Introduction
Ovarian carcinoma is one of the most common types of
malignant tumors in gynecology: Annually, there are ~204,449 novel
cases of ovarian carcinoma worldwide and ~124,860 cases of patient
mortality due to associated diseases (1). The early lesion may not be identified
easily since the ovaries are located deep in the pelvic cavity. The
onset of symptoms is delayed which means that when a patient
presents with symptoms, the patient is typically already in
end-stage disease, particularly for patients with epithelial
ovarian carcinoma (2,3). Ovarian carcinoma exhibits multi-drug
resistance, recurrence and metastasis which causes it to be the
most fatal type of gynecological malignant tumor (2).
The endoplasmic reticulum (ER) is an important
organelle which has an important role in protein conformation and
post-translational modification, enfoldment and oligomerization
(4,5).
In addition, ER serves a role in lipid metabolism, steroid hormone
synthesis and storage. Under a variety of conditions (including
anoxia, alimentary deficiency, glycosylation, oxidative stress,
metabolic disorders and mutant protein expression) cells cause
non-folding or misfolding protein aggregation in the ER and result
in the expression of the ER stress protein glucose-regulated
protein 78 (GRP78). GRP78 is a primary molecular chaperone in the
ER and is a member of the heat-shock protein-70 family (5). Multiple stimuli may disturb ER functions
and induce GRP78 expression (6),
which serves a protective role and enables the survival of tumor
cells (7). A previous study
demonstrated that GPR78 is expressed in a number of types of tumor
including breast, liver, lung, gastric, esophageal and skin cancer.
Furthermore, by conducting studies involving overexpression and
short interfering RNA knockouts, the tolerance, invasion and
irradiation of cancer cells may be determined (8). GRP78 serves an important role in tumor
survival which indicates a novel target for antineoplastic drugs
(9).
The observed failure of chemotherapy in patients
with ovarian carcinoma is attributed to the ability of ovarian
carcinoma cells to exhibit drug resistance (10) by mechanisms including unregulated
apoptosis (11). A previous study
indicated that administration of anticancer drugs, including
cisplatin, results in tumor cells releasing cytochrome c,
activating caspase-3 and undergoing apoptosis (12). Abnormal expression of genes which
regulate apoptosis cause caspase-3 to become dependent on abnormal
apoptotic conduction and therefore alter the sensitivity of
chemotherapeutics (13).
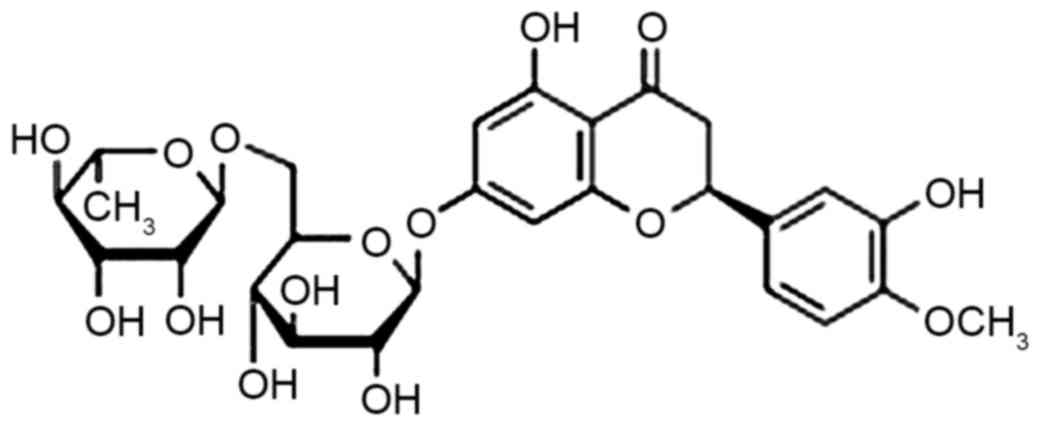
Sources of citrus, a generic term for the retaceous
plant citrus, are abundant in China and hesperidin (chemical
structure presented in Fig. 1) is an
important factor in processing the by-product pomace (14). Hesperidin functions in cancer
prevention, decreasing cholesterol, and serves a role in
anti-anaphylaxis, antihypertension and as an antioxidant (15,16). A
previous study has demonstrated that hesperidin has broad-spectrum
bacteriostatic actions on Bacillus subtilis,
Salmonella, Shigella and Streptococcus
haemolyticus (17). Hesperidin is
therefore widely applied as a food additive and in food processing.
The aim of the present study was to investigate whether hesperidin
exhibited an effect on ovarian cancer cell viability through
endoplasmic reticulum stress signaling pathways.
Materials and methods
Cell culture and treatment
Human ovarian cancer cell line A2780 was obtained
from the Shanghai Cell Bank of Chinese Academy of Sciences
(Shanghai, China). A2780 cells were cultured in Dulbecco's modified
Eagle's medium (Invitrogen; Thermo Fisher Scientific Inc., Waltham,
MA, USA), 100 µg/ml streptomycin and 100 U/ml penicillin at 37°C in
a humidified incubator containing 5% CO2.
Cell viability and cytotoxicity
assay
A2780 cells were seeded in 96-well plates at
1×104 cells'well for 6 h and subsequently treated with
various concentrations of hesperidin (0, 0.1, 1 and 10 µM for 6, 12
and 24 h). Subsequently, 5 mg/ml MTT (Sigma Aldrich; Merck KGaA,
Darmstadt, Germany) was added to the cells prior to incubation at
37°C for 4 h. MTT solution was removed and 150 µl dimethyl
sulfoxide was added (Sigma Aldrich; Merck KGaA). Cell viability was
measured at 490 nm using a microplate reader (Tecan Group Ltd.,
Zurich, Switzerland) and cells treated with 0 µM were used as a
control for comparison. Next, cytotoxicity was evaluated by the
lactate dehydrogenase (LDH) assay according to the manufacturer's
protocol (Beyotime Institute of Biotechnology, Jiangsu, China).
Cytotoxicity was measured at 490 nm using the aforementioned
microplate reader.
Apoptosis analysis
A2780 cells in 6-well plates were treated with
various concentrations of hesperidin (0, 0.1, 1 and 10 µM) for 48 h
following seeding at 1×106 cells/well for 12 h.
Following treatment with hesperidin, A2780 cells were suspended
with 100 µl binding buffer (IMGENEX; Novus Biologicals, LLC,
Littleton, CO, USA). A total of 10 µl Annexin V-fluorescein
isothiocyanate (BD Biosciences, Franklin Lakes, NJ, USA) was added
to the cells prior to incubation for 30 min in the dark at room
temperature. Subsequently, 5 µl propidium iodide was added to the
cells prior to incubation for 5 min in the dark. Apoptosis was
analyzed using a flow cytometer (C6; BD Biosciences).
Western blot analysis
A2780 cells in 6-well plates were treated with
various concentrations of hesperidin (0, 0.1, 1 and 10 µM) for 48 h
following seeding at 1×106 cells'well for 12 h.
Subsequently, A2780 cells were lysed with lysis buffer
(radioimmunoprecipitation assay buffer) containing protease
inhibitor cocktail (phenylmethanesulfonyl fluoride) and EDTA, at
4°C for 30 min. Cell lysates were centrifuged at 12,000 × g for 30
min at 4°C. Total protein was extracted and protein concentration
was determined using the Bradford assay. Extracted proteins (40
µg'lane) were subjected to SDS-PAGE (10–12% gel) and subsequently
transferred onto a polyvinylidene difluoride membrane (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). The membrane was blocked
with 5% non-fat milk, in Tris-buffered saline containing Tween-20
for 1 h at 37°C and subsequently incubated with the following
primary antibodies: Anti-cleaved caspase-3 (dilution, 1:500; cat.
no. sc-98785), anti-growth arrest- and DNA damage-inducible gene
(GADD) 153 (dilution, 1:500; cat. no. sc-575), anti-78 kDa
glucose-regulated protein (GRP-78; dilution, 1:500; cat. no.
sc-13968), anti-cytochrome c (dilution, 1:500; cat. no.
sc-7159) and anti-β-actin (dilution, 1:2,000; cat. no. A2780; all
from Santa Cruz Biotechnology, Inc., Dallas, TX, USA), and
anti-CCAAT'enhancer-binding protein homologous protein (CHOP;
dilution, 1:2,000; cat. no. 2895; Cell Signaling Technology, Inc.)
overnight at 4°C. Following this, membranes were incubated with the
anti-mouse or anti-rabbit immunoglobulin G secondary antibody
(dilution, 1:5,000; catalog no. 14709 and 14708, respectively; Cell
Signaling Technology, Inc.) at 37°C for 2 h. Western blots were
developed using an enhanced chemiluminescence kit (GE Healthcare
Bio-Sciences, Pittsburgh, PA, USA). Western blots were quantified
using the Odyssey infrared imaging system (LI-COR Biosciences,
Lincoln, NE, USA) and Bio-Rad Laboratories Quantity One software
(version 3.0; Bio-Rad Laboratories, Inc.).
Statistical analysis
Results are presented as the mean ± standard error
of the mean of at least three independent experiments. Statistical
analyses were carried out using Student's t-test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Hesperidin inhibits ovarian cancer
cell proliferation
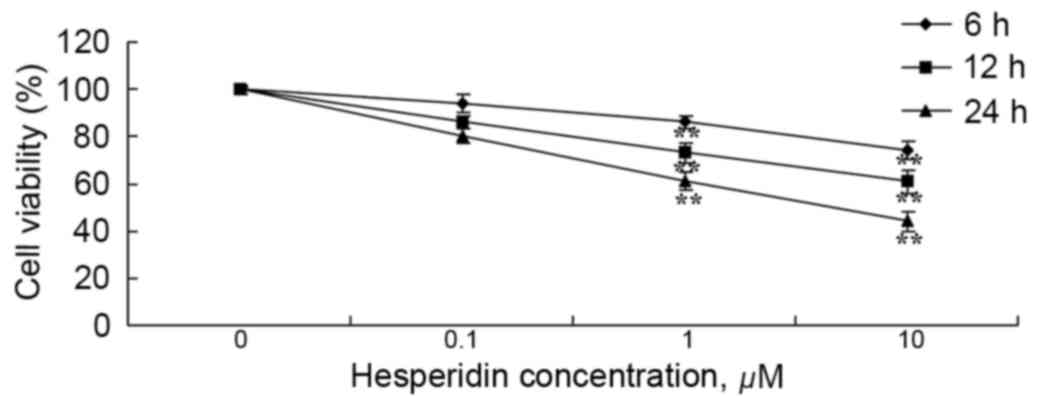
Human ovarian cancer A2780 cells were incubated with
various concentrations of hesperidin (0, 0.1, 1 and 10 µM) for 6,
12 and 24 h. As presented in Fig. 2,
treatment with hesperidin decreased the viability of A2780 cells in
a time- and dose-dependent manner. At hesperidin concentrations of
1 and 10 µM, A2780 cell viability was significantly decreased
(Fig. 2).
Hesperidin induces ovarian cancer
cytotoxicity
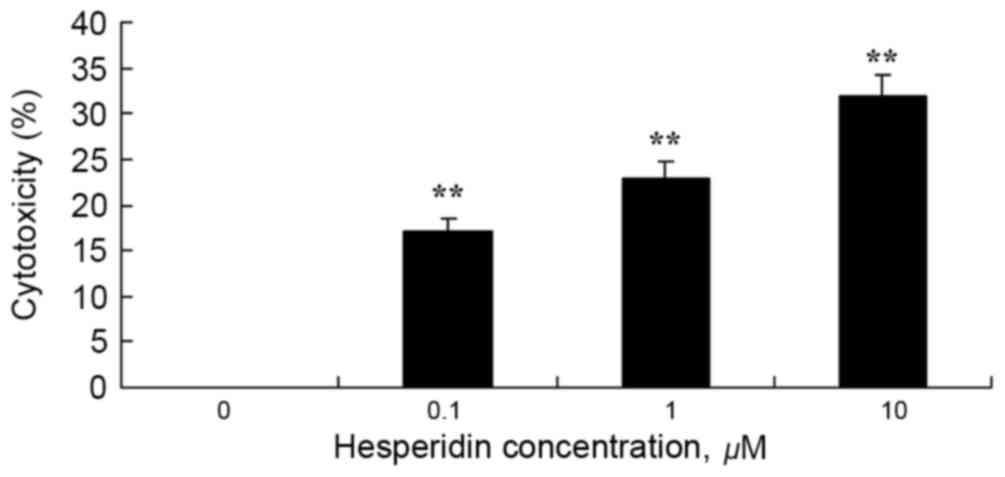
To investigate whether hesperidin exhibits cytotoxic
effects on ovarian cancer A2780 cells using an LDH assay. As
presented in Fig. 3, 1 and 10 µM
hesperidin exhibited significantly increased cytotoxicity in A2780
cells.
Hesperidin induces ovarian cancer cell
apoptosis
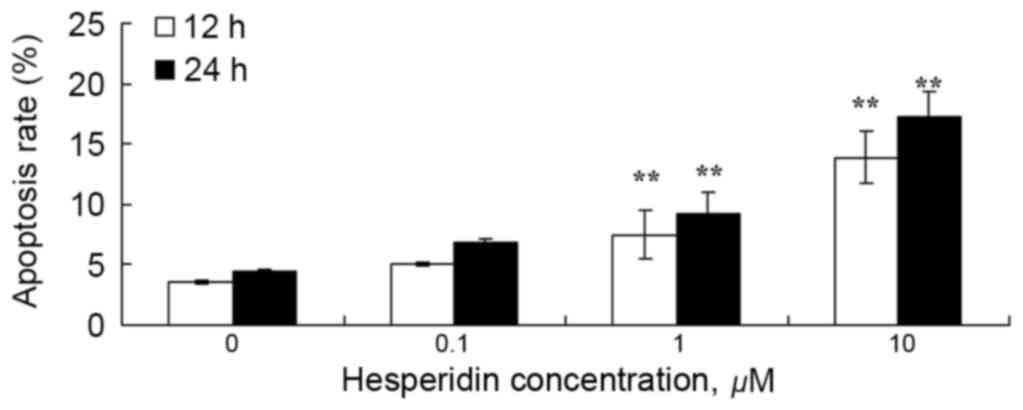
To examine the effect of hesperidin on ovarian
cancer cell apoptosis, the apoptotic rate was analyzed using a flow
cytometer. As presented in Fig. 4, 1
and 10 µM hesperidin significantly induced apoptosis in A2780
cells.
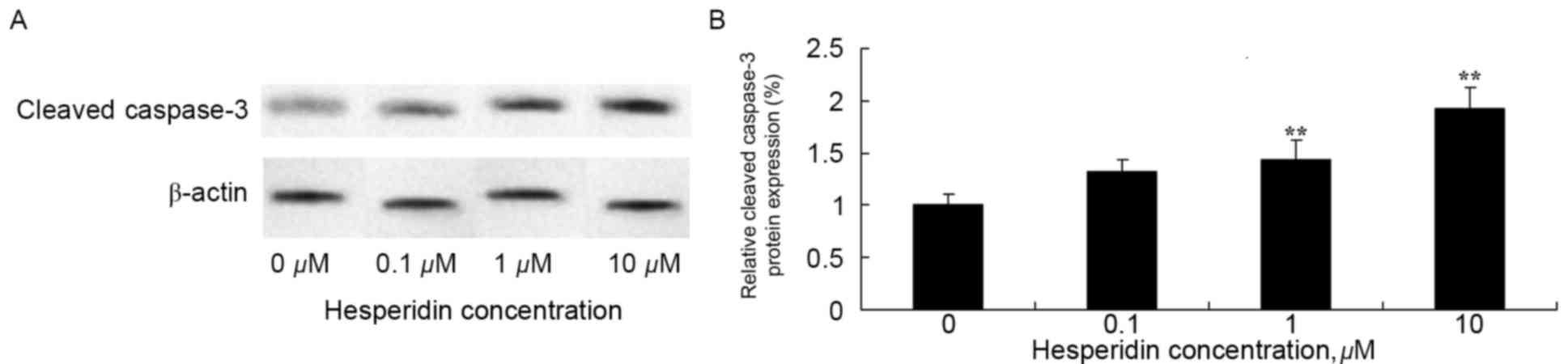
Hesperidin induces cleaved caspase-3
protein expression in ovarian cancer cells
To investigate whether cleaved caspase-3 may be
involved in the anticancer effect of hesperidin, expression levels
of cleaved caspase-3 protein was determined using western blot
analysis. The results of the western blot analysis identified that
1 and 10 µM hesperidin significantly increased the protein
expression levels of cleaved caspase-3 in A2780 cells (Fig. 5).
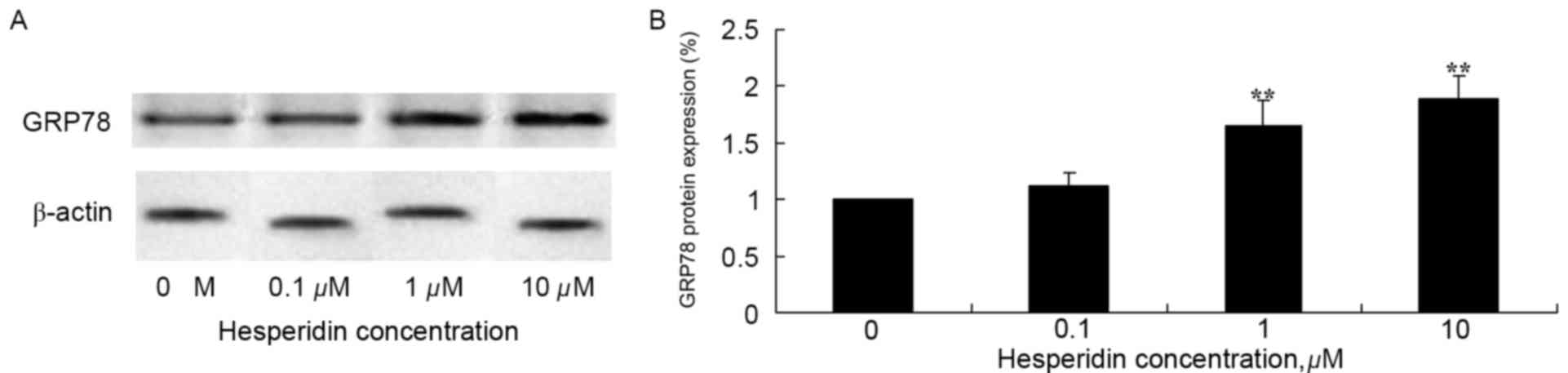
Hesperidin induces GRP78 protein
expression in ovarian cancer cells
To investigate the effect of hesperidin on protein
expression levels of GRP78 in ovarian cancer cells, western blot
analysis of A2780 cells was performed. As presented in Fig. 6, GRP78 protein expression levels in
A2780 cells was significantly increased following treatment with 1
and 10 µM hesperidin, compared with the control (0 µM
hesperidin).
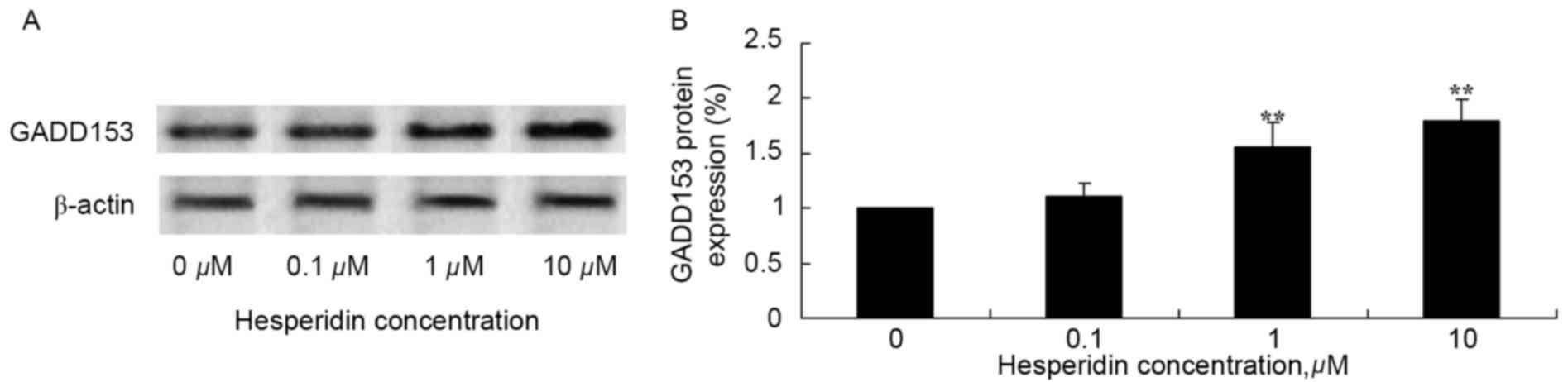
Hesperidin induces GADD153 protein
expression in ovarian cancer cells
The effect of hesperidin on GADD153 protein
expression levels in ovarian cancer cells was investigated using
western blot analysis. Hesperidin concentrations of 1 and 10 µM
significantly increased the protein expression levels of GADD153 in
A2780 cells, compared with the control (0 µM hesperidin; Fig. 7).
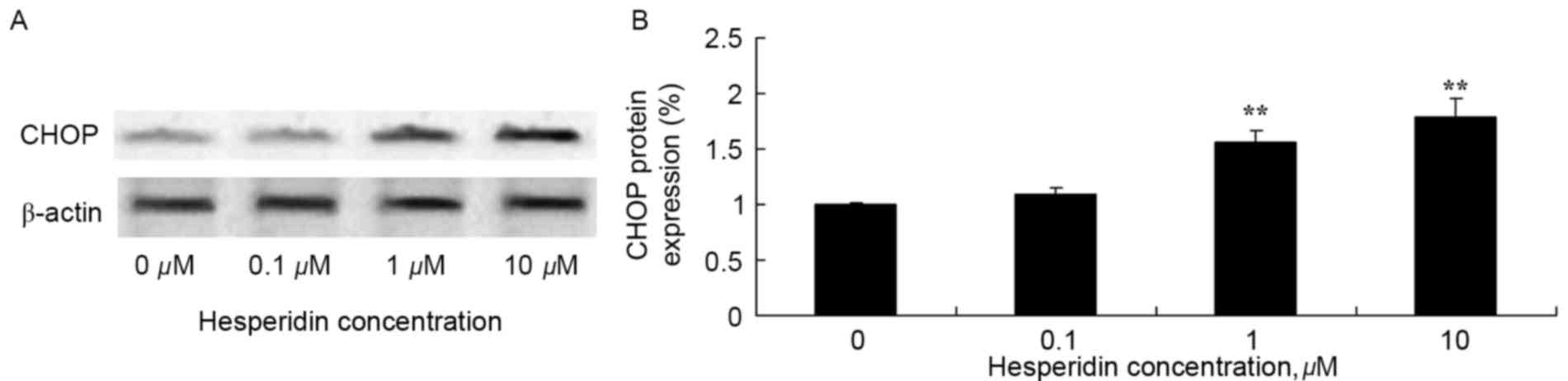
Hesperidin induces CHOP protein
expression in ovarian cancer cells
The effect of hesperidin on CHOP protein expression
levels in ovarian cancer cell was determined using western blot
analysis. As presented in Fig. 8,
treatment with 1 and 10 µM hesperidin significantly increased
expression levels of CHOP protein in A2780 cells, compared with the
control (0 µM hesperidin).
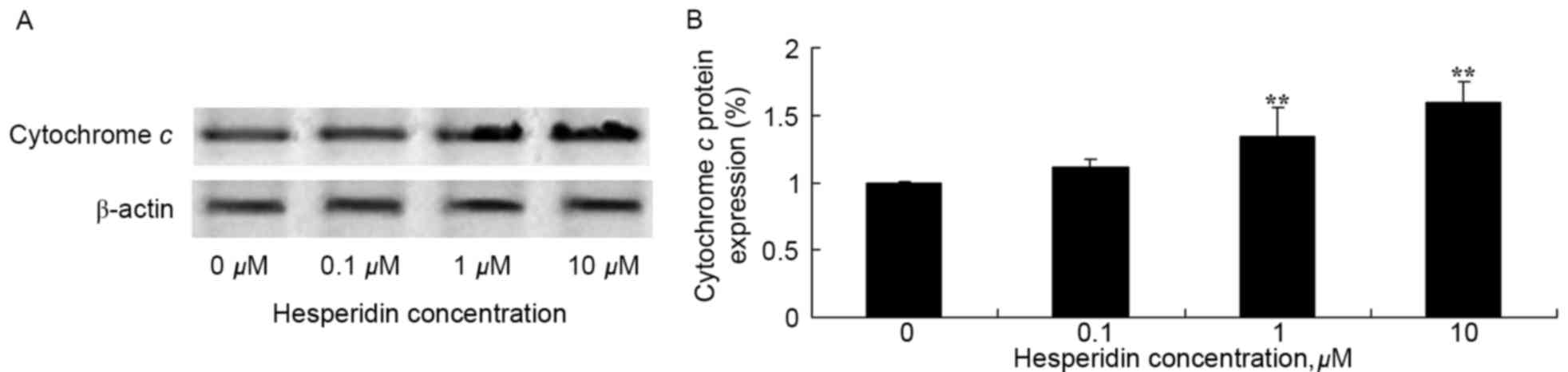
Hesperidin induces cytochrome c
protein expression in ovarian cancer cells
To investigate the effect of hesperidin on
cytochrome c protein expression in ovarian cancer cells,
levels of cytochrome c protein expression were determined
using western blot analysis. As presented in Fig. 9, cytochrome c protein
expression levels were significantly increased by treatment with
hesperidin (1 and 10 µM) in A2780 cells, compared with the control
(0 µM hesperidin).
Discussion
Ovarian carcinoma is the most fatal type of
malignant gynaecological tumor. If epithelial ovarian carcinoma is
identified to be in phase II or phase III of the disease, the
mortality rate within 5 years is estimated to be ~70% (18). Currently, the standard treatment for
patients with later-stage ovarian carcinoma, is cytoreductive
surgery and postoperative combined chemotherapy (19). There are successful treatments;
however, chemotherapy is not well tolerated which restricts the
efficacy and prognosis of patients with ovarian carcinomas
(20). The results of the present
study identified that hesperidin significantly decreased cell
viability and increased ovarian cancer cytotoxicity, apoptosis and
protein expression levels of cleaved caspase-3 in A2780 cells.
Birsu Cincin et al (21)
indicated that hesperidin exhibits anti-proliferative, apoptotic
and signal transduction effects in non-small cell lung cancer cells
through the activation of caspase-3. Ghorbani et al
(14) demonstrated that hesperidin
induces apoptosis in acute lymphoblastic leukemia NALM-6 cells
through the activation of p53 and suppression of nuclear factor
κB.
The abnormal viability and uncontrolled apoptosis of
cells are important factors which enable the development of
malignant tumors. GADD153 is a DNA damage protein which inhibits
cell viability and apoptosis (22).
Following DNA damage, GADD153 expression is increased, and cells
are unable to progress through the cell cycle and exhibit decreased
viability. If damage continues, GADD153 may induce apoptosis
(23). GADD153 has increased
expression and is accompanied by inhibiting the viability of the
human ovarian cancer cell line CAOV3. Cell cycle arrest, triggered
by GADD153, prevents cells progressing from G1 to S
phase and occurs in numerous apoptotic cells (23). This indicates that long-term
starvation results in damage to DNA, inducing increased expression
of GADD153 and results in decreased cell viability (24). The results of the present study
indicated that hesperidin induces GADD153 protein expression in
ovarian cancer cells.
Protein kinase R-like endoplasmic reticulum kinase
(PERK)-mediated apoptosis depends primarily on the activation of
the downstream apoptosis-promoting transcription factor CHOP
(25). Activation of CHOP may be
induced by downstream transcription factors of PERK including
activating transcription factor (ATF)4, ATF6 and X-box-binding
protein 1. PERK-eukaryotic initiation factor (eIF)2-ATF4 serves an
important role in the decreased expression of CHOP (26). Under the condition of ER stress, for
CHOP to be expressed, PERK must be present and ATF4 absent, by
phosphorylation of 51Ser of eIF2α. CHOP may be regulated
at the mRNA level by post-transcription modifications including
phosphorylation by p38 mitogen-activation protein kinase (MAPK)
(27), which may increase the
apoptosis-promoting activity of CHOP. The downstream target of the
serine/threonine-protein kinase/endoribonuclease inositol-requiring
enzyme (IRE)1'tumor necrosis factor receptor-associated factor
2/apoptosis signal-regulating kinase 1 signaling pathway is p38
MAPK; therefore, p38 MAPK phosphorylation of CHOP may link the two
signaling pathways of PERK and IRE1 (28). A previous study identified that the
loss of CHOP function protected cells, whereas the acquisition of
CHOP increased the sensitivity of cells to external stress which
caused more disturbance of the ER (29). The results of the present study
demonstrated that hesperidin induces CHOP protein expression in
ovarian cancer cell.
Compared with normal tissues, development of tumors
results in insufficient blood supply and alimentary deficiency,
causing the establishment of an anoxic and acidotic
microenvironment low in sugar (6).
The tumor microenvironment may result in ER stress, activation of
the unfolded protein response and induction of increased GRP78
expression. Increased GRP78 expression is an important protein
marker of ER stress. A previous study indicated that GRP78
expression was increased in a number of tumors including breast,
gastric, prostate, liver and colon cancer (30). In the aforementioned types of tumors,
increased expression of GRP78 serves an important role in tumor
cell survival, apoptosis, tumor lymphatic metastasis and
chemotherapy resistance (31). The
results of the present study demonstrated that hesperidin induces
GRP78 protein expression in ovarian cancer cells. Wang et al
(32) revealed that hesperidin
inhibits HeLa cell viability via the promotion of GADD153, CHOP,
GRP78 and cytochrome c.
Apoptosis-associated genes may regulate the
accumulation of cytochrome c in the cytoplasm by altering
the permeability of the mitochondrial membrane to affect
mitochondrial contents including caspase-3 (33). B-cell lymphoma (Bcl)-2-associated X
protein, B-cell lymphoma-2-antagonist'killer 1 and Bcl-2 homology 3
domain-interacting death agonist proteins may induce mitochondria
to release cytochrome c, whereas Bcl-2 and Bcl-extra large
(xL) inhibit the release of cytochrome c (34). Activation of caspase-3 results in the
release of cytochrome c from mitochondria (35). When cells are stimulated by
chemotherapeutics, apoptotic factors, including Bcl-xL, may inhibit
mitochondria to release cytochrome c to prevent apoptosis
(36). The apoptotic signal
conduction induced caspase-3 through the promotion of cytochrome
c, which caused a tumor-cytotoxicity effect (37). Wang et al (32) identified that hesperidin inhibits HeLa
cell viability by increasing cytochrome c protein.
Additionally, Saiprasad et al (38) demonstrated that hesperidin induces
apoptosis in colon carcinogenesis via the phosphoinositide
3-kinase/protein kinase B/glycogen synthase kinase-3β and
cytochrome c signaling pathways (38). The results of the present study
identified that hesperidin induces cytochrome c protein
expression in ovarian cancer cells.
From the results of the present study, it may be
concluded that hesperidin markedly decreases A2780 cell viability,
and induces tumor cytotoxicity and apoptosis. Additionally,
hesperidin activated protein expression of cleaved caspase-3 in
ovarian cancer cells, through GADD153'CHOP'GRP78 and cytochrome
c signaling pathways in A2780 cell. Further studies are
required.
References
|
1
|
Tillmanns TD, Lowe MP, Walker MS,
Stepanski EJ and Schwartzberg LS: Phase II clinical trial of
bevacizumab with albumin-bound paclitaxel in patients with
recurrent, platinum-resistant primary epithelial ovarian or primary
peritoneal carcinoma. Gynecol Oncol. 128:221–228. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Köbel M, Kalloger SE, Baker PM, Ewanowich
CA, Arseneau J, Zherebitskiy V, Abdulkarim S, Leung S, Duggan MA,
Fontaine D, et al: Diagnosis of ovarian carcinoma cell type is
highly reproducible: A transcanadian study. Am J Surg Pathol.
34:984–993. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Eilati E, Hales K, Zhuge Y, Fricano
Ansenberger K, Yu R, van Breemen RB and Hales DB: Flaxseed enriched
diet-mediated reduction in ovarian cancer severity is correlated to
the reduction of prostaglandin E(2) in laying hen ovaries.
Prostaglandins Leukot Essent Fatty Acids. 89:179–187. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bobak Y, Kurlishchuk Y,
Vynnytska-Myronovska B, Grydzuk O, Shuvayeva G, Redowicz MJ,
Kunz-Schughart LA and Stasyk O: Arginine deprivation induces
endoplasmic reticulum stress in human solid cancer cells. Int J
Biochem Cell Biol. 70:29–38. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yadunandam AK, Yoon JS, Seong YA, Oh CW
and Kim GD: Prospective impact of 5-FU in the induction of
endoplasmic reticulum stress, modulation of GRP78 expression and
autophagy in Sk-Hep1 cells. Int J Oncol. 41:1036–1042. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang LY, Li PL, Xu A and Zhang XC:
Involvement of GRP78 in the resistance of ovarian carcinoma cells
to paclitaxel. Asian Pac J Cancer Prev. 16:3517–3522. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huang LW, Lin CY, Lee CC, Liu TZ and Jeng
CJ: Overexpression of GRP78 is associated with malignant
transformation in epithelial ovarian tumors. Appl Immunohistochem
Mol Morphol. 20:381–385. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Koomägi R, Mattern J and Volm M:
Glucose-related protein (GRP78) and its relationship to the
drug-resistance proteins P170, GST-pi, LRP56 and angiogenesis in
non-small cell lung carcinomas. Anticancer Res. 19:4333–4336.
1999.PubMed/NCBI
|
|
9
|
Yacoub A, Liu R, Park MA, Hamed HA, Dash
R, Schramm DN, Sarkar D, Dimitriev IP, Bell JK, Grant S, et al:
Cisplatin enhances protein kinase R-like endoplasmic reticulum
kinase- and CD95-dependent melanoma differentiation-associated
gene-7/interleukin-24-induced killing in ovarian carcinoma cells.
Mol Pharmacol. 77:298–310. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee CS, Kwak SW, Kim YJ, Lee SA, Park ES,
Myung SC, Kim W, Lee MS and Lee JJ: Guanylate cyclase activator
YC-1 potentiates apoptotic effect of licochalcone A on human
epithelial ovarian carcinoma cells via activation of death receptor
and mitochondrial pathways. Eur J Pharmacol. 683:54–62. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang HL, Lin KY, Juan YC, Kumar KJ, Way
TD, Shen PC, Chen SC and Hseu YC: The anti-cancer activity of
Antrodia camphorata against human ovarian carcinoma (SKOV-3)
cells via modulation of HER-2′neu signaling pathway. J
Ethnopharmacol. 148:254–265. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu Y, Zhang J, Shi W and Liu Y: Anticancer
effects of 3,3′-diindolylmethane are associated with G1 arrest and
mitochondria-dependent apoptosis in human nasopharyngeal carcinoma
cells. Oncol Lett. 5:655–662. 2013.PubMed/NCBI
|
|
13
|
Liu Y and Luo W: Betulinic acid induces
Bax'Bak-independent cytochrome c release in human nasopharyngeal
carcinoma cells. Mol Cells. 33:517–524. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ghorbani A, Nazari M, Jeddi-Tehrani M and
Zand H: The citrus flavonoid hesperidin induces p53 and inhibits
NF-κB activation in order to trigger apoptosis in NALM-6 cells:
Involvement of PPARγ-dependent mechanism. Eur J Nutr. 51:39–46.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Parhiz H, Roohbakhsh A, Soltani F, Rezaee
R and Iranshahi M: Antioxidant and anti-inflammatory properties of
the citrus flavonoids hesperidin and hesperetin: An updated review
of their molecular mechanisms and experimental models. Phytother
Res. 29:323–331. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Garg A, Garg S, Zaneveld LJ and Singla AK:
Chemistry and pharmacology of the Citrus bioflavonoid hesperidin.
Phytother Res. 15:655–669. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Stermitz FR, Cashman KK, Halligan KM,
Morel C, Tegos GP and Lewis K: Polyacylated neohesperidosides from
Geranium caespitosum: Bacterial multidrug resistance pump
inhibitors. Bioorg Med Chem Lett. 13:1915–1918. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ueda Y, Miyatake T, Nagamatsu M, Yamasaki
M, Nishio Y, Yoshino K, Fujita M, Tsutsui T, Enomoto T and Kimura
T: A phase II study of combination chemotherapy using docetaxel and
irinotecan for TC-refractory or TC-resistant ovarian carcinomas
(GOGO-OV2 study) and for primary clear or mucinous ovarian
carcinomas (GOGO-OV3 Study). Eur J Obstet Gynecol Reprod Biol.
170:259–263. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Emons G, Gorchev G, Sehouli J, Wimberger
P, Stähle A, Hanker L, Hilpert F, Sindermann H, Gründker C and
Harter P: Efficacy and safety of AEZS-108 (INN: Zoptarelin
doxorubicin acetate) an LHRH agonist linked to doxorubicin in women
with platinum refractory or resistant ovarian cancer expressing
LHRH receptors: A multicenter phase II trial of the ago-study group
(AGO GYN 5). Gynecol Oncol. 133:427–432. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gelmon KA, Tischkowitz M, Mackay H,
Swenerton K, Robidoux A, Tonkin K, Hirte H, Huntsman D, Clemons M,
Gilks B, et al: Olaparib in patients with recurrent high-grade
serous or poorly differentiated ovarian carcinoma or
triple-negative breast cancer: A phase 2, multicentre, open-label,
non-randomised study. Lancet Oncol. 12:852–861. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cincin Birsu Z, Unlu M, Kiran B, Bireller
Sinem E, Baran Y and Cakmakoglu B: Anti-proliferative, apoptotic
and signal transduction effects of hesperidin in non-small cell
lung cancer cells. Cell Oncol (Dordr). 38:195–204. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Delmastro DA, Li J, Vaisman A, Solle M and
Chaney SG: DNA damage inducible-gene expression following platinum
treatment in human ovarian carcinoma cell lines. Cancer Chemother
Pharmacol. 39:245–253. 1997.PubMed/NCBI
|
|
23
|
Kandala PK and Srivastava SK: Regulation
of macroautophagy in ovarian cancer cells in vitro and in vivo by
controlling glucose regulatory protein 78 and AMPK. Oncotarget.
3:435–449. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gately DP, Jones JA, Christen R, Barton
RM, Los G and Howell SB: Induction of the growth arrest and DNA
damage-inducible gene GADD153 by cisplatin in vitro and in vivo. Br
J Cancer. 70:1102–1106. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang J, Hu X and Jiang H: ERS-PERK
signaling pathway-mediated Nrf2′ARE-HO-1 axis: A novel therapeutic
target for attenuating myocardial ischemia and reperfusion injury.
Int J Cardiol. 203:779–780. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang Y, Kuramitsu Y, Baron B, Kitagawa T,
Tokuda K, Akada J and Nakamura K: CGK733-induced LC3 II formation
is positively associated with the expression of cyclin-dependent
kinase inhibitor p21Waf1′Cip1 through modulation of the AMPK and
PERK'CHOP signaling pathways. Oncotarget. 6:39692–39701. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jiang Q, Li F, Shi K, Wu P, An J, Yang Y
and Xu C: ATF4 activation by the p38MAPK-eIF4E axis mediates
apoptosis and autophagy induced by selenite in Jurkat cells. FEBS
Lett. 587:2420–2429. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zheng QY, Li PP, Jin FS, Yao C, Zhang GH,
Zang T and Ai X: Ursolic acid induces ER stress response to
activate ASK1-JNK signaling and induce apoptosis in human bladder
cancer T24 cells. Cell Signal. 25:206–213. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chang CC, Kuan CP, Lin JY, Lai JS and Ho
TF: Tanshinone IIA Facilitates TRAIL Sensitization by Up-regulating
DR5 through the ROS-JNK-CHOP signaling axis in human ovarian
carcinoma cell lines. Chem Res Toxicol. 28:1574–1583. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhu X, Wang F, Lin MC, Tian L, Fan W, Ng
SS, Liu M, Huang J, Xu Z, Li D and Kung H: The 3′ UTR variants in
the GRP78 are not associated with overall survival in resectable
hepatocellular carcinoma. PLoS One. 6:e177832011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhu X, Chen MS, Tian LW, Li DP, Xu PL, Lin
MC, Xie D and Kung HF: Single nucleotide polymorphism of rs430397
in the fifth intron of GRP78 gene and clinical relevance of primary
hepatocellular carcinoma in Han Chinese: Risk and prognosis. Int J
Cancer. 125:1352–1357. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang Y, Yu H, Zhang J, Gao J, Ge X and Lou
G: Hesperidin inhibits HeLa cell proliferation through apoptosis
mediated by endoplasmic reticulum stress pathways and cell cycle
arrest. BMC Cancer. 15:6822015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu G, Wang T, Wang T, Song J and Zhou Z:
Effects of apoptosis-related proteins caspase-3, Bax and Bcl-2 on
cerebral ischemia rats. Biomed Rep. 1:861–867. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yuan Z, Cao K, Lin C, Li L, Liu HY, Zhao
XY, Liu L, Deng HX, Li J, Nie CL and Wei YQ: The p53 upregulated
modulator of apoptosis (PUMA) chemosensitizes intrinsically
resistant ovarian cancer cells to cisplatin by lowering the
threshold set by Bcl-x(L) and Mcl-1. Mol Med. 17:1262–1274. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chen H, Liang ZW, Wang ZH, Zhang JP, Hu B,
Xing XB and Cai WB: Akt activation and inhibition of cytochrome C
release: Mechanistic insights into Leptin-promoted survival of type
II alveolar epithelial cells. J Cell Biochem. 116:2313–2324. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Adam AC, Scriba A, Ortmann M, Huss S, Kahl
P, Steiner S, Störkel S and Büttner R: Immunohistochemical analysis
of cytochrome C oxidase facilitates differentiation between
oncocytoma and chromophobe renal cell carcinoma. Appl
Immunohistochem Mol Morphol. 23:54–59. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Feng X, Ching CB and Chen WN: EBV
up-regulates cytochrome c through VDAC1 regulations and decreases
the release of cytoplasmic Ca2+ in the NPC cell line. Cell Biol
Int. 36:733–738. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Saiprasad G, Chitra P, Manikandan R and
Sudhandiran G: Hesperidin induces apoptosis and triggers autophagic
markers through inhibition of Aurora-A mediated
phosphoinositide-3-kinase/Akt/mammalian target of rapamycin and
glycogen synthase kinase-3 beta signalling cascades in experimental
colon carcinogenesis. Eur J Cancer. 50:2489–2507. 2014. View Article : Google Scholar : PubMed/NCBI
|