards human
tumor xenografts and cell lines in vitro. European J Cancer.
37:S122001. View Article : Google Scholar
|
|
11
|
Langer M.MB, Wilhelm-Ogunbiyi K, Witthohn
K and Lentzen H: Antitumour activity of rViscumin in vitro and in
vivo. 26:3942003.
|
|
12
|
Wilhelm-Ogunbiyi K, Möckel B, Burger A,
Langer M, Zinke H, Fiebig HH, et al: rViscumin, a novel anticancer
agent-preclinical and clinical development status. Eur J Cancer. 37
Supplement 3:S52001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Abuharbeid S, Apel J, Sander M, Fiedler B,
Langer M, Zuzarte ML, Czubayko F and Aigner A: Cytotoxicity of the
novel anti-cancer drug rViscumin depends on HER-2 levels in SKOV-3
cells. Biochem Biophys Res Commun. 321:403–412. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hostanska K, Vuong V, Rocha S, Soengas MS,
Glanzmann C, Saller R, Bodis S and Pruschy M: Recombinant mistletoe
lectin induces p53-independent apoptosis in tumour cells and
cooperates with ionising radiation. Br J Cancer. 88:1785–1792.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schöffski P, Riggert S, Fumoleau P,
Campone M, Bolte O, Marreaud S, Lacombe D, Baron B, Herold M,
Zwierzina H, et al: Phase I trial of intravenous aviscumine
(rViscumin) in patients with solid tumors: A study of the European
Organization for Research and Treatment of Cancer New Drug
Development Group. Ann Oncol. 15:1816–1824. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schöffski P, Breidenbach I, Krauter J,
Bolte O, Stadler M, Ganser A, Wilhelm-Ogunbiyi K and Lentzen H:
Weekly 24 h infusion of aviscumine (rViscumin): A phase I study in
patients with solid tumours. Eur J Cancer. 41:1431–1438. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Trefzer U, Gutzmer R, Wilhelm T, Schenck
F, Kähler KC, Jacobi V, Witthohn K, Lentzen H and Mohr P: Treatment
of unresectable stage IV metastatic melanoma with aviscumine after
anti-neoplastic treatment failure: A phase II, multi-centre study.
J Immunother Cancer. 2:272014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zwierzina H, Bergmann L, Fiebig H, Aamdal
S, Schöffski P, Witthohn K and Lentzen H: The preclinical and
clinical activity of aviscumine: A potential anticancer drug. Eur J
Cancer. 47:1450–1457. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bergmann L, Aamdal S, Marreaud S, Lacombe
D, Herold M, Yamaguchi T, Wilhelm-Ogunbiyi K, Lentzen H and
Zwierzina H: European Organisation for Research and Treatment of
Cancer: Phase I trial of r viscumin (INN: Aviscumine) given
subcutaneously in patients with advanced cancer: A study of the
European Organisation for Research and Treatment of Cancer (EORTC
protocol number 13001). Eur J Cancer. 44:1657–1662. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rose A, El-Leithy T, vom Dorp F, Zakaria
A, Eisenhardt A, Tschirdewahn S and Rübben H: Mistletoe Plant
Extract in Patients with Nonmuscle Invasive Bladder Cancer: Results
of a Phase Ib/IIa Single Group Dose Escalation Study. J Urol.
194:939–943. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kutikhin AG and Yuzhalin AE: Editorial:
Pattern Recognition Receptors and Cancer. Frontiers in Immunology.
6:1–2. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kirsch A and Hajto T: Case reports of
sarcoma patients with optimized lectin-oriented mistletoe extract
therapy. J Altern Complement Med. 17:973–979. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Müthing J, Meisen I, Bulau P, Langer M,
Witthohn K, Lentzen H, Neumann U and Peter-Katalinić J: Mistletoe
lectin I is a sialic acid-specific lectin with strict preference to
gangliosides and glycoproteins with terminal Neu5Ac alpha 2–6Gal
beta 1–4GlcNAc residues. Biochemistry. 43:2996–3007. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kiessling R, Klein E, Pross H and Wigzell
H: ‘Natural’ killer cells in the mouse. II. Cytotoxic cells with
specificity for mouse Moloney leukemia cells. Characteristics of
the killer cell. Eur J Immunol. 5:117–121. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ströhlein MA, Grützner KU, Schildberg FW
and Heiss MM: Induction of cytotoxicity against autologous tumour
cells by interleukin-12: Evidence for intrinsic anti-tumor immune
capacity in curatively resected gastrointestinal tumour patients.
Cancer Immunol Immunother. 51:505–512. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Konjevic G, Jurisic V, Jovic V, Vuletic A,
Martinovic Mirjacic K, Radenkovic S and Spuzic I: Investigation of
NK cell function and their modulation in different malignancies.
Immunol Res. 52:139–156. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fregni G, Perier A, Avril MF and Caignard
A: NK cells sense tumors, course of disease and treatments:
Consequences for NK-based therapies. Oncoimmunology. 1:38–47. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Vitale M, Cantoni C, Pietra G, Mingari MC
and Moretta L: Effect of tumor cells and tumor microenvironment on
NK-cell function. Eur J Immunol. 44:1582–1592. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Borghaei H, Paz-Ares L, Horn L, Spigel DR,
Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al:
Nivolumab versus docetaxel in advanced nonsquamous non-small-cell
lung cancer. N Engl J Med. 373:1627–1639. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Larkin J, Chiarion-Sileni V, Gonzalez R,
Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M,
Rutkowski P, et al: Combined Nivolumab and Ipilimumab or
Monotherapy in Untreated Melanoma. N Engl J Med. 373:23–34. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
McDowell KA, Hank JA, DeSantes KB,
Capitini CM, Otto M and Sondel PM: NK Cell-based immunotherapies in
pediatric oncology. J Pediatr Hematol Oncol. 37:79–93. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Poggi A, Massaro AM, Negrini S, Contini P
and Zocchi MR: Tumor-induced apoptosis of human IL-2-activated NK
cells: Role of natural cytotoxicity receptors. J Immunol.
174:2653–2660. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hajto T, Baranyai L, Kirsch A, Kuzma M and
Perjési P: Can a synergistic activation of pattern recognition
receptors by plant immunomodulators enhance the effect of oncologic
therapy? Case Report of a patient with uterus and ovary sarcoma.
Clin Case Rep Rev. 1:235–238. 2015. View Article : Google Scholar
|