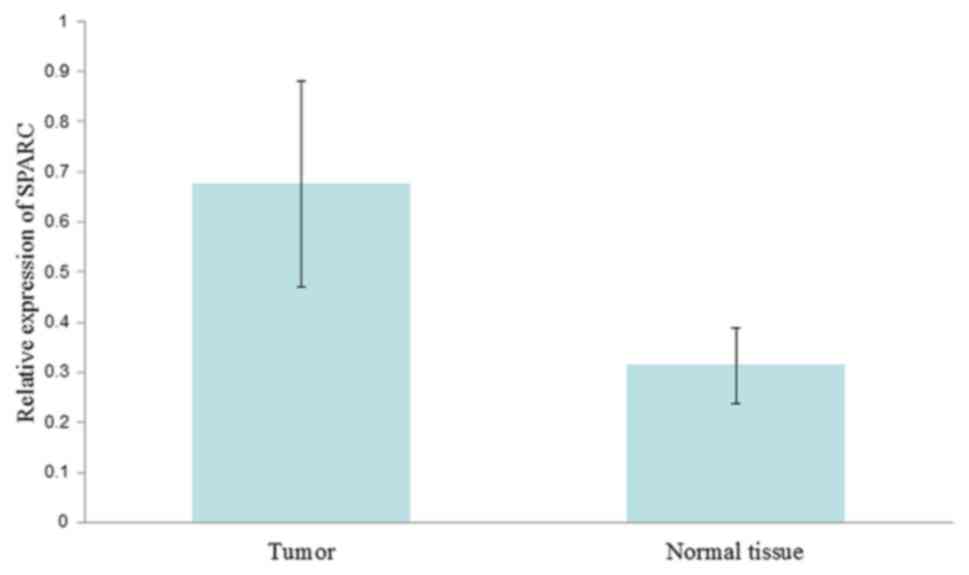
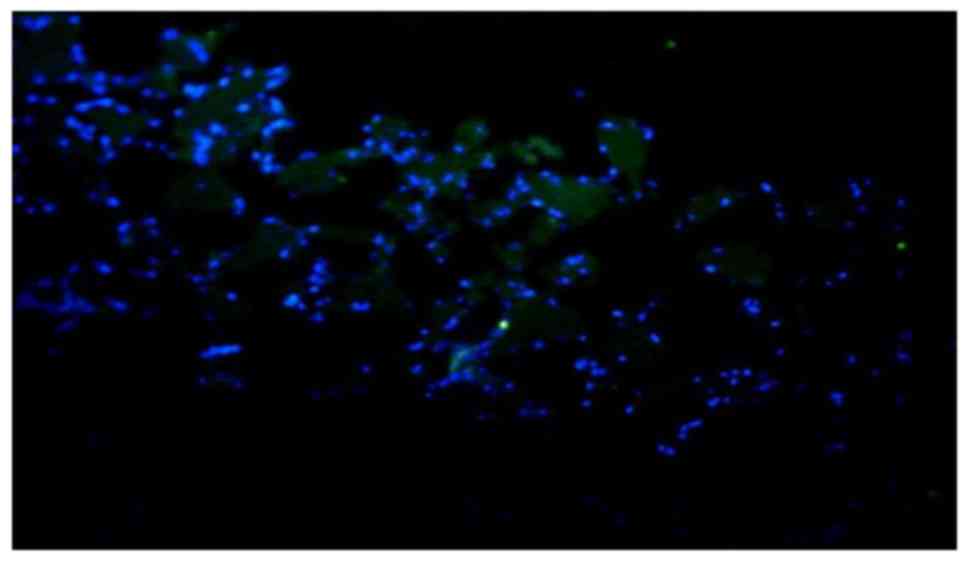
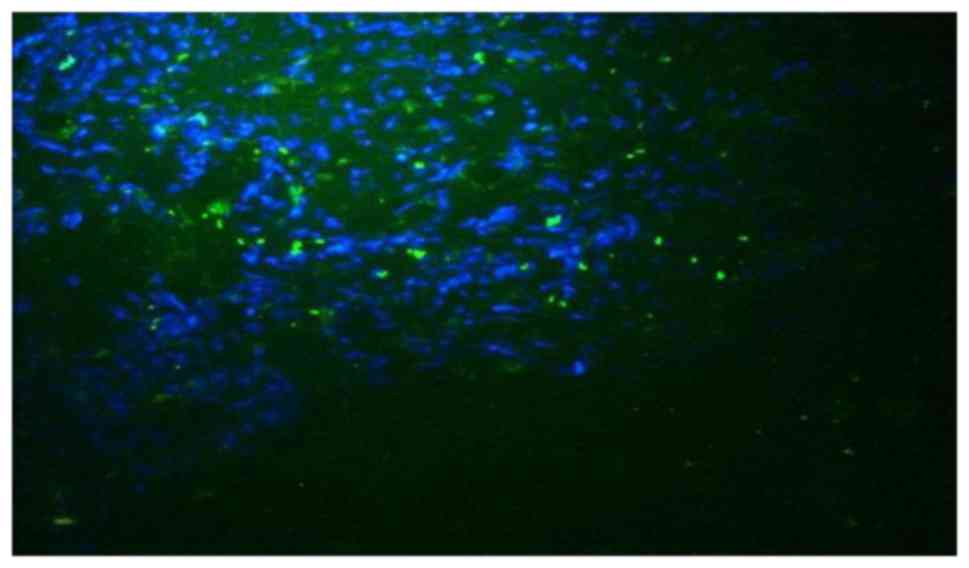
|
1
|
Dahlin DC and Coventry MB: Osteogenic
Sarcoma. A study of 600 cases. J Bone Joint Surg. 49A:101–110.
1967. View Article : Google Scholar
|
|
2
|
Serra M, Reverter-Branchat G, Maurici D,
Benini S, Shen JN, Chano T, Hattinger CM, Manara MC, Pasello M,
Scotlandi K and Picci P: Analysis of dihydrofolate reductase and
reduced folate carrier gene status in relation to methotrexate
resistance in osteosarcoma cells. Ann Oncol. 15:151–160. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schwartz CL, Gorlick R, Teot L, Krailo M,
Chen Z, Goorin A, Grier HE, Bernstein ML and Meyers P: Children's
Oncology Group: Multiple drug resistance in osteogenic sarcoma:
INTO133 from the Children's Oncology Group. J Clin Oncol.
25:2057–2062. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Massi D, Franchi A, Borgognoni L, Reali UM
and Santucci M: Osteonectin expression correlates with clinical
outcome in thin cutaneous malignant melanomas. Hum Pathol.
30:339–344. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Porte H, Chastre E, Prevot S, Nordlinger
B, Empereur S, Basset P, Chambon P and Gespach C: Neoplastic
progression of human colorectal cancer is associated with
overexpression of the stromelysin-3 and BM-40⁄SPARC genes. Int J
Cancer. 64:70–75. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Koukourakis MI, Giatromanolaki A, Brekken
RA, Sivridis E, Gatter KC, Harris AL and Sage EH: Enhanced
expression of SPARC⁄osteonectin in the tumor-associated stroma of
non-small cell lung cancer is correlated with markers of
hypoxia⁄acidity and with poor prognosis of patients. Cancer Res.
63:5376–5380. 2003.PubMed/NCBI
|
|
7
|
Le Bail B, Faouzi S, Boussarie L,
Guirouilh J, Blanc JF, Carles J, Bioulac-Sage P, Balabaud C and
Rosenbaum J: Osteonectin⁄SPARC is overexpressed in human
hepatocellular carcinoma. J Pathol. 189:46–52. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ledda F, Bravo AI, Adris S, Bover L,
Mordoh J and Podhajcer OL: The expression of the secreted protein
acidic and rich in cysteine (SPARC) is associated with the
neoplastic progression of human melanoma. J Invest Dermatol.
108:210–214. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Paley PJ, Goff BA, Gown AM, Greer BE and
Sage EH: Alterations in SPARC and VEGF immunoreactivity in
epithelial ovarian cancer. Gynecol Oncol. 78:336–341. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Podhajcer OL, Benedetti LG, Girotti MR,
Prada F, Salvatierra E and Llera AS: The role of the matricellular
protein SPARC in the dynamic interaction between the tumor and the
host. Cancer Metastasis Rev. 27:691–705. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sakai N, Baba M, Nagasima Y, Kato Y, Hirai
K, Kondo K, Kobayashi K, Yoshida M, Kaneko S, Kishida T, et al:
SPARC expression in primary human renal cell carcinoma:
Upregulation of SPARC in sarcomatoid renal carcinoma. Hum Pathol.
32:1064–1070. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Thomas R, True LD, Bassuk JA, Lange PH and
Vessella RL: Differential expression of osteonectin⁄SPARC during
human prostate cancer progression. Clin Cancer Res. 6:1140–1149.
2000.PubMed/NCBI
|
|
13
|
Wang CS, Lin KH, Chen SL, Chan YF and
Hsueh S: Overexpression of SPARC gene in human gastric carcinoma
and its clinic-pathologic significance. Br J Cancer. 91:1924–1930.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yamanaka M, Kanda K, Li NC, Fukumori T,
Oka N, Kanayama HO and Kagawa S: Analysis of the gene expression of
SPARC and its prognostic value for bladder cancer. J Urol.
166:2495–2499. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yamashita K, Upadhay S, Mimori K, Inoue H
and Mori M: Clinical significance of secreted protein acidic and
rich in cystein in esophageal carcinoma and its relation to
carcinoma progression. Cancer. 97:2412–2419. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang YK, Niu XH, Zhang Q, Hao L, Ding Y
and Xu H: The Efficacy of abraxane on osteosarcoma xenografts in
nude mice and expression of secreted protein, acidic and rich in
cysteine. Am J Med Sci. 344:199–205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jaffe N: Recent advance in the
chemotherapy of metastatic osteogenic sarcoma. Cancer. 30:621–627.
1972. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cortes EP, Holland JF, Wang JJ and Sinks
LF: Doxorubicin in disseminated osteosarcoma. JAMA. 221:1132–1138.
1972. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rosen G, Suwansirikul S, Kwon C, Tan C, Wu
SJ, Beattie EJ Jr and Murphy ML: High-dose methotrexate with
citrovorum factor rescue and adriamycin in childhood osteogenic
sarcoma. Cancer. 33:1151–1163. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Meyers PA, Schwartz CL, Krailo MD, Healey
JH, Bernstein ML, Betcher D, Ferguson WS, Gebhardt MC, Goorin AM,
Harris M, et al: Osteosarcoma: The addition of muramyl tripeptide
to chemotherapy improves overall survival-a report from the
Children's Oncology Group. J Clin Oncol. 26:633–638. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mori K, Ando K and Heymann D: Liposomal
muramyl tripeptide phosphatidyl ethanolamine: A safe and effective
agent against osteosarcoma pulmonary metastases. Expert Rev
Anticancer Ther. 8:151–159. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
ESMO Guidelines Working Group and Saeter
G: Osteosarcoma: ESMO clinical recommendations for diagnosis,
treatment and follow-up. Ann Oncol. 18 (Suppl 2):ii77–ii78.
2007.PubMed/NCBI
|
|
24
|
Lewis IJ, Weeden S, Machin D, Stark D and
Craft AW: Received dose and dose-intensity of chemotherapy and
outcome in nonmetastatic extremity osteosarcoma. European
Osteosarcoma Intergroup. J Clin Oncol. 15:4028–4037. 2000.
View Article : Google Scholar
|
|
25
|
Lewis IJ, Nooij MA, Whelan J, Sydes MR,
Grimer R, Hogendoorn PC, Memon MA, Weeden S, Uscinska BM, van
Glabbeke M, et al: Improvement in histologic response but not
survival in osteosarcoma patients treated with intensified
chemotherapy: A randomized phase III trial of the European
Osteosarcoma Intergroup. J Natl Cancer Inst. 99:112–128. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bielack S, Kempf-Bielack B and Winkler K:
Osteosarcoma: Relationship of response to preoperative chemotherapy
and type of surgery to local recurrence. J Clin Oncol. 14:683–684.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bielack S, Kempf-Bielack B, Heise U,
Schwenzer D and Winkler K: Combined modality treatment for
osteosarcoma occurring as a second malignant disease. Cooperative
German-Austrian-Swiss Osteosarcoma Study Group. J Clin Oncol.
17:11641999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lourda M, Trougakos IP and Gonos ES:
Development of resistance to chemotherapeutic drugs in human
osteosarcoma cell lines largely depends on up-regulation of
Clusterin/Apolipoprotein J. Int J Cancer. 120:611–622. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
LaRocque J, Bharali DJ and Mousa SA:
Cancer detection and treatment: The role of nanomedicines. Mol
Biotechnol. 42:358–366. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Huang G, Mills L and Worth LL: Expression
of human glutathione S-transferase P1 mediates the chemosensitivity
of osteosarcoma cells. Mol Cancer Ther. 6:1610–1619. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Desai N, Trieu V, Damascelli B and
Soon-Shiong P: SPARC expression correlates with tumor response to
albumin-bound paclitaxel in head and neck cancer patients. Transl
Oncol. 2:59–64. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Trieu V, Damascelli B, Soon-Shiong P and
Desai N: SPARC expression in head and neck cancer correlates with
tumor response to nanoparticle albumin-bound paclitaxel
(nab-paclitaxel, ABI-007, Abraxane). Proc Amer Assoc Cancer Res.
47:1050–1051. 2006.
|
|
33
|
Desai NP, Trieu V, Hwang LY, Wu R,
Soon-Shiong P and Gradishar WJ: Improved effectiveness of
nanoparticle albumin-bound (nab) paclitaxel versus
polysorbate-based docetaxel in multiple xenografts as a function of
HER2 and SPARC status. Anticancer Drugs. 19:899–909. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Huang Y, Liang W, Yang Y, Zhao L, Zhao H,
Wu X, Zhao Y, Zhang Y and Zhang L: Phase I/II dose-finding study of
nanoparticle albumin-bound paclitaxel (nab®-Paclitaxel)
plus Cisplatin as treatment for metastatic nasopharyngeal
carcinoma. BMC Cancer. 16:4642016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yang YK, Niu XH, Zhang Q, et al: In vitro
inhibiting effect of albumin-bound paclitaxel on human osteosarcoma
cell OS-732. Shandong Med J. 50:24–26. 2010.(In Chinese).
|
|
36
|
Fanburg-Smith JC, Bratthauer GL and
Miettinen M: Osteocalcin and osteonectin immunoreactivity in
extraskeletal osteosarcoma: A study of 28 cases. Hum Pathol.
30:32–38. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Dalla-Torre C, Yoshimoto M, Lee C, Joshua
AM, de Toledo SR, Petrilli AS, Andrade JA, Chilton-MacNeill S,
Zielenska M and Squire JA: Effects of THBS3, SPARC and SPP1
expression on biological behavior and survival in patients with
osteosarcoma. BMC Cance. 6:2372006. View Article : Google Scholar
|
|
38
|
Fanburg JC, Rosenberg AE, Weaver DL,
Leslie KO, Mann KG, Taatjes DJ and Tracy RP: Osteocalcin and
osteonectin immunoreactivity in the diagnosis of osteosarcoma. Am J
Clin Pathol. 108:464–473. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wuisman P, Roessner A, Bosse A, Ueda Y,
Winkelmann W and Enneking WF: Osteonectin in osteosarcomas: A
marker for differential diagnosis and/or prognosis? Ann Oncol. 3
(Suppl 2):S33–S35. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bullock MD, Sayan AE, Packham GK and
Mirnezami AH: MicroRNAs: Critical regulators of epithelial to
mesenchymal (EMT) and mesenchymal to epithelial transition (MET) in
cancer progression. Biol Cell. 104:3–12. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Cock-Rada A and Weitzman JB: The
methylation landscape of tumour metastasis. Biol Cell. 105:73–90.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Grunewald TG, Herbst SM, Heinze J and
Burdach S: Understanding tumor heterogeneity as functional
compartments-superorganisms revisited. J Transl Med. 9:792011.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Merlo LM, Pepper JW, Reid BJ and Maley CC:
Cancer as an evolutionary and ecological process. Nat Rev Cancer.
6:924–935. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lane TF, Iruela-Arispe ML and Sage EH:
Regulation of gene expression by SPARC during angiogenesis in
vitro. Changes in fibronectin, thrombospondin-1, and plasminogen
activator inhibitor-1. J Biol Chem. 267:16736–16745.
1992.PubMed/NCBI
|
|
45
|
Jendraschak E and Sage EH: Regulation of
angiogenesis by SPARC and angiostatin: Implications for tumor cell
biology. Semin Cancer Biol. 7:139–146. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Tai IT and Tang MJ: SPARC in cancer
biology: Its role in cancer progression and potential for therapy.
Drug Resist Updat. 11:231–246. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Tremble PM, Lane TF, Sage EH and Werb Z:
SPARC, a secreted protein associated with morphogenesis and tissue
remodeling, induces expression of metalloproteinases in fibroblasts
through a novel extracellular matrix-dependent pathway. J Cell
Biol. 121:1433–1444. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ledda MF, Adris S, Bravo AI, Kairiyama C,
Bover L, Chernajovsky Y, Mordoh J and Podhajcer OL: Suppression of
SPARC expression by antisense RNA abrogates the tumorigenicity of
human melanoma cells. Nat Med. 3:171–176. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Gilles C, Bassuk JA, Pulyaeva H, Sage EH,
Foidart JM and Thompson EW: SPARC/osteonectin induces matrix
metalloproteinase 2 activation in human breast cancer cell lines.
Cancer Res. 58:5529–5536. 1998.PubMed/NCBI
|