Introduction
Primary central nervous system lymphoma (PCNSL) is
an aggressive neoplasm of the central nervous system with poor
prognosis, accounting for 2–3% of all brain tumors worldwide
(1,2).
The incidence of PCNSL has increased markedly in immunocompetent
patients for unknown reasons over the previous decades, whereas the
incidence of human immunodeficiency virus (HIV)-associated PCNSLs
has declined, possibly due to the development of highly active
antiretroviral therapies (3,4). Morphologically, ~95% of these tumors are
diffuse large B cell lymphoma (DLBCL), according to the new World
Health Organization classification (5). Although the prognosis of PCNSL has been
improved by optimal systemic treatment based on high-dose
methotrexate (HD-MTX) (6–8), the overall survival (OS) of the majority
of patients remains poor. This underlines the need to identify
prognostic biomarkers for potential therapeutic targets and
risk-stratified treatment.
In systemic DLBCL, based on cDNA microarray and
immunohistochemical staining with various markers, including
cluster of differentiation (CD)10, B cell lymphoma (BCL)-6 and
multiple myeloma-1/interferon regulatory factor-4 (MUM-1), previous
studies have identified two subtypes of DLBCL by Hans algorithm,
namely, germinal center (GC) B cell-like (GCB) and non-GCB
(9–13). Patients in the GCB subgroup showed an
improved prognosis compared with ABC (activated B cell-like;
including activated GCB and activated non-GCB, according to Chang's
classification) (9,10,12,13). In
PCNSL, numerous studies have been performed to observe the
prognostic significance of the variable biological markers widely
used in systemic DLBCL (14–24). The present study aimed to analyze the
expression profile of immunohistochemical markers and their
potential prognostic significance in 89 Chinese PCNSL cases.
Materials and methods
Patients and tumor specimens
The clinical data of 89 immunocompetent patients
with PCNSL were retrospectively reviewed at the Department of
Hematology, Beijing Tiantan Hospital, Capital Medical University
(Beijing, China) and the Department of Neurosurgery, Navy General
Hospital (Beijing, China), between July 2009 and April 2015. Of the
total 89 patients, 53 were male and 36 were female (male-female sex
ratio of 1.47:1). The median age was 56 years (range, 11–85 years;
≤60 years, 54 patients; >60 years, 35 patients). All specimens
were obtained by stereotactic biopsy or surgery for pathological
diagnosis prior to treatment. Diagnosis of DLBCL was made by
histological review of all specimens by two pathologists using
light microscopy. The pathologists assessed the immunohistochemical
markers including CD20, CD10, BCL-6, BCL-2, MUM1, CD138 and Ki-67
independently and between 15 and 20 fields were analyzed/specimen
(magnification, ×400). A total of 16/89 patients were lost to
follow-up. In the follow-up of the remaining 73 patients, 39
received HD-MTX plus cytarabine [3.5 g/m2 intravenous
(i.v.) in 3 h on day 1 + 0.5–1 g/m2 i.v. on day 2
according to age and Karnofsky Performance Status] every 3 weeks,
and the other patients received HD-MTX plus tomozolomide (3.5
g/m2 i.v. in 3 h on day 1 + 100 mg/m2
administered orally on days 1–5) every 3 weeks.
The present study protocol was approved by the
Ethics Committees of Beijing Tiantan Hospital and Navy General
Hospital. All patients gave written informed consent.
Immunohistochemical analysis
Tumor specimens were fixed with 10% formalin at room
temperature for 24 h and paraffin-embedded. A series of 4-µm
sections were obtained for conventional hematoxylin and eosin
(H&E) and immunohistochemical staining. Sections were
deparaffinized in xylene and dehydrated with ethanol. Endogenous
peroxidase was blocked with 0.1% hydrogen peroxide-methanol for 30
min at room temperature. Sections were washed with PBS, and the
specimens were then incubated for antigen retrieval in a microwave
oven for 15 min, followed by washing with PBS. The sections were
treated with 3% H2O2 for 5 min at room
temperature to block endogenous peroxidase activity, and sections
were then incubated with the working dilution of each monoclonal
antibody in a moist box (100% humidity) at 4°C overnight.
Monoclonal antibodies against CD20 (UM800002, 1:100 dilution), CD10
(UM870127, 1:600 dilution), BCL-6 (TA804186, 1:150 dilution), MUM1
(TA327705; 1:100-1:500 dilution), BCL-2 (UM870117, 1:500 dilution),
Ki-67 (TA352729, 1:100), CD138 (TA327619, 1:25-1:200) were used and
all purchased from OriGene Technologies, Inc. (Rockville, MD, USA).
Subsequent to washing the specimens with PBS, they were incubated
with corresponding secondary antibodies at a dilution of 1:2,000
[horseradish peroxidase-conjugated monoclonal goat anti-mouse IgG
(HS201-01) or horseradish peroxidase-conjugated monoclonal goat
anti-rabbit IgG (HS101-01); TransGen Biotech Co., Ltd., Beijing,
China] for 1 h at room temperature. The EnVision kit was purchased
from OriGene Technologies, Inc., and immunohistochemistry (EnVision
method) was performed according to the manufacturer's protocol.
Systemic DLBCLs were used as positive controls.
The staining for each marker was scored by two
pathologists independently. Images were captured using a LEITZ DMR
microscope (between 15 and 20 fields; magnification, ×400; Leica
Microsystems GmbH, Wetzlar, Germany). Staining was considered
positive for CD10, BCL-6, MUM-1 and CD138 when >30% of cells
were positively stained (25). For
BCL-2, staining was considered positive when >50% of cells were
positively stained (26) (between 15
and 20 fields; magnification, ×400). Ki-67 expression was evaluated
by semi-quantitative method and on the basis of the proportion of
positive tumor cells (between 0 and 100%). High expression was
considered when >90% of cells were positively stained for Ki-67.
Low expression was considered when ≤90% of cells were positively
stained for Ki-67.
Immunophenotype classification
Hans' method
Using a decision tree, Hans et al (12) divided tumors into two main subgroups
according to three markers: CD10, BCL-6 and MUM-1. All
CD10+ tumors and those with a CD10−
BCL-6+ MUM-1− phenotype were considered as
the GCB subgroup. The non-GCB subgroup included CD10−
BCL-6+ MUM-1+, CD10−
BCL-6− MUM-1+ and CD10−
BCL-6− MUM-1− immunophenotypes.
Chang's method
Using the immunohistochemical markers CD10 and BCL-6
for GCB markers, and MUM-1 and CD138 for ABC markers, Chang et
al (13) classified the tumors as
GCB and activated GBC subgroups (activated GCB and activated
non-GCB subgroups). At least one positive GCB marker without the
expression of activation markers was considered as the GCB
subgroup. The activated GCB subgroup expressed CD10 and/or BCL-6
and one activation marker. The activated non-GCB subgroup expressed
at least one activation marker without the expression of GCB
markers.
Statistical analysis
OS time was counted from the start of treatment to
the time of mortality due to any cause. Progression-free survival
(PFS) time was counted from the start of treatment to the time of
disease progression or mortality due to PCNSL. Kaplan-Meier
survival curves were obtained, and differences in OS or PFS times
were performed using the log-rank test. Multivariate analysis for
OS and PFS times using the Cox proportional hazards regression
models. Distribution of the characteristics of patients examined
using the χ2 test. All statistical analyses were
performed using SPSS 17.0 (SPSS, Inc., Chicago, IL, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
Characteristics and clinical outcomes
of patients
The characteristics of patients with PCNSL are
described in Table I. All patients
with PCNSL were immunocompetent patients who were HIV-negative. Of
the total 89 patients, 53 were male and 36 were female (male-female
sex ratio of 1.47:1). The median age was 56 years (range, 11–85
years; ≤60 years, 54 patients; >60 years, 35 patients). In
total, 21 patients (23.6%) had an Eastern Cooperative Oncology
Group (ECOG) performance status (27)
of 0 or 1 and 68 patients (76.4%) had an ECOG performance status of
2–4. Multiple brain lesions were observed in 67.4% (60/89) of
patients and the presence of deep brain structures was observed in
68.5% (61/89) of patients. The concentration of serum lactate
dehydrogenase (LDH) was elevated in 30 (39.5%) of 76 patients.
 | Table I.Clinical characteristics of patients
with PCNSL. |
Table I.
Clinical characteristics of patients
with PCNSL.
|
Characteristics | Patients |
|---|
| Median age
(range) | 56 (11–85) |
| Age, n (%),
years |
|
|
>60 | 35/89 |
|
≤60 | 54/89 |
| Sex, n (%) |
|
|
Male | 53/89 (59.6) |
|
Female | 36/89 (40.4) |
| ECOG, n (%) |
|
|
0–1 | 21/89 (23.6) |
|
2–4 | 68/89 (76.4) |
| LDH, n (%) |
|
|
Elevated | 30/76 (39.5) |
|
Normal | 46/76 (60.5) |
| No. of lesions, n
(%) |
|
| 1 | 29/89 (32.6) |
| ≥2 | 60/89 (67.4) |
| Deep brain lesions,
n (%) |
|
|
Absent | 28/89 (31.5) |
|
Present | 61/89 (68.5) |
| Chemotherapy, n
(%) |
|
|
HD-MTX+Ara-C | 39/73 (53.4) |
|
HD-MTX+TMZ | 34/73 (46.6) |
| Median OS (95%
CI) | 45.3
(25.01–65.59) |
| Median PFS (95%
CI) | 30.0
(13.43–46.57) |
Follow-up was performed for 73 patients, as 16 were
lost to follow-up. The median follow-up time was 13 months [95%
confidence interval (CI), 10.93–15.08]. The median OS time was 45.3
months (95% CI, 25.01–65.59) and the median PFS time was 30.0
months (95% CI, 13.43–46.57).
Cytological and immunohistochemical
analysis
In tumor cells with a diffuse distribution, the
nuclei were 2 times greater than normal lymphocytes. CD20 staining
showed diffuse patterns; CD20, CD10 and CD138 showed cell membrane
staining; BCL-6, MUM-1 and Ki-67 showed nuclei staining; and BCL-2
showed cytoplasmic staining of cells (Fig. 1).
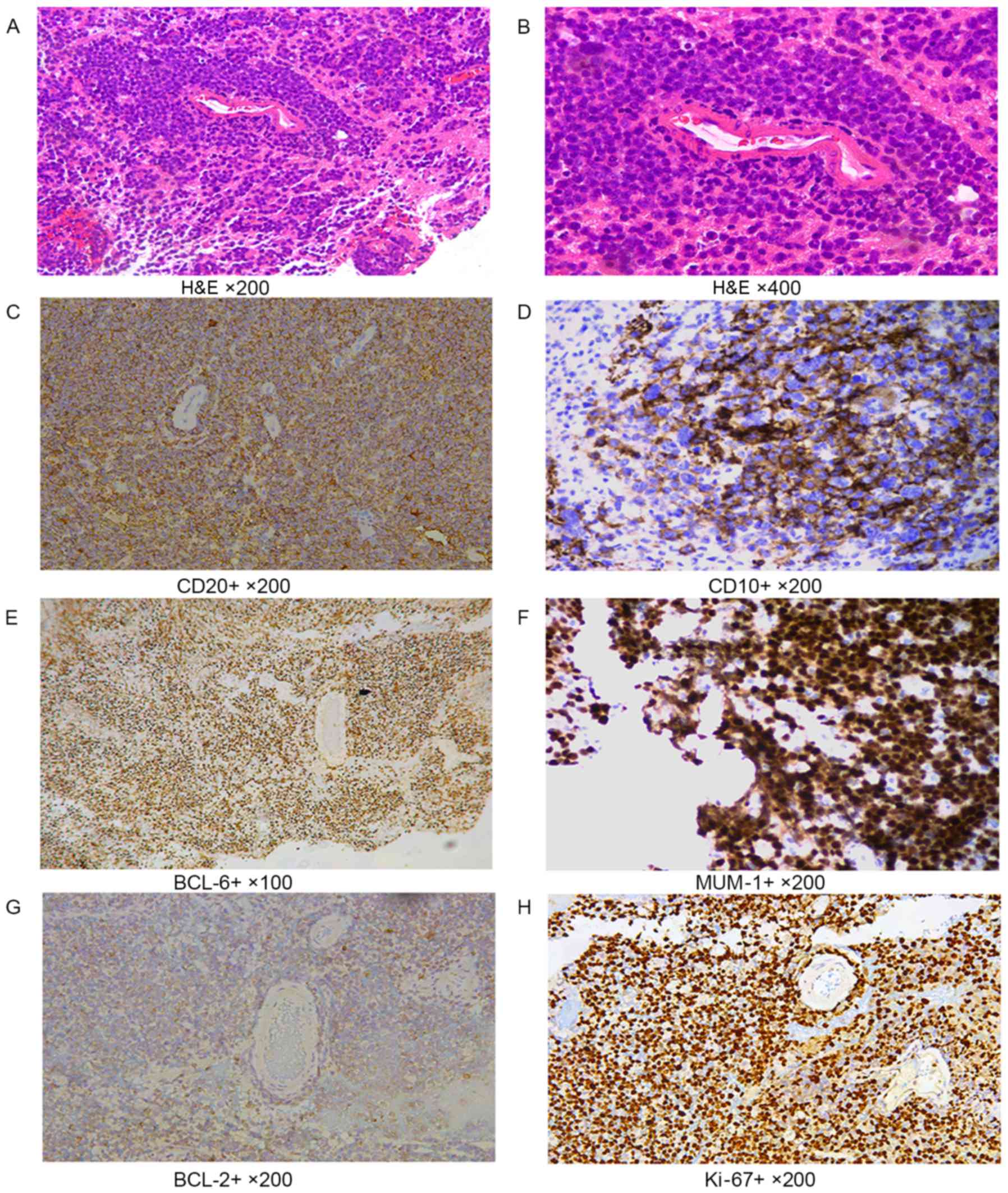 | Figure 1.Immunohistochemical labeling. (A and
B) H&E staining. In tumor cells with the diffuse distribution,
the size of nuclei were 2 times greater than of normal lymphocytes.
(A) Magnification, ×200. (B) Magnification, ×400. (C) CD20 cell
membrane staining performed using the EnVision method.
Magnification, ×200. (D) CD10 cell membrane staining performed
using the EnVision method. Magnification, ×200. (E) BCL-6, nuclei
staining, EnVision method, ×100. (F) MUM-1 nuclei staining
performed using the EnVision method. Magnification, ×200. (G) BCL-2
cytoplasmic staining performed using the EnVision method.
Magnification, ×200. (H) Ki-67 nuclei staining performed using the
EnVision method. Magnification, ×200. H&E, hematoxylin and
eosin; CD, cluster of differentiation; BCL, B cell lymphoma; MUM-1,
multiple myeloma-1. |
CD10, BCL-6, and MUM-1 were positive in 16.9
(15/89), 51.7 (46/89) and 92.1% (82/89) of patients. Among 86
tested samples, BCL-2 was positive in 73.3% (63/86). In total, 42
PCNSLs showed >90% Ki-67 expression. CD138 was negative in 100%
(65/65).
Immunophenotype classification
According to the Hans classification, 18 tumors
(20.2%) were classified in the GCB subgroup: 8 (9.0%) were
CD10+ BCL-6+ MUM-1+; 2 (2.2%) were
CD10+ BCL-6+ MUM-1−; 5 (5.6%) were
CD10+ BCL-6− MUM-1+; 3 (3.4%) were
CD10− BCL-6+ MUM-1−; and none were
CD10+ BCL-6− MUM-1−. A total of 71
tumors (79.8%) were considered as non-GCB: 33 (37.1%) were
CD10− BCL-6+ MUM-1+; 36 (40.5%)
were MUM-1+ only; and 2 (2.2%) were negative for all
markers tested.
According to the Chang classification, the 87
patients were classified into the three subgroups: 5 (5.7%) tumors
were CD10+ BCL-6+/− MUM-1− and
CD10− BCL-6+ MUM-1−, and were
classified as GCB; 46 (52.9%) tumors were CD10+
BCL-6+ MUM-1+, CD10−
BCL-6+ MUM-1+ or CD10+
BCL-6− MUM-1+ and were classified as
activated GCB; and 36 (41.4%) tumors were CD10−
BCL-6− MUM-1+ and were classified as
activated non-GCB.
Analysis of prognosis
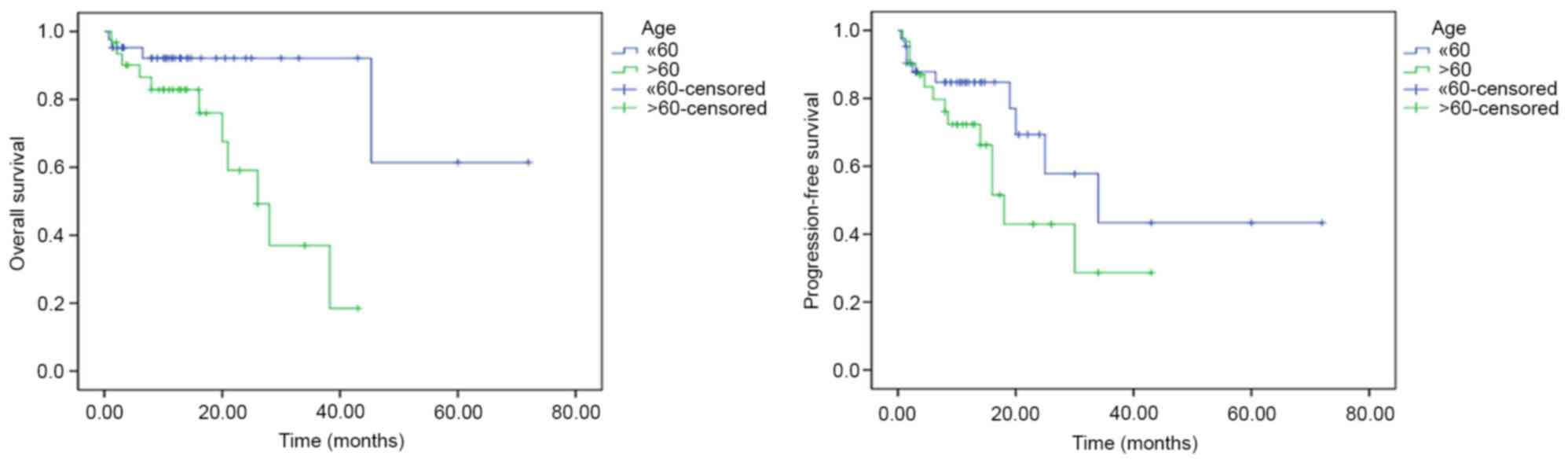
Among the clinical characteristics of patients, an
age >60 years was associated with a shorter OS time compared
with an age of ≤60 years (univariate analysis, P=0.009;
multivariate analysis, hazard ratio=0.229; 95% CI, 0.057–0.922;
P=0.038; Fig. 2). Among the
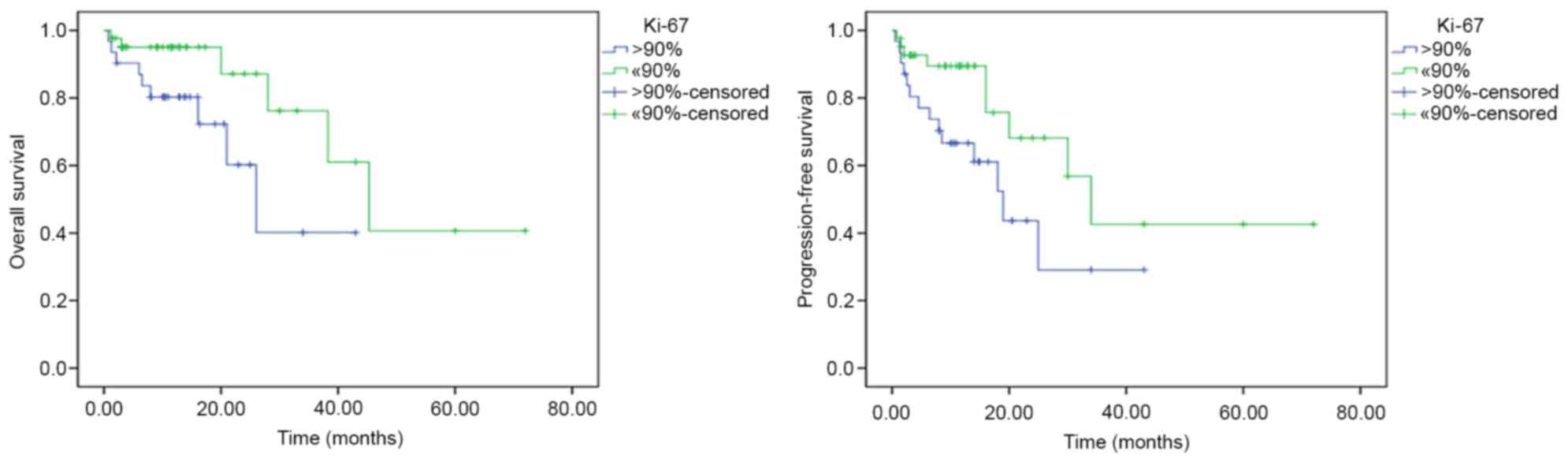
biological markers, based on univariate analysis, Ki-67 expression
(>90%) was associated with a shorter OS (P=0.037) and shorter
PFS (P=0.039) times, compared with ≤90% Ki-67 expression (Fig. 3). However, on multivariate analysis,
no biological markers were associated with OS and PFS time. No
significant prognostic effect on OS or PFS was observed for the
other clinical or biological parameters (sex, ECOG, CD10, BCL-6,
BCL-2, LDH concentration, number of lesions, chemotherapy regimens
and GCB/non-GCB subgroups) (Table
II).
 | Table II.Univariate and multivariate analyses
for overall survival and progression-free survival. |
Table II.
Univariate and multivariate analyses
for overall survival and progression-free survival.
| A, Overall
survival |
|
|
|
|
|---|
|
|
|
|
|
|---|
|
| Univariate analysis
(log-rank test) | Multivariate
analysis (Cox test) |
|---|
|
|
|
|
|---|
| Characteristic | P-value | HR | P-value | 95% CI |
|---|
| Age (≤60 vs. >60
years) | 0.009 | 0.229 | 0.038 | 0.057–0.922 |
| Sex (male vs.
female) | 0.525 | – | – | – |
| ECOG (0–1 vs.
2–4) | 0.377 | – | – | – |
| CD10 (positive vs.
negative) | 0.924 | – | – | – |
| BCL-6 (positive vs.
negative) | 0.453 | 0.612 | 0.468 | 0.163–2.303 |
| BCL-2 (positive vs.
negative) | 0.328 | 0.549 | 0.427 | 0.125–2.409 |
| Ki-67 (>90 vs.
≤90%) | 0.037 | 0.414 | 0.162 | 0.120–1.424 |
| Immunophenotype
(GCB vs. non-GCB) | 0.410 | 0.506 | 0.365 | 0.116–2.209 |
| Chemotherapy
(HD-MTX+Ara-C vs. HD-MTX+TMZ) | 0.671 | 0.993 | 0.990 | 0.309–3.191 |
| LDH (elevated vs.
normal) | 0.442 | – | – | – |
| No. of lesions (1
vs. ≥2) | 0.592 | 0.880 | 0.835 | 0.262–2.954 |
|
| B, Progression-free
survival |
|
|
| Univariate analysis
(log-rank test) | Multivariate
analysis (Cox test) |
|
|
|
|
| Characteristic | P-value | HR | P-value | 95% CI |
|
| Age (≤60 vs. >60
years) | 0.141 | 0.566 | 0.237 | 0.220–1.456 |
| Sex (male vs.
female) | 0.957 | – | – | – |
| ECOG (0–1 vs.
2–4) | 0.313 | – | – | – |
| CD10 (positive vs.
negative) | 0.264 | – | – | – |
| BCL-6 (positive vs.
negative) | 0.304 | 0.736 | 0.571 | 0.255–2.126 |
| BCL-2 (positive vs.
negative) | 0.463 | 0.649 | 0.438 | 0.218–1.934 |
| Ki-67 (>90 vs.
≤90%) | 0.039 | 0.437 | 0.075 | 0.176–1.086 |
| Immunophenotype
(GCB vs. non-GCB) | 0.131 | 0.398 | 0.109 | 0.129–1.228 |
| Chemotherapy
(HD-MTX+Ara-C vs. HD-MTX+TMZ) | 0.459 | 1.063 | 0.898 | 0.422–2.675 |
| LDH (elevated vs.
normal) | 0.779 | – | – | – |
| No. of lesions (1
vs. ≥2) | 0.740 | 1.021 | 0.967 | 0.387–2.696 |
Discussion
In the present study, it was revealed that, unlike
systemic DLBCLs, the majority of PCNSLs originate from post-GC,
with a low expression of the GC marker CD10, expression of the GC
marker BCL-6 and high expression of the activated B cell-like
marker MUM-1, which is consistent with the majority of previous
studies (14–24,28,29)
(Table III). In addition, the
co-expression of BCL-6 and MUM-1 and the absence of late post-GC
marker CD138 expression indicated the activated immunophenotype and
the early post-GC origin of PCNSL, which was in accordance with
Camilleri-Broёt et al (24).
 | Table III.Comparison of immunohistochemical
marker expression in previous studies and the present study. |
Table III.
Comparison of immunohistochemical
marker expression in previous studies and the present study.
| First author
(year) | Country | Cases, no. | CD10, n (%) | BCL-6, n (%) | MUM-1, n (%) | BCL-2, n (%) | Algorithm | GCB, n (%) | Non-GCB, n (%) | (Refs.) |
|---|
| Mahadevan et
al (2015) | India | 24 | 0 | 12/24 (50.0) | 22/24 (91.7) | – | Hans and Chang | – | 22/24 (91.7) | (14) |
| Aki et al
(2013) | Turkey | 35 | 6/35 (17.1) | 15/35 (42.8) | 27/35 (77.1) | 15/35 (42.8) | Hans | 6/35 (17.1) | 29/35 (82.9) | (15) |
| Hattab et al
(2010) | USA | 31 | 4/31 (12.9) | 26/31 (83.9) | 27/31 (87.1) | 21/30 (70.0) | Hans | 5/31 (16.1) | 26/31 (83.9) | (16) |
| Raoux et al
(2010) | France | 39 | 8/39 (20.5) | 14/39 (35.9) | 23/39 (60.0) | 33/39 (84.6) | Hans | 13/39 (25.7) | 26/39 (74.3) | (17) |
| Momota et al
(2010) | Japan | 27 | 6/27 (22.2) | 13/27 (48.1) | 22/27 (81.5) | – | Hans and Chang | 1/23 (4.4) | 22/23 (95.6) | (18) |
| Kinoshita et
al (2010) | Japan | 32 | 6/32 (18.8) | 21/32 (65.6) | 27/32 (84.4) | – | Hans and Chang | 8/29 (27.6) | 21/29 (72.4) | (19) |
| Bhagavathi et
al (2008) | USA | 21 | 1/21 (4.8) | 19/21 (90.5) | 19/21 (90.5) | 17/21 (81.0) | Hans | 2/21 (9.5) | 19/21 (90.5) | (20) |
| Levy et al
(2008) | USA | 38 | 3/38 (7.9) | 18/38 (47.4) | 36/38 (94.7) | – | Hans | 5/38 (13.0) | 33/38 (87.0) | (21) |
| Cheng et al
(2008) | China | 47 | 3/47 (6.4) | 25/47 (53.2) | 43/47 (91.5) | – | Chang | 4/47 (8.5) | 43/47 (91.5) | (22) |
| Lin et al
(2006) | Taiwan | 51 | 9/51 (17.6) | 30/51 (58.9) | 43/51 (84.3) | 25/51 (49.0) | Hans | 11/51 (21.6) | 40/51 (78.4) | (23) |
| Camilleri-Broët
et al (2006) | France | 82 | 2/82 (2.4) | 45/81 (55.5) | 75/81 (92.6) | 45/81 (55.5) | Hans and Chang | 3/82 (3.7) | 79/82 (96.3) | (24) |
| Present study | China | 89 | 15/89 (16.9) | 46/89 (51.7) | 82/89 (92.1) | 63/86 (73.3) | Hans and Chang | 18/89 (20.2) | 71/89 (80.9) |
|
A number of previous studies (21,23,24,28–30)
have utilized immunohistochemical markers to predict the prognosis
of PCNSL. However, the significance of immunohistochemical markers
on the prognosis of PCNSL remains questionable due to limitations,
including a small sample size, heterogeneous treatment regimens or
different methods and standards of immunohistochemistry. The
present study analyzed the expression of biological markers and
evaluated their prognostic significance in the largest
retrospective studies at present. All the patients uniformly
received HD-MTX based chemotherapy as the first line of treatment.
The Ki-67 proliferative index, a nuclear antigen present in all
stages of the cell cycle, with the exception of G0,
represents the active growth fraction of the tumor (31–33). Ki-67
is a valuable immunohistochemical marker to distinguish indolent
from aggressive lymphomas, particularly in small needle biopsies
where exact typing may not be possible (31). Several studies have demonstrated that
high expression of Ki-67 is an adverse prognostic marker in
systemic DLBCL (34–36). In the present study, it was also
observed that Ki-67 expression is a significant prognostic
parameter of poor prognosis in patients with PCNSL. Unlike systemic
DLBCLs, the mean Ki-67 index for PCNSL was high (mean, 88%). Patel
et al (28) revealed that the
proliferative index was high (60–98%) in their study of 73 PCNSL
cases. Hashmi et al (31)
showed that the mean Ki-67 index for indolent non-Hodgkin lymphoma
(NHL) included 23% for small cell, 25% for mantle cell, 28.5% for
marginal zone and 34.6% for follicular lymphoma. By contrast, the
mean Ki-67 index for aggressive lymphomas was 66.4, 66.9, 80.3,
83.3 and 94.4% for DLBCL, T cell, anaplastic large cell,
lymphoblastic and Burkitt's lymphoma, respectively (31). A uniform high expression of Ki-67 is a
notable feature of PCNSL, which may explain the poor outcome of
PCNSLs. Previous studies did not reveal the prognostic significance
of Ki-67, which may be due to the small number of patients with a
uniform high expression of Ki-67 or the different
immunohistochemical methods used.
CD10 is expressed in pre-B cells and germinal center
B cells (37,38). MUM-1 performs an important role in the
terminal stages of B cell differentiation and can be used as a
post-GC cell or activation marker (39,40). Due
to the low expression of CD10 and the high expression of MUM-1,
CD10 and MUM-1 where considered to be characteristics of PCNSL, but
not prognostic indicators.
BCL-2, a proto-oncogene, localizes to mitochondria
and enhances cell survival by blocking programmed cell death
(41). BCL-2 protein expression is an
important independent predictor of survival in patients with
systemic DLBCL (42,43). However, in the present study, no
association between BCL-2 and prognosis was observed, which is in
accordance with the studies by Krogh-Jensen et al (44) and Preusser et al (45).
The BCL-6 gene encoding a nuclear-located
Krüppel-type zinc finger protein is rearranged in ~30% of DLBCLs
and is expressed predominantly in normal GCB cells and associated
lymphomas (46,47). BCL-6 may have an important role in
regulating the differentiation of normal GCB cells, and its
deregulated expression may contribute to lymphomagenesis (30). Previous studies about the prognostic
significance of BCL-6 expression remain controversial. Survival
analyses revealed BCL-6 expression as an independent prognostic
parameter of DLBCL associated with favorable outcomes, and its
positivity indicates an improved disease course (21,29,45). The
CALGB 50202 study of the prospective G-PCNSL-SG1 trial disclosed
that BCL-6 may assume clinical relevance as an unfavorable
prognostic biomarker in PCNSL (7,30). In the
present study, BCL-6 expression was not associated with OS or PFS.
The present study is a retrospective study with a short follow-up
time, and these limitations may explain the discrepancy between the
present study and previous studies.
In the present study, no significant difference was
observed between GCB and non-GCB subgroups on OS or PFS time, which
is in accordance with earlier studies (12,17,18,24,30).
These results may indicate that PCNSL has a common immunophenotype
classification, but this subtype classification may not have an
effect on prognosis.
In conclusion, the present study confirmed the
activated immunophenotype and the early post-GC origin of PCNSL,
and determined that older age (>60 years) was associated with a
shorter OS time. In addition, high Ki-67 expression was found to be
a valuable biological marker for poor prognosis. Considering the
short follow-up time of the present retrospective study and the
controversial results of previous studies, additional prospective
studies are required.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81272842 to Y.L.
and 81500157 to Q.C.) and the Beijing Natural Science Foundation
(grant no. 7172071). The authors thank Dr Young Whang (Lineberger
Comprehensive Cancer Center, University of North Carolina, Chapel
Hill, NC, USA) for a review of the manuscript before
submission.
References
|
1
|
Batchelor T and Loeffler JS: Primary CNS
lymphoma. J Clin Oncol. 24:1281–1288. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Abrey LE: Primary central nervous system
lymphoma. Curr Opin Neurol. 22:675–680. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Corn BW, Marcus SM, Topham A, Hauck W and
Curran WJ Jr: Will primary central nervous system lymphoma be the
most frequent brain tumor diagnosed in the year 2000? Cancer.
79:2409–2413. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kadan-Lottick NS, Skluzacek MC and Gurney
JG: Decreasing incidence rates of primary central nervous system
lymphoma. Cancer. 95:193–202. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Campo E, Swerdlow SH, Harris NL, Pileri S,
Stein H and Jaffe ES: The 2008 WHO classification of lymphoid
neoplasms and beyond: Evolving concepts and practical applications.
Blood. 117:5019–5032. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Omuro A, Correa DD, DeAngelis LM,
Moskowitz CH, Matasar MJ, Kaley TJ, Gavrilovic IT, Nolan C,
Pentsova E, Grommes CC, et al: R-MPV followed by high-dose
chemotherapy with TBC and autologous stem-cell transplant for newly
diagnosed primary CNS lymphoma. Blood. 125:1403–1410. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rubenstein JL, Hsi ED, Johnson JL, Jung
SH, Nakashima MO, Grant B, Cheson BD and Kaplan LD: Intensive
chemotherapy and immunotherapy in patients with newly diagnosed
primary CNS lymphoma: CALGB 50202 (Alliance 50202). J Clin Oncol.
31:3061–3068. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu J, Sun XF, Qian J, Bai XY, Zhu H, Cui
QU, Li XY, Chen YD, Wang YM and Liu YB: Immunochemotherapy for
primary central nervous system lymphoma with rituximab,
methotrexate, cytarabine and dexamethasone: Retrospective analysis
of 18 cases. Mol Clin Oncol. 3:949–953. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Alizadeh AA, Eisen MB, Davis RE, Ma C,
Lossos IS, Rosenwald A, Boldrick JC, Sabet H, Tran T, Yu X, et al:
Distinct types of diffuse large B-cell lymphoma identified by gene
expression profiling. Nature. 403:503–511. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rosenwald A, Wright G, Chan WC, Connors
JM, Campo E, Fisher RI, Gascoyne RD, Muller-Hermelink HK, Smeland
EB, Giltnane JM, et al: The use of molecular profiling to predict
survival after chemotherapy for diffuse large-B-cell lymphoma. N
Engl J Med. 346:1937–1947. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shipp MA, Ross KN, Tamayo P, Weng AP,
Kutok JL, Aguiar RC, Gaasenbeek M, Angelo M, Reich M, Pinkus GS, et
al: Diffuse large B-cell lymphoma outcome prediction by
gene-expression profiling and supervised machine learning. Nat Med.
8:68–74. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hans CP, Weisenburger DD, Greiner TC,
Gascoyne RD, Delabie J, Ott G, Müller-Hermelink HK, Campo E,
Braziel RM, Jaffe ES, et al: Confirmation of the molecular
classification of diffuse large B-cell lymphoma by
immunohistochemistry using a tissue microarray. Blood. 103:275–282.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chang CC, McClintock S, Cleveland RP,
Trzpuc T, Vesole DH, Logan B, Kajdacsy-Balla A and Perkins SL:
Immunohistochemical expression patterns of germinal center and
activation B-cell markers correlate with prognosis in diffuse large
B-cell lymphoma. Am J Surg Pathol. 28:464–470. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mahadevan A, Rao CR, Shanmugham M and
Shankar SK: Primary central nervous system diffuse large B-cell
lymphoma in the immunocompetent: Immunophenotypic subtypes and
Epstein-Barr virus association. J Neurosci Rural Pract. 6:8–14.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Aki H, Uzunaslan D, Saygin C, Batur S,
Tuzuner N, Kafadar A, Ongoren S and Oz B: Primary central nervous
system lymphoma in immunocompetent individuals: A single center
experience. Int J Clin Exp Pathol. 6:1068–1075. 2013.PubMed/NCBI
|
|
16
|
Hattab EM, Martin SE, Al-Khatib SM, Kupsky
WJ, Vance GH, Stohler RA, Czader M and Al-Abbadi MA: Most primary
central nervous system diffuse large B-cell lymphomas occurring in
immunocompetent individuals belong to the nongerminal center
subtype: A retrospective analysis of 31 cases. Mod Pathol.
23:235–243. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Raoux D, Duband S, Forest F, Trombert B,
Chambonnière ML, Dumollard JM, Khaddage A, Gentil-Perret A and
Péoc'h M: Primary central nervous system lymphoma:
Immunohistochemical profile and prognostic significance.
Neuropathology. 30:232–240. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Momota H, Narita Y, Maeshima AM, Miyakita
Y, Shinomiya A, Maruyama T, Muragaki Y and Shibui S: Prognostic
value of immunohistochemical profile and response to high-dose
methotrexate therapy in primary CNS lymphoma. J Neurooncol.
98:341–348. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kinoshita M, Hashimoto N, Izumoto S, Okita
Y, Kagawa N, Maruno M, Ohnishi T, Arita N and Yoshimine T:
Immunohistological profiling by B-cell differentiation status of
primary central nervous system lymphoma treated by high-dose
methotrexate chemotherapy. J Neurooncol. 99:95–101. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bhagavathi S, Sharathkumar A, Hunter S,
Sung L, Kanhere R, Venturina MD and Wilson JD: Activated B-cell
immunophenotype might be associated with poor prognosis of primary
central nervous system lymphomas. Clin Neuropathol. 27:13–20. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Levy O, Deangelis LM, Filippa DA, Panageas
KS and Abrey LE: Bcl-6 predicts improved prognosis in primary
central nervous system lymphoma. Cancer. 112:151–156. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cheng J, Tu P, Shi QL, Zhou HB, Zhou ZY,
Zhao YC, Ma HH and Zhou XJ: Primary diffuse large B-cell lymphoma
of central nervous system belongs to activated B-cell-like
subgroup: A study of 47 cases. Zhonghua Bing Li Xue Za Zhi.
37:384–389. 2008.(In Chinese). PubMed/NCBI
|
|
23
|
Lin CH, Kuo KT, Chuang SS, Kuo SH, Chang
JH, Chang KC, Hsu HC, Tien HF and Cheng AL: Comparison of the
expression and prognostic significance of differentiation markers
between diffuse large B-Cell lymphoma of central nervous system
origin and peripheral nodal origin. Clin Cancer Res. 12:1152–1156.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Camilleri-Broët S, Crinière E, Broët P,
Delwail V, Mokhtari K, Moreau A, Kujas M, Raphaël M, Iraqi W,
Sautès-Fridman C, et al: A uniform activated B-cell-like
immunophenotype might explain the poor prognosis of primary central
nervous system lymphomas: Analysis of 83 cases. Blood. 107:190–196.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Patel B, Chacko G, Nair S, Anandan J,
Chacko AG, Rajshekhar V and Turel M: Clinicopathological correlates
of primary central nervous system lymphoma: Experience from a
tertiary care center in South India. Neurol India. 63:77–82. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mounier N, Briere J, Gisselbrecht C, Emile
JF, Lederlin P, Sebban C, Berger F, Bosly A, Morel P, Tilly H, et
al: Rituximab plus CHOP (R-CHOP) overcomes BCL-2-associated
resistance to chemotherapy in elderly patients with diffuse large
B-cell lymphoma (DLBCL). Blood. 101:4279–4284. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the Eastern Cooperative Oncology Group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Patel B, Chacko G, Nair S, Anandan J,
Chacko AG, Rajshekhar V and Turel M: Clinicopathological correlates
of primary central nervous system lymphoma: Experience from a
tertiary care center in South India. Neurol India. 63:77–82. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Braaten KM, Betensky RA, de Leval L, Okada
Y, Hochberg FH, Louis DN, Harris NL and Batchelor TT: BCL-6
expression predicts improved survival in patients with primary
central nervous system lymphoma. Clin Cancer Res. 9:1063–1069.
2003.PubMed/NCBI
|
|
30
|
Kreher S, Jöhrens K, Strehlow F, Martus P,
Borowiec K, Radke J, Heppner F, Roth P, Thiel E, Pietsch T, et al:
Prognostic impact of B-cell lymphoma 6 in primary CNS lymphoma.
Neuro Oncol. 17:1016–1021. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hashmi AA, Hussain ZF, Faridi N and
Khurshid A: Distribution of Ki67 proliferative indices among WHO
subtypes of non-Hodgkin's lymphoma: Association with other clinical
parameters. Asian Pac J Cancer Prev. 15:8759–8763. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gerdes J, Lemke H, Baisch H, Wacker HH,
Schwab U and Stein H: Cell cycle analysis of a cell
proliferation-associated human nuclear antigen defined by
monoclonal antibody Ki67. J Immunol. 133:1710–1715. 1984.PubMed/NCBI
|
|
33
|
Niikura N, Iwamoto T, Masuda S, Kumaki N,
Xiaoyan T, Shirane M, Mori K, Tsuda B, Okamura T, Saito Y, et al:
Immunohistochemical Ki67 labeling index has similar proliferation
predictive power to various gene signatures in breast cancer.
Cancer Sci. 103:1508–1512. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jovanović MP, Jaković L, Bogdanović A,
Marković O, Martinović VC and Mihaljević B: Poor outcome in
patients with diffuse large B-cell lymphoma is associated with high
percentage of bcl-2 and Ki 67-positive tumor cells. Vojnosanit
Pregl. 66:738–743. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Miller TP, Grogan TM, Dahlberg S, Spier
CM, Braziel RM, Banks PM, Foucar K, Kjeldsberg CR, Levy N, Nathwani
BN, et al: Prognostic significance of the Ki-67-associated
proliferative antigen in aggressive non-Hodgkin's lymphomas: A
prospective Southwest Oncology Group trial. Blood. 83:1460–1466.
1994.PubMed/NCBI
|
|
36
|
Broyde A, Boycov O, Strenov Y, Okon E,
Shpilberg O and Bairey O: Role and prognositic significance of the
Ki-67 index in non-Hodgkin's lymphoma. Am J Hematol. 84:338–343.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Dogan A, Bagdi E, Munson P and Isaacson
PG: CD10 and BCL-6 expression in paraffin sections of normal
lymphoid tissue and B-cell lymphomas. Am J Surg Pathol. 24:846–852.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Falini B and Mason DY: Proteins encoded by
genes involved in chromosomal alterations in lymphoma and leukemia:
Clinical value of their detection by immunocytochemistry. Blood.
99:409–426. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Falini B, Fizzotti M, Pucciarini A,
Bigerrna B, Marafioti T, Gambacorta M, Pacini R, Alunni C,
Natali-Tanci L, Ugolini B, et al: A monoclonal antibody (MUM1p)
detects expression of the MUM1/IRF4 protein in a subset of germinal
center B cells, plasma cells, and activated T cells. Blood.
95:2084–2092. 2000.PubMed/NCBI
|
|
40
|
Rosenwald A, Wright G, Chan WC, Connors
JM, Campo E, Fisher RI, Gascoyne RD, Muller-Hermelink HK, Smeland
EB, Giltnane JM, et al: The use of molecular profiling to predict
survival after chemotherapy for diffuse large B-cell lymphoma. N
Engl J Med. 346:1937–1947. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hockenbery D, Nuñez G, Milliman C,
Schreiber R and Korsmeyer SJ: Bcl-2 is an inner mitochondrial
membrane protein that blocks programmed cell death. Nature.
348:334–336. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Goscoyne RD, Adomat SA, Krajewski S,
Krajewska M, Horsman DE, Tolcher AW, O'Reilly SE, Hoskins P,
Coldman AJ, Reed JC and Connors JM: Prognostic significance of
BCL-2 protein expression and BCL-2 gene rearrangement in diffuse
aggressive non-Hodgkin' s lymphoma. Blood. 90:244–251.
1997.PubMed/NCBI
|
|
43
|
Mahmoud HM and EI-Sakhawy YN: Significance
of Bcl-2 and Bcl-6 immunostaining in B-Non Hodgkin's lymphoma.
Hematol Rep. 3:e262011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Krogh-Jensen M, Johansen P and D'Amore F:
Primary central nervous system lymphomas in immunocompetent
individuals: Histology, Epstein-Barr virus genome, Ki-67
proliferation index, p53 and bcl-2 gene expression. Leuk Lymphoma.
30:131–142. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Preusser M, Woehrer A, Koperek O,
Rottenfusser A, Dieckmann K, Gatterbauer B, Roessler K, Slavc I,
Jaeger U, Streubel B, et al: Primary central nervous system
lymphoma: A clinicopathological study of 75 cases. Pathology.
42:547–552. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Cattoretti G, Chang CC, Cechova K, Zhang
J, Ye BH, Falini B, Louie DC, Offit K, Chaganti RS and Dalla-Favera
R: BCL-6 protein is expressed in germinal-center B cells. Blood.
86:45–53. 1995.PubMed/NCBI
|
|
47
|
Falini B, Bigerna B, Pasqualucci L,
Fizzotti M, Martelli MF, Pileri S, Pinto A, Carbone A, Venturi S,
Pacini R, et al: Distinctive expression pattern of the BCL-6
protein in nodular lymphocyte predominance Hodgkin's disease.
Blood. 87:465–471. 1996.PubMed/NCBI
|