Introduction
Osteosarcoma is the most common primary malignancy
of the bone (1), and is a high-grade
malignant mesenchymal tumor with high recurrence and metastatic
rates (1). Classical chemotherapy
drugs include methotrexate, cisplatin, doxorubicin and ifosfamide.
However, multidrug resistance is the main problem of chemotherapy,
and its associated mechanism is not clear. A number of factors may
be associated with tumor resistance to classical chemotherapy
(2,3).
For patients with drug resistance, effective treatment and tumor
markers for prognosis are lacking. Therefore, studies investigating
treatments, tumor markers and targets for osteosarcoma treatment
are essential. Abraxane® [paclitaxel for injection
(albumin-bound)] contains paclitaxel nanoparticles and albumin. As
a vector, albumin combines with secreted protein, acidic and rich
in cysteine (SPARC), which is expressed in various malignant tumors
and is associated with the occurrence and progression of tumors
(4–15). Increased expression of SPARC also
indicates recurrence and poor prognosis in a number of malignances
(4–15). However, the expression level of SPARC
in human osteosarcoma and its associated mechanism remains unclear.
Therefore, the present study was designed based on our previous
study (16) and the hypothesis that
there is high SPARC expression in human osteosarcoma, to elucidate
the possibility of SPARC as a tumor marker and therapeutic target
for osteosarcoma. The present study focused on SPARC protein and
gene expression in human osteosarcoma. A selection of clinical
factors was analyzed and positive results were demonstrated.
Materials and methods
Tumor sample processing and clinical
characteristics
The inclusion criteria for samples were: Specimens
which had been preserved well; pathological confirmation of primary
malignant osteosarcoma; well-preserved normal tissues 2 cm away
from tumor margin; no chemotherapy prior to operation; and complete
clinical data. Between January 2013 and September 2013, a total of
20 osteosarcoma specimens and normal tissues in the Department of
Orthopedic Oncology Surgery, Beijing Ji Shui Tan Hospital (Beijing,
China) matched these conditions. All cases were confirmed as
osteosarcoma by a pathologist through post-operative examination.
All samples were excised from fresh osteosarcoma tumors and
immediately snap-frozen in liquid nitrogen. The frozen samples were
stored at −80°C in tissue bank of the Department of Orthopedic
Oncology Surgery, Beijing Ji Shui Tan Hospital. All patient data,
including age, sex, tumor site and size, laboratory tests,
metastasis and survival were collected (Table I). The patients in the present study
received direct amputation due to tumor invasion of major vessels,
so the potential interruption of chemotherapy drugs on protein
expression was avoided. The study was approved by the Institutional
Review Board of Beijing Ji Shui Tan Hospital (Beijing, China) and
all patients provided written informed consent for the use of their
samples.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Case | Sex | Age, years | Tumor site | Follow-up time,
months | Tumor volume,
cm3 | Maximum tumor
diameter, cm | Metastasis | Recurrence | Alive |
|---|
| 1 | M | 23 | Pelvis | 26 | 1,400 | 14 | N | N | Y |
| 2 | M | 19 | Femur | 13 | 4,056 | 24 | Y | N | N |
| 3 | F | 9 | Femur | 27 | 1,215 | 15 | N | N | Y |
| 4 | M | 51 | Humerus | 24 | 1,980 | 15 | Y | N | Y |
| 5 | M | 26 | Humerus | 22 |
360 | 12 | Y | N | Y |
| 6 | M | 16 | Tibia | 19 |
187 | 8.5 | Y | N | Y |
| 7 | M | 14 | Tibia | 19 |
702 | 12 | N | N | Y |
| 8 | M | 28 | Tibia | 18 |
140 | 8 | N | N | Y |
| 9 | F | 10 | Femur | 24 |
264 | 8 | N | N | Y |
| 10 | M | 18 | Tibia | 23 |
640 | 10 | N | N | Y |
| 11 | M | 19 | Femur | 24 |
885 | 11 | N | N | Y |
| 12 | M | 21 | Femur | 21 | 3,127 | 22 | Y | N | N |
| 13 | F | 9 | Femur | 18 | 1,654 | 14 | N | N | Y |
| 14 | M | 23 | Humerus | 18 |
580 | 13 | N | N | Y |
| 15 | M | 14 | Tibia | 22 |
430 | 9 | Y | N | Y |
| 16 | F | 17 | Humerus | 21 | 1,650 | 13 | Y | N | Y |
| 17 | M | 21 | Tibia | 20 |
260 | 7 | N | N | Y |
| 18 | M | 28 | Tibia | 24 |
346 | 9 | N | N | Y |
| 19 | F | 10 | Femur | 12 | 1,540 | 11 | Y | N | Y |
| 20 | F | 18 | Femur | 18 | 2,132 | 15 | N | N | Y |
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
The reaction was performed with preliminary
incubation for 2 min at 95°C, followed by 45 cycles of denaturation
at 95°C for 20 sec and annealing/extension at 59°C for 25 sec and
72°C for 30 sec. The final melting lasted 50 sec from 70 to 95°C at
an interval of 0.5°C/s. Total RNA was extracted from tumor and
normal tissue using TRIzol (Beijing Solarbio Science and Technology
Co., Ltd., Beijing, China). The RNA was denatured at 70°C for 5 min
using template RNA and oligo(dT) (both from Beijing Solarbio
Science and Technology Co., Ltd.). The reverse transcription
reaction solution was incubated at 42°C for 120 min in 5 µl 5X
Moloney murine leukemia virus buffer (Beijing Solarbio Science and
Technology Co., Ltd.), 1.25 µl deoxyribonucleoside triphosphate
mixture and 25 units RNase inhibitor (Beijing Solarbio Science and
Technology Co., Ltd.). Subsequently, qPCR was performed with the
following primer sequences of SPARC (GenBank accession no. NM
009242): Forward, 5-CATCAAGGAGCAGGACATCAAC-3 and reverse,
5-GCAGCAGGAGGCGTGAA-3 (Primer Premier 5.0 and Oligo 6.0). A PCR
detection system (Bioer Technology Co., Ltd., Hangzhou, China) was
applied to measure the fluorescence emitted with SYBR-Green
(Beijing Solarbio Science and Technology Co., Ltd.). Cq was set as
the cycle at which fluorescence was significantly increased
compared with background groups. GAPDH was used as a control for
normalization. The internal control was β-actin (18S RNA) and the
external control was the tumor sample. Thus, ΔCq was
Cq (sample) - Cq (external control) and
ΔΔCq was ΔCq (SPARC gene) - Cq
(18S). The relative quantification of SPARC gene was calculated as
2−ΔΔCq (17) and the
result was presented as the fold of tumor tissue over normal
tissue.
Immunofluorescence detection
Tissues were taken from −80°C environment and
reheated in a Leica CM1850 cryostat (Leica Microsystems GmbH,
Wetzlar, Germany) for 30 min. Tissues were embedded with tissue
freezing medium (Leica Microsystems GmbH) and placed in the
cryostat. The embedded tissues were cut into 6-µm thick slices and
fixed in pre-cooling polyformaldehyde for 1 min. They were then
immersed in PBS five times and incubated in 5% bovine serum albumin
(Beijing Solarbio Science and Technology Co., Ltd.) for 20 min at
37°C. The slices were incubated with anti-SPARC antibody (cat. no.
SC-25574, 1:50; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) at
4°C overnight. Absorbent paper was used to remove the primary
antibody, and the slices were then immersed in PBS several times.
Then, the slices were incubated with secondary antibody (dilution
1:100, cat. no. 111-035-003) tagged with fluorescein isothiocyanate
(1:100) (both from Jackson ImmunoResearch Laboratories, Inc., West
Grove, PA, USA) for 1 h at 37°C and immersed in PBS three times.
The nuclei were stained by DAPI (5 µg/ml) for 3 min and immersed in
PBS containing Tween-20 three times. The slides were mounted and
images were captured using a fluorescence microscope. The staining
intensity of SPARC protein was evaluated as following: No
fluorescence (−); fluorescence suspicious extremely weak (±);
fluorescence is weak but clearly visible (+); bright fluorescence
(++); extremely bright fluorescent (+++/++++). The distribution
area of SPARC was calculated as the area with intensity: ±, +, ++
and +++/++++ divided by the total area of view under the microscope
(green area/total area).
Statistical analysis
All statistical analyses were performed using SPSS
(version 17.0; SPSS, Inc., Chicago, IL, USA). SPARC expression
level in the tumor and normal control groups were compared with a
mean-value paired Student's t-test; correlation tests were
performed to analyze the correlation of SPARC and clinical factors.
The Pearson method was applied for parametric tests, and the
Spearman method was applied for non-parametric tests. P<0.05 was
considered to indicate a statistically significant difference.
Results
SPARC expression level
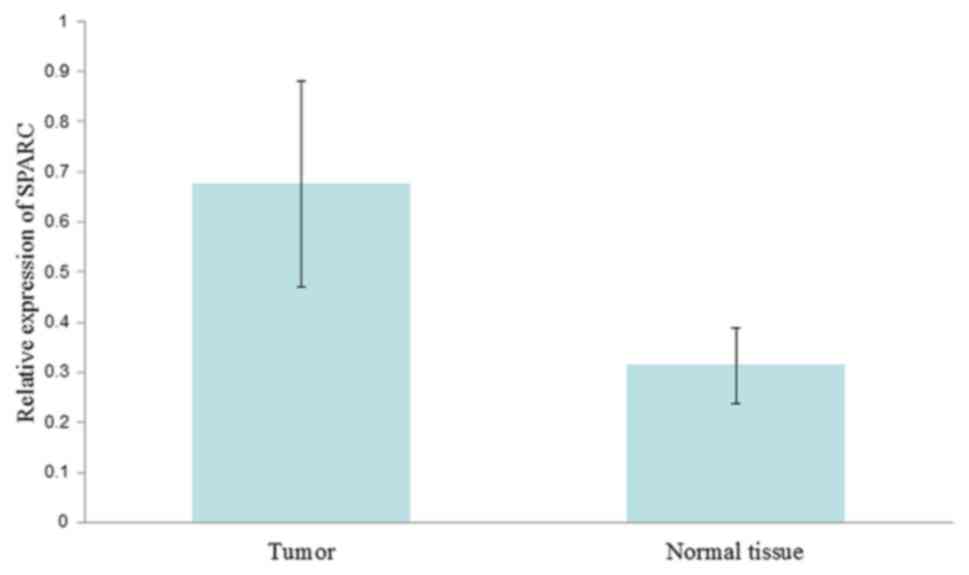
The SPARC gene level was also examined in 20 tumor
and normal samples. The gene amplification curve exhibited an
exponential growth phase following between 22 and 25 cycles (mean,
23.5 cycles). SPARC RNA expression in the tumor tissues was
increased 2.15-fold compared with that in normal tissues (0.676 and
0.314, respectively; P=0.002; Fig.
1).
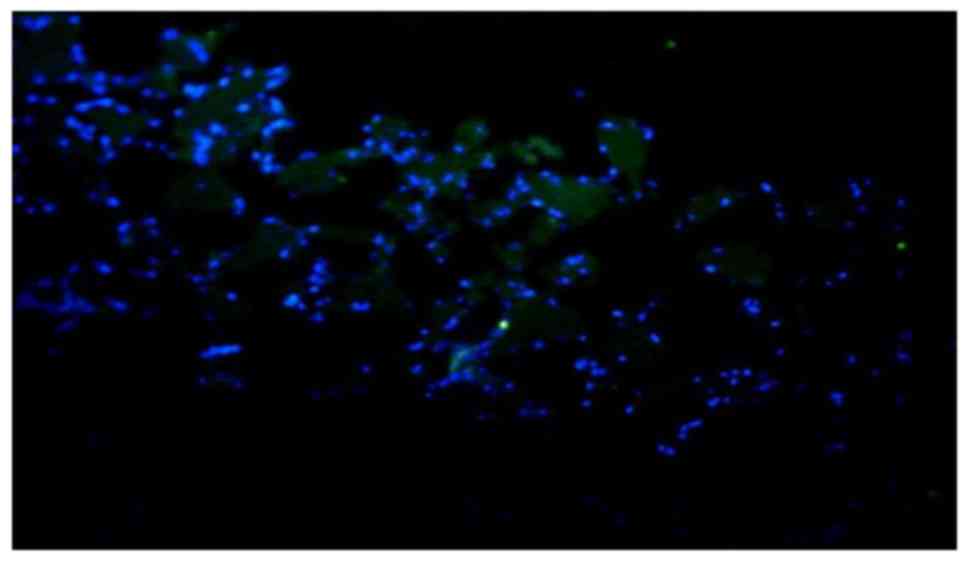
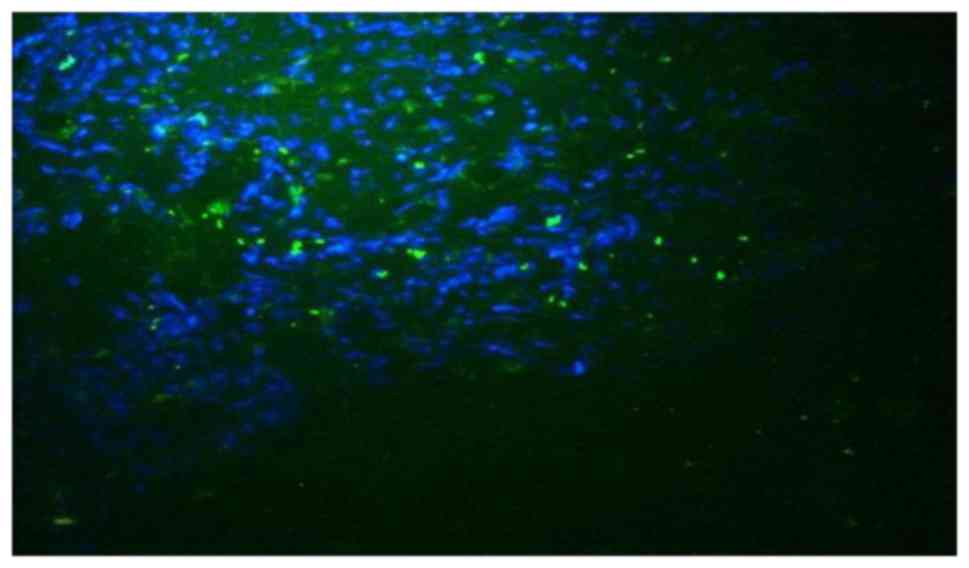
Immunofluorescence detection
The immunofluorescence detection results suggested
that the SPARC protein was widely distributed in tumor tissues.
Additionally, SPARC was observed in the tumor stroma, and not
confined to the tumor cell or nucleus. These results indicate the
distribution characteristics of the secreted protein. The staining
intensity of SPARC protein in tumor tissues was increased compared
with that in normal tissues. The staining intensity of SPARC
protein in tumor tissues was (++) or brighter and the staining
intensity of SPARC protein in normal tissues was (−) or (±). The
distribution area of SPARC protein in tumor tissues was also
increased compared with normal tissues (Figs. 2 and 3).
Correlation analysis
Clinical characteristics including tumor size, tumor
site, laboratory tests and metastasis were analyzed (Table II). The SPARC protein expression
level was positively associated with lung metastasis (P=0.016). The
SPARC protein level was negatively associated with the blood
neutrophil level (P=0.003). The Pearson test demonstrated a
marginal association between SPARC protein level and tumor site
(femur or humerus) (P=0.058). To address whether the tumor site was
associated with the SPARC protein level, mean values of SPARC level
in femur and humerus groups were compared. The SPARC level in the
femur group was significantly decreased compared with that in the
humerus group (P=0.005). The distinct tumor sizes in these two
groups were also measured: Mean tumor volumes were 1,859
cm3 in the femur group and 1,143 cm3 in the
humerus group.
 | Table II.Association between patient
characteristics and secreted protein, acidic and rich in cysteine
expression. |
Table II.
Association between patient
characteristics and secreted protein, acidic and rich in cysteine
expression.
| Characteristic | Value | Pearson
correlation | Spearman
correlation | P-value |
|---|
| Sex |
|
| −0.348 | 0.324 |
|
Male | 14 |
|
|
|
|
Female | 6 |
|
|
|
| Mean age, years
(range) | 20.5 (9–51) | 0.094 |
| 0.796 |
| Mean tumor volume,
cm3 (range) | 1,185.8
(140–4056) | −0.352 |
| 0.319 |
| Maximum tumor
diameter, cm (range) | 12.5 (7–24) | −0.326 |
| 0.357 |
| Tumor site |
| 0.866 |
| 0.058 |
|
Femur | 8 |
|
|
|
|
Humerus | 4 |
|
|
|
| Metastasis |
|
| 0.709 | 0.016 |
|
Yes | 8 |
|
|
|
| No | 12 |
|
|
|
| Mean alkaline
phosphatase level, U/l (range) | 202.1 (97–360) | −0.413 |
| 0.310 |
| Mean lactate
dehydrogenase level, U/l (range) | 410.4
(213–954) | −0.665 |
| 0.072 |
| Mean hemoglobin
level, g/l (range) | 124.1 (77–156) | 0.359 |
| 0.382 |
| Mean erythrocyte
number, ×1013 cells/l (range) | 4.1
(2.65–5.08) | 0.349 |
| 0.397 |
| Mean white blood
cell number, ×109 cells/l (range) | 7.1
(4.35–12.08) | −0.549 |
| 0.159 |
| Mean neutrophilic
granulocytes, % (range) | 67.7
(46.4–86.0) | −0.885 |
| 0.003 |
| Mean erythrocyte
sedimentation rate, mm/h (range) | 33.9 (8–120) | −0.082 |
| 0.862 |
| Mean C-reactive
protein level, mg/l (range) | 53.5
(2.2–302.0) | −0.434 |
| 0.331 |
| Mean D-dimer level,
mg/l (range) | 1.9
(0.29–4.92) | 0.041 |
| 0.923 |
Discussion
Since the 1970s, Jaffe and Cortes began to apply
methotrexate and doxorubicin to osteosarcoma chemotherapy (18,19). Rosen
first suggested neoadjuvant chemotherapy in the early 1980s
(20). Subsequently, the 5-year
survival rate of osteosarcoma patients increased to >60%
(21,22). At present, the widely used therapeutic
module is: Neoadjuvant chemotherapy + surgery + (adjuvant)
chemotherapy (18). However, in the
previous 20 years, despite concerted efforts, the survival rate of
osteosarcoma has not markedly improved (23–25). A
number of studies have indicated that the poor response to
first-line chemotherapy was not altered by longer durations or
higher doses of chemotherapy (26,27). A
lack of response to first-line chemotherapy and metastases are poor
prognostic factors affecting long-term survival. Although there are
a number of effective drugs for lung and breast cancer, and other
malignant tumors, the development of a novel drug for osteosarcoma
has proven difficult.
SPARC is a multifunctional glycoprotein. It was
identified to be highly expressed in a number of malignant tumors
including head and neck cancer, breast cancer, melanoma and colon
cancer (4–15). SPARC is associated with tumor
development, invasion, metastasis and prognosis (4–15). It is
an important protein in the regulation of tumor cell proliferation,
invasion and survival, and may interact with vascular endothelial
growth factor and basic fibroblast growth factor (10). Paclitaxel for injection
(albumin-bound; nab-paclitaxel, Abraxane®) is targeted
paclitaxel with the application of albumin nanoparticle technology.
Previously, a number of studies (28–30)
confirmed that its safety and efficacy are increased compared with
paclitaxel, which may enable it to become a novel option for the
treatment of osteosarcoma.
The high affinity of SPARC for albumin is a valuable
characteristic. Previous studies have indicated that the efficacy
of Abraxane® is associated with the expression level of
SPARC (31,32). Increased expression of SPARC in
metastatic nasopharyngeal carcinoma and breast cancer tissues may
improve the distribution concentration of nab-paclitaxel in tumor
tissues and the effects of the treatment (33,34), which
makes SPARC a potential novel antitumor target and predictive
marker. Thus, a series of experiments was performed in the present
study. Preclinical studies demonstrated that nab-paclitaxel exerted
a significant inhibitory effect on osteosarcoma in vitro and
in vivo (16,35). A tendency of increased expression
tendency of SPARC in osteosarcoma was also identified in mice
(35), which provided a theoretical
basis for the present study.
The SPARC expression level in human osteosarcoma and
the associated mechanism remains unclear. The immunohistochemical
study of SPARC expression in extraskeletal osteosarcoma by
Fanburg-Smith et al (36)
identified that the SPARC-positive rate in tumor cells was higher
compared with that in the tumor matrix. Dalla-Torre et al
(37) demonstrated a high expression
of the SPARC gene in osteosarcoma specimens, which is
consistent with the hypotheses and results of the present study.
However, that study (37) did not
detect the protein expression level and the distribution. Certain
studies also revealed SPARC expression in osteosarcoma; however,
these studies focused only on immunohistochemical tests, not SPARC
protein and gene expression, as in the present study (38,39).
In the present study, SPARC protein and gene
expression was examined concomitantly in human osteosarcoma tissues
and compared with adjacent normal tissues. The RT-qPCR analysis
demonstrated a significantly higher expression of SPARC protein in
human osteosarcoma samples compared with adjacent normal tissues.
To improve the scientific value and decrease the potential effect
of various chemotherapeutic drugs on the expression level of SPARC
protein, the present study only enrolled patients who did not
receive any chemotherapy prior to surgery. These results provide
the basis for additional studies.
To explore the value of SPARC in osteosarcoma,
potential factors including clinical features, tumor
characteristics and laboratory data were analyzed. The results
indicated that the expression level of SPARC was significantly
associated with lung metastasis, and these patients exhibited poor
prognosis. Therefore, SPARC may be a potential novel marker for the
prognosis of osteosarcoma. A number of molecular mechanisms may be
involved in tumor progression and metastasis. MicroRNAs exhibit
fundamental roles in the regulation of intracellular processes and
serve important roles in tumor invasion and metastasis. Epithelial
to mesenchymal transition (EMT) allows malignant epithelial cells
to become detached from each other and invade the surrounding
stroma (40). DNA methylation and
histone-tail methylation are also involved in tumor metastasis
(36). The potential reversibility of
these molecular makes them potential biomarkers and therapeutic
targets (41). The present study
identified that the expression level of SPARC was negatively
associated with the level of blood neutrophils, yet the reason
remains unclear. Neutrophil levels represent the degree of immune
system activation in the body. It is assumed that activation of the
immune system and the resultant number of neutrophils may inhibit
the secretion of SPARC in tumor tissues.
There were also differences of SPARC expression in
different skeletal sites. The reason that SPARC expression in the
humerus was significantly increased compared with in the femur may
be that a larger tumor exhibits more necrosis and edema components,
which means relatively less tumor cells and protein expression.
Therefore, it is hypothesized that the differential expression of
SPARC protein in osteosarcoma is associated with intra-tumor
heterogeneity, as solid neoplasms are superorganisms with complex
compartments and functions. Tumors are highly heterogeneous
populations derived from one common progenitor (42). Within a neoplasm, a mosaic of mutant
cells competes for space and resources, evades predation by the
immune system and may even cooperate to disperse and metastasize to
new organs (43). The evolution of
tumor cells in a solid tumor may be the most significant obstacle
to eliminating them. The understanding of the evolution of
neoplastic cells may assist in identifying novel therapeutic
targets of tumors. However, the statistical analysis of the present
study demonstrated no significant association between SPARC and
tumor size or maximum diameter. This is probably due to the small
sample size, which is a limitation of the present study. SPARC
expression in tumors may be enhanced by intra-tumoral hypoxia and
acidity, which indicates poor prognosis (6).
According to previous studies, the role and
mechanism of SPARC in the progression of tumors is complicated. It
has been suggested that SPARC may exhibit important effects in
angiogenesis that are necessary for tumor invasion and metastasis
(44,45). SPARC also facilitates tumor invasion
and metastasis through disrupting the adhesive interactions between
neoplastic cells and the extracellular matrix (5). SPARC may also reduce the adhesion of
tumor cells to the extracellular matrix through the degradation of
the extracellular matrix and cytoskeletal rearrangement, thereby
promoting tumor progression and metastasis (46,47). In
melanoma and breast cancer, the association of SPARC expression
with the expression, secretion and function of matrix
metalloproteinases indicated that SPARC may enhance the
invasiveness of tumor cells through the activation of
matrix-degrading enzymes (10,48,49).
Although the present study showed some positive and
meaningful results on the expression of SPARC in human
osteosarcoma, but there is still limitation such as the Western
blot test of SPARC expression was not analyzed.
In conclusion, the results of the present study
identified increased expression levels of SPARC in human
osteosarcoma, and the SPARC expression level was positively
associated with lung metastasis. Combined with studies
investigating other malignant tumors, SPARC may lead to tumor
progression and indicate a poor outcome. Conversely, although
patients with increased SPARC expression may be insensitive to
conventional therapy, they may be sensitive to
Abraxane®, which has not yet been applied to the
treatment of osteosarcoma. The value of SPARC in the prognosis and
prediction of the treatment outcomes of osteosarcoma by
nab-paxlitaxel remains to be evaluated in future studies.
Acknowledgements
This study was supported by Beijing Talents Fund
(grant no. 2015000021469G181).
References
|
1
|
Dahlin DC and Coventry MB: Osteogenic
Sarcoma. A study of 600 cases. J Bone Joint Surg. 49A:101–110.
1967. View Article : Google Scholar
|
|
2
|
Serra M, Reverter-Branchat G, Maurici D,
Benini S, Shen JN, Chano T, Hattinger CM, Manara MC, Pasello M,
Scotlandi K and Picci P: Analysis of dihydrofolate reductase and
reduced folate carrier gene status in relation to methotrexate
resistance in osteosarcoma cells. Ann Oncol. 15:151–160. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schwartz CL, Gorlick R, Teot L, Krailo M,
Chen Z, Goorin A, Grier HE, Bernstein ML and Meyers P: Children's
Oncology Group: Multiple drug resistance in osteogenic sarcoma:
INTO133 from the Children's Oncology Group. J Clin Oncol.
25:2057–2062. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Massi D, Franchi A, Borgognoni L, Reali UM
and Santucci M: Osteonectin expression correlates with clinical
outcome in thin cutaneous malignant melanomas. Hum Pathol.
30:339–344. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Porte H, Chastre E, Prevot S, Nordlinger
B, Empereur S, Basset P, Chambon P and Gespach C: Neoplastic
progression of human colorectal cancer is associated with
overexpression of the stromelysin-3 and BM-40⁄SPARC genes. Int J
Cancer. 64:70–75. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Koukourakis MI, Giatromanolaki A, Brekken
RA, Sivridis E, Gatter KC, Harris AL and Sage EH: Enhanced
expression of SPARC⁄osteonectin in the tumor-associated stroma of
non-small cell lung cancer is correlated with markers of
hypoxia⁄acidity and with poor prognosis of patients. Cancer Res.
63:5376–5380. 2003.PubMed/NCBI
|
|
7
|
Le Bail B, Faouzi S, Boussarie L,
Guirouilh J, Blanc JF, Carles J, Bioulac-Sage P, Balabaud C and
Rosenbaum J: Osteonectin⁄SPARC is overexpressed in human
hepatocellular carcinoma. J Pathol. 189:46–52. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ledda F, Bravo AI, Adris S, Bover L,
Mordoh J and Podhajcer OL: The expression of the secreted protein
acidic and rich in cysteine (SPARC) is associated with the
neoplastic progression of human melanoma. J Invest Dermatol.
108:210–214. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Paley PJ, Goff BA, Gown AM, Greer BE and
Sage EH: Alterations in SPARC and VEGF immunoreactivity in
epithelial ovarian cancer. Gynecol Oncol. 78:336–341. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Podhajcer OL, Benedetti LG, Girotti MR,
Prada F, Salvatierra E and Llera AS: The role of the matricellular
protein SPARC in the dynamic interaction between the tumor and the
host. Cancer Metastasis Rev. 27:691–705. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sakai N, Baba M, Nagasima Y, Kato Y, Hirai
K, Kondo K, Kobayashi K, Yoshida M, Kaneko S, Kishida T, et al:
SPARC expression in primary human renal cell carcinoma:
Upregulation of SPARC in sarcomatoid renal carcinoma. Hum Pathol.
32:1064–1070. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Thomas R, True LD, Bassuk JA, Lange PH and
Vessella RL: Differential expression of osteonectin⁄SPARC during
human prostate cancer progression. Clin Cancer Res. 6:1140–1149.
2000.PubMed/NCBI
|
|
13
|
Wang CS, Lin KH, Chen SL, Chan YF and
Hsueh S: Overexpression of SPARC gene in human gastric carcinoma
and its clinic-pathologic significance. Br J Cancer. 91:1924–1930.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yamanaka M, Kanda K, Li NC, Fukumori T,
Oka N, Kanayama HO and Kagawa S: Analysis of the gene expression of
SPARC and its prognostic value for bladder cancer. J Urol.
166:2495–2499. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yamashita K, Upadhay S, Mimori K, Inoue H
and Mori M: Clinical significance of secreted protein acidic and
rich in cystein in esophageal carcinoma and its relation to
carcinoma progression. Cancer. 97:2412–2419. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang YK, Niu XH, Zhang Q, Hao L, Ding Y
and Xu H: The Efficacy of abraxane on osteosarcoma xenografts in
nude mice and expression of secreted protein, acidic and rich in
cysteine. Am J Med Sci. 344:199–205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jaffe N: Recent advance in the
chemotherapy of metastatic osteogenic sarcoma. Cancer. 30:621–627.
1972. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cortes EP, Holland JF, Wang JJ and Sinks
LF: Doxorubicin in disseminated osteosarcoma. JAMA. 221:1132–1138.
1972. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rosen G, Suwansirikul S, Kwon C, Tan C, Wu
SJ, Beattie EJ Jr and Murphy ML: High-dose methotrexate with
citrovorum factor rescue and adriamycin in childhood osteogenic
sarcoma. Cancer. 33:1151–1163. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Meyers PA, Schwartz CL, Krailo MD, Healey
JH, Bernstein ML, Betcher D, Ferguson WS, Gebhardt MC, Goorin AM,
Harris M, et al: Osteosarcoma: The addition of muramyl tripeptide
to chemotherapy improves overall survival-a report from the
Children's Oncology Group. J Clin Oncol. 26:633–638. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mori K, Ando K and Heymann D: Liposomal
muramyl tripeptide phosphatidyl ethanolamine: A safe and effective
agent against osteosarcoma pulmonary metastases. Expert Rev
Anticancer Ther. 8:151–159. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
ESMO Guidelines Working Group and Saeter
G: Osteosarcoma: ESMO clinical recommendations for diagnosis,
treatment and follow-up. Ann Oncol. 18 (Suppl 2):ii77–ii78.
2007.PubMed/NCBI
|
|
24
|
Lewis IJ, Weeden S, Machin D, Stark D and
Craft AW: Received dose and dose-intensity of chemotherapy and
outcome in nonmetastatic extremity osteosarcoma. European
Osteosarcoma Intergroup. J Clin Oncol. 15:4028–4037. 2000.
View Article : Google Scholar
|
|
25
|
Lewis IJ, Nooij MA, Whelan J, Sydes MR,
Grimer R, Hogendoorn PC, Memon MA, Weeden S, Uscinska BM, van
Glabbeke M, et al: Improvement in histologic response but not
survival in osteosarcoma patients treated with intensified
chemotherapy: A randomized phase III trial of the European
Osteosarcoma Intergroup. J Natl Cancer Inst. 99:112–128. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bielack S, Kempf-Bielack B and Winkler K:
Osteosarcoma: Relationship of response to preoperative chemotherapy
and type of surgery to local recurrence. J Clin Oncol. 14:683–684.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bielack S, Kempf-Bielack B, Heise U,
Schwenzer D and Winkler K: Combined modality treatment for
osteosarcoma occurring as a second malignant disease. Cooperative
German-Austrian-Swiss Osteosarcoma Study Group. J Clin Oncol.
17:11641999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lourda M, Trougakos IP and Gonos ES:
Development of resistance to chemotherapeutic drugs in human
osteosarcoma cell lines largely depends on up-regulation of
Clusterin/Apolipoprotein J. Int J Cancer. 120:611–622. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
LaRocque J, Bharali DJ and Mousa SA:
Cancer detection and treatment: The role of nanomedicines. Mol
Biotechnol. 42:358–366. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Huang G, Mills L and Worth LL: Expression
of human glutathione S-transferase P1 mediates the chemosensitivity
of osteosarcoma cells. Mol Cancer Ther. 6:1610–1619. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Desai N, Trieu V, Damascelli B and
Soon-Shiong P: SPARC expression correlates with tumor response to
albumin-bound paclitaxel in head and neck cancer patients. Transl
Oncol. 2:59–64. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Trieu V, Damascelli B, Soon-Shiong P and
Desai N: SPARC expression in head and neck cancer correlates with
tumor response to nanoparticle albumin-bound paclitaxel
(nab-paclitaxel, ABI-007, Abraxane). Proc Amer Assoc Cancer Res.
47:1050–1051. 2006.
|
|
33
|
Desai NP, Trieu V, Hwang LY, Wu R,
Soon-Shiong P and Gradishar WJ: Improved effectiveness of
nanoparticle albumin-bound (nab) paclitaxel versus
polysorbate-based docetaxel in multiple xenografts as a function of
HER2 and SPARC status. Anticancer Drugs. 19:899–909. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Huang Y, Liang W, Yang Y, Zhao L, Zhao H,
Wu X, Zhao Y, Zhang Y and Zhang L: Phase I/II dose-finding study of
nanoparticle albumin-bound paclitaxel (nab®-Paclitaxel)
plus Cisplatin as treatment for metastatic nasopharyngeal
carcinoma. BMC Cancer. 16:4642016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yang YK, Niu XH, Zhang Q, et al: In vitro
inhibiting effect of albumin-bound paclitaxel on human osteosarcoma
cell OS-732. Shandong Med J. 50:24–26. 2010.(In Chinese).
|
|
36
|
Fanburg-Smith JC, Bratthauer GL and
Miettinen M: Osteocalcin and osteonectin immunoreactivity in
extraskeletal osteosarcoma: A study of 28 cases. Hum Pathol.
30:32–38. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Dalla-Torre C, Yoshimoto M, Lee C, Joshua
AM, de Toledo SR, Petrilli AS, Andrade JA, Chilton-MacNeill S,
Zielenska M and Squire JA: Effects of THBS3, SPARC and SPP1
expression on biological behavior and survival in patients with
osteosarcoma. BMC Cance. 6:2372006. View Article : Google Scholar
|
|
38
|
Fanburg JC, Rosenberg AE, Weaver DL,
Leslie KO, Mann KG, Taatjes DJ and Tracy RP: Osteocalcin and
osteonectin immunoreactivity in the diagnosis of osteosarcoma. Am J
Clin Pathol. 108:464–473. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wuisman P, Roessner A, Bosse A, Ueda Y,
Winkelmann W and Enneking WF: Osteonectin in osteosarcomas: A
marker for differential diagnosis and/or prognosis? Ann Oncol. 3
(Suppl 2):S33–S35. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bullock MD, Sayan AE, Packham GK and
Mirnezami AH: MicroRNAs: Critical regulators of epithelial to
mesenchymal (EMT) and mesenchymal to epithelial transition (MET) in
cancer progression. Biol Cell. 104:3–12. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Cock-Rada A and Weitzman JB: The
methylation landscape of tumour metastasis. Biol Cell. 105:73–90.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Grunewald TG, Herbst SM, Heinze J and
Burdach S: Understanding tumor heterogeneity as functional
compartments-superorganisms revisited. J Transl Med. 9:792011.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Merlo LM, Pepper JW, Reid BJ and Maley CC:
Cancer as an evolutionary and ecological process. Nat Rev Cancer.
6:924–935. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lane TF, Iruela-Arispe ML and Sage EH:
Regulation of gene expression by SPARC during angiogenesis in
vitro. Changes in fibronectin, thrombospondin-1, and plasminogen
activator inhibitor-1. J Biol Chem. 267:16736–16745.
1992.PubMed/NCBI
|
|
45
|
Jendraschak E and Sage EH: Regulation of
angiogenesis by SPARC and angiostatin: Implications for tumor cell
biology. Semin Cancer Biol. 7:139–146. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Tai IT and Tang MJ: SPARC in cancer
biology: Its role in cancer progression and potential for therapy.
Drug Resist Updat. 11:231–246. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Tremble PM, Lane TF, Sage EH and Werb Z:
SPARC, a secreted protein associated with morphogenesis and tissue
remodeling, induces expression of metalloproteinases in fibroblasts
through a novel extracellular matrix-dependent pathway. J Cell
Biol. 121:1433–1444. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ledda MF, Adris S, Bravo AI, Kairiyama C,
Bover L, Chernajovsky Y, Mordoh J and Podhajcer OL: Suppression of
SPARC expression by antisense RNA abrogates the tumorigenicity of
human melanoma cells. Nat Med. 3:171–176. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Gilles C, Bassuk JA, Pulyaeva H, Sage EH,
Foidart JM and Thompson EW: SPARC/osteonectin induces matrix
metalloproteinase 2 activation in human breast cancer cell lines.
Cancer Res. 58:5529–5536. 1998.PubMed/NCBI
|