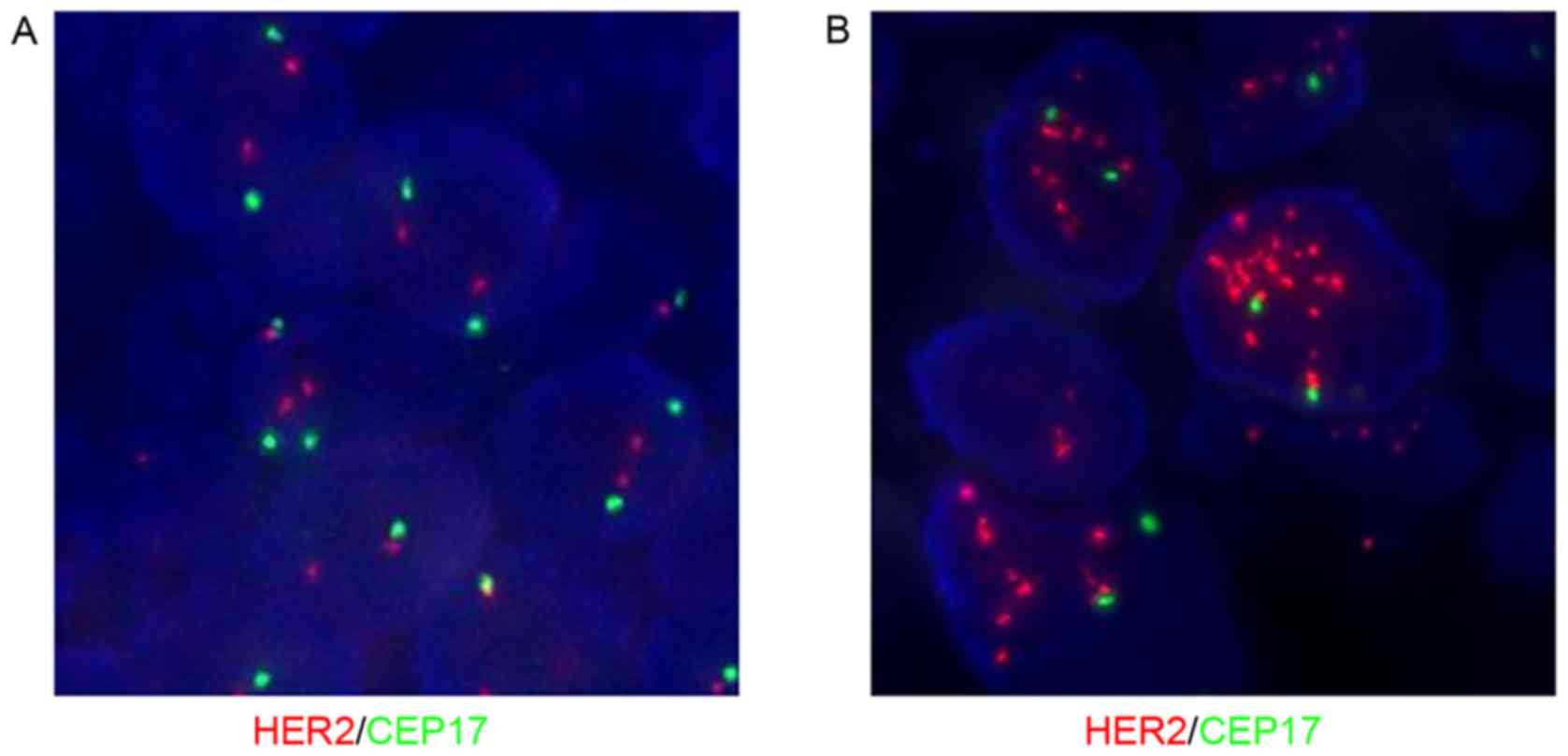
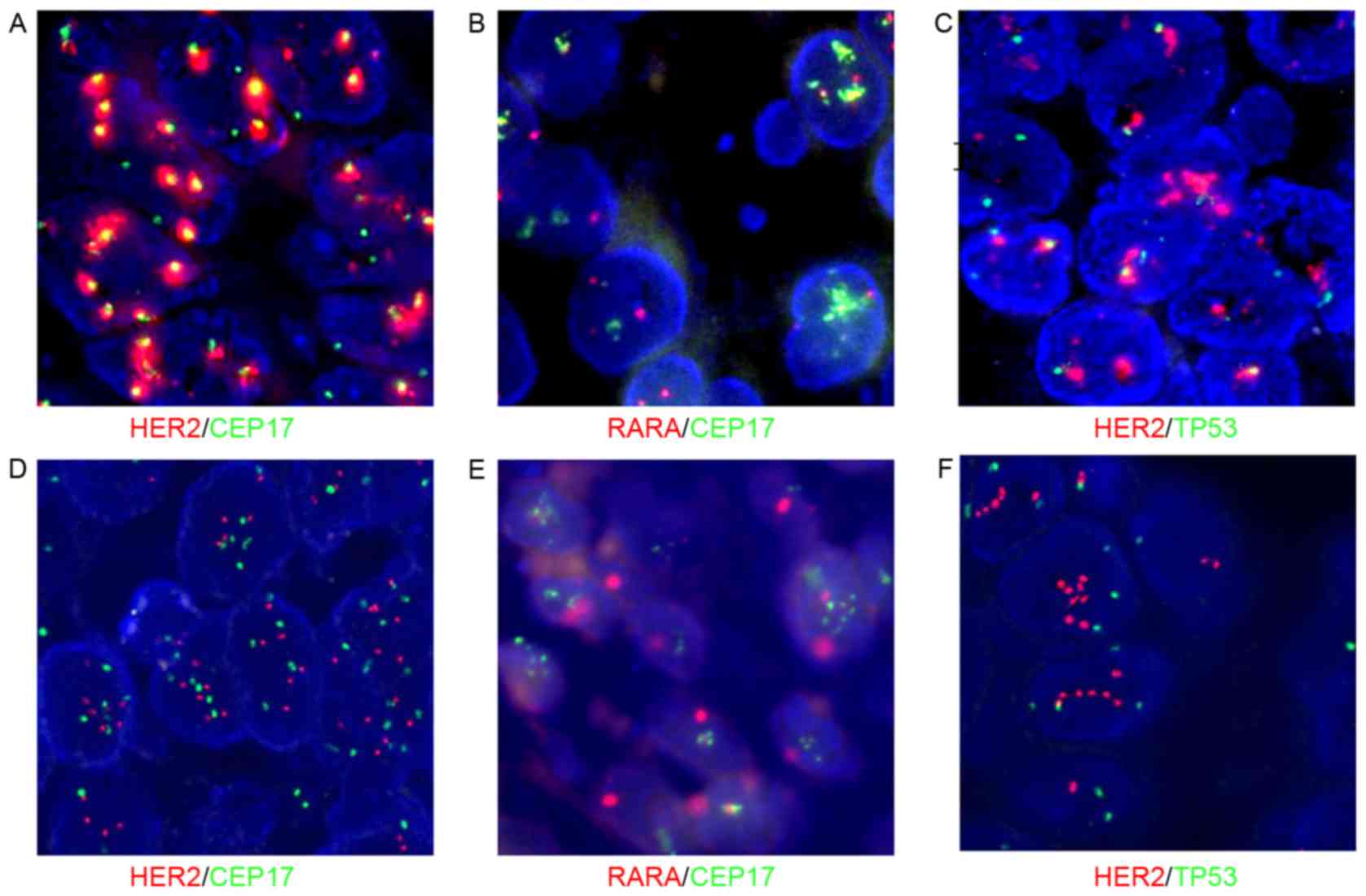
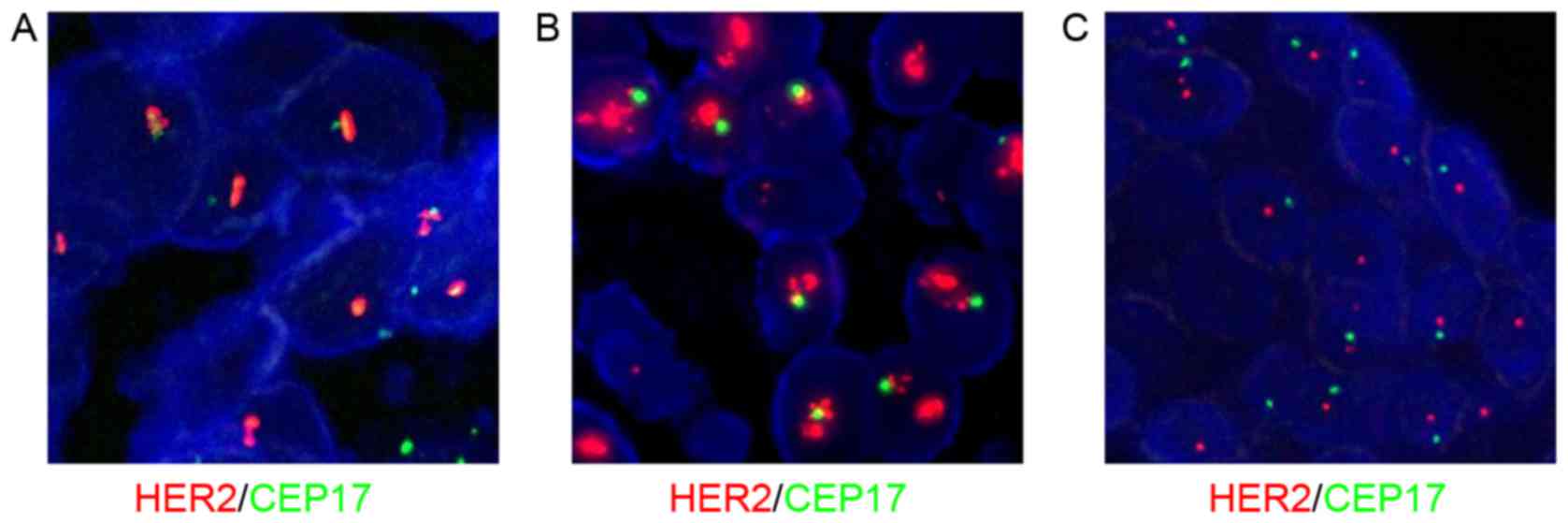
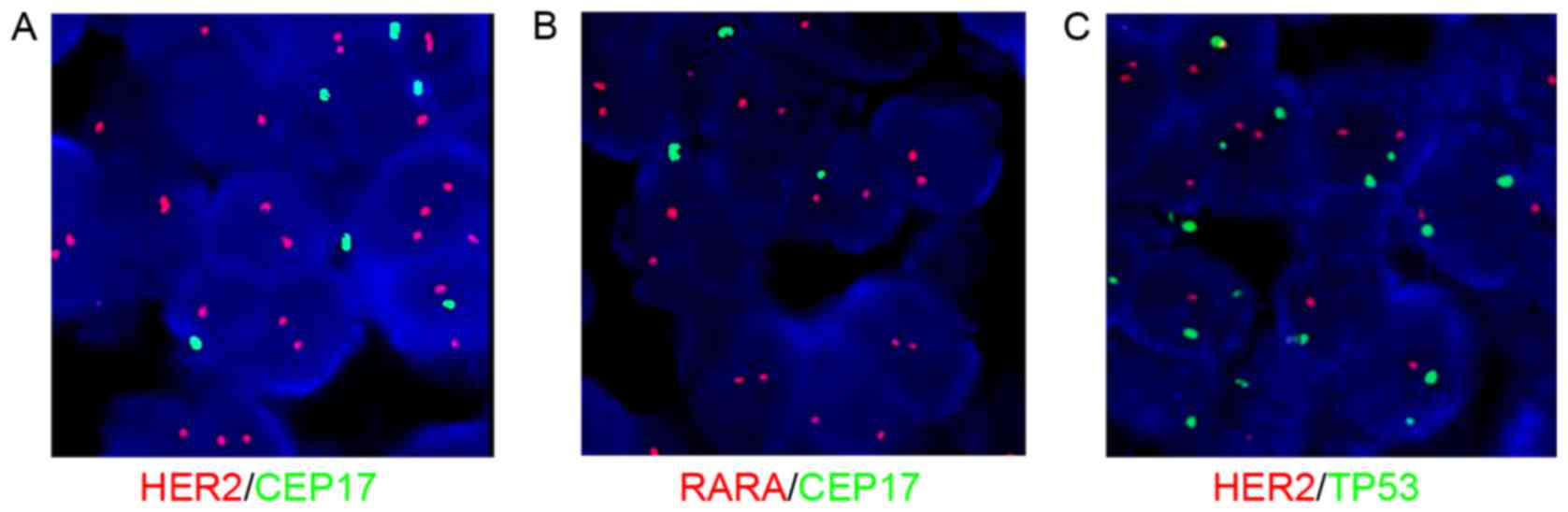
|
1
|
Slamon DJ, Clark GM, Wong SG, Levin WJ,
Ullrich A and McGuire WL: Human breast cancer: Correlation of
relapse and survival with amplification of the HER-2/neu oncogene.
Science. 235:177–182. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pauletti G, Dandekar S, Rong H, Ramos L,
Peng H, Seshadri R and Slamon DJ: Assessment of methods for
tissue-based detection of the HER-2/neu alteration in human breast
cancer: A direct comparison of fluorescence in situ hybridization
and immunohistochemistry. J Clin Oncol. 18:3651–3664. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ross JS, Fletcher JA, Bloom KJ, Linette
GP, Stec J, Clark E, Ayers M, Symmans WF, Pusztai L and Hortobagyi
GN: HER-2/neu testing in breast cancer. Am J Clin Pathol. 120
Suppl:S53–S71. 2003.PubMed/NCBI
|
|
4
|
Winston JS, Ramanaryanan J and Levine E:
HER-2/neu evaluation in breast cancer: Are we there yet? Am J Clin
Pathol. 121 (Suppl):S33–S49. 2004.PubMed/NCBI
|
|
5
|
Slamon DJ, Leyland-Jones B, Shak S, Fuchs
H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M,
et al: Use of chemo therapy plus a monoclonal antibody against HER2
for metastatic breast cancer that overexpresses HER2. N Engl J Med.
344:783–792. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vogel CL, Cobleigh MA, Tripathy D, Gutheil
JC, Harris LN, Fehrenbacher L, Slamon DJ, Murphy M, Novotny WF,
Burchmore M, et al: Efficacy and safety of trastuzumab as a single
agent in first-line treatment of HER2-overexpressing metastatic
breast cancer. J Clin Oncol. 20:719–726. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cobleigh MA, Vogel CL, Tripathy D, Robert
NJ, Scholl S, Fehrenbacher L, Wolter JM, Paton V, Shak S, Lieberman
G and Slamon DJ: Multinational study of the efficacy and safety of
humanized anti-HER2 monoclonal antibody in women who have
HER2-overexpressing metastatic breast cancer that has progressed
after chemotherapy for metastatic disease. J Clin Oncol.
17:2639–2648. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Romond EH, Perez EA, Bryant J, Suman VJ,
Geyer CE Jr, Davidson NE, Tan-Chiu E, Martino S, Paik S, Kaufman
PA, et al: Trastuzumab plus adjuvant chemotherapy for operable
HER2-positive breast cancer. N Engl J Med. 353:1673–1684. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Piccart-Gebhart MJ, Procter M,
Leyland-Jones B, Goldhirsch A, Untch M, Smith I, Gianni L, Baselga
J, Bell R, Jackisch C, et al: Trastuzumab after adjuvant
chemotherapy in HER2-positive breast cancer. N Engl J Med.
353:1659–1672. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wolff AC, Hammond ME, Schwartz JN, Hagerty
KL, Allred DC, Cote RJ, Dowsett M, Fitzgibbons PL, Hanna WM, Langer
A, et al: American Society of Clinical Oncology/College of American
Pathologists guideline recommendations for human epidermal growth
factor receptor 2 testing in breast cancer. J Clin Oncol.
25:118–145. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wolff AC, Hammond ME, Hieks DG, Dowsett M,
McShane LM, Allison KH, Allred DC, Bartlett JM, Bilous M,
Fitzgibbons P, et al: Recommendations for human epidermal growth
factor receptor 2 testing in breast cancer: American Society of
Clinical Oncology/College of American Pathologists clinical
practice guideline update. J Clin Oncol. 31:3997–4013. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Troxell ML, Bangs CD, Lawce HJ, Galperin
IB, Baiyee D, West RB, Olson SB and Cherry AM: Evaluation of
Her-2/neu status in carcinomas with amplified chromosome 17
centromere locus. Am J Clin Pathol. 126:709–716. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Varga Z, Tubbs RR, Wang Z, Sun Y, Noske A,
Kradolfer D, Bosshard G, Jochum W, Moch H and Öhlschlegel C:
Co-amplification of the HER2 gene and chromosome 17 centromere: A
potential diagnostic pitfall in HER2 testing in breast cancer.
Breast Cancer Res Treat. 132:925–935. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Press MF: How Is Her-2/neu Status
Established When Her-2/neu gene and chromosome 17 centromere are
both amplified? Am J Clin Pathol. 126:673–674. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gunn S, Yeh IT, Lytvak I, Tirtorahardjo B,
Dzidic N, Zadeh S, Kim J, McCaskill C, Lim L, Gorre M and Mohammed
M: Clinical array-based karyotyping of breast cancer with equivocal
HER2 status resolves gene copy number and reveals chromosome 17
complexity. BMC Cancer. 10:3962010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Marchiò C, Lambros MB, Gugliotta P, Di
Cantogno LV, Botta C, Pasini B, Tan DS, Mackay A, Fenwick K, Tamber
N, et al: Does chromosome 17 centromere copy number predict
polysomy in breast cancer? A fluorescence in situ hybridization and
microarray-based CGH analysis. J Pathol. 219:16–24. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tse CH, Hwang HC, Goldstein LC, Kandalaft
PL, Wiley JC, Kussick SJ and Gown AM: Determining true HER2 gene
status in breast cancers with polysomy by using alternative
chromosome 17 reference genes: Implications for Anti-HER2 targeted
therapy. J Clin Oncol. 29:4168–4174. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Egervari K, Kosa C and Szollosi Z: Impact
of chromosome 17 centromere region assessment on HER2 status
reported in breast cancer. Pathol Res Pract. 207:468–471. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zeng X, Liang ZY, Wu SF, Gao J, Zhou WX
and Liu TH: HER2 status in breast cancer of Chinese women: A study
of 1,170 cases using fluorescence in-situ hybridization. Zhonghua
Bing Li Xue Za Zhi. 37:594–598. 2008.(In Chinese). PubMed/NCBI
|
|
20
|
Orsaria M, Khelifa S, Buza N, Kamath A and
Hui P: Chromosome 17 polysomy: Correlation with histological
parameters and HER2NEU gene amplification. J Clin Pathol.
66:1070–1075. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jiang H, Bai X, Zhao T, Zhang C and Zhang
X: Fluorescence in situ hybridization of chromosome polysomy
in breast cancer using thin tissue sections causes the loss of
CEP17 and HER2 signals. Oncol Rep. 32:1889–1896. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jiang H, Bai X, Meng F, Zhang C and Zhang
X: Evaluation of chromosome 17 polysomy in breast cancer by FISH
analysis of whole nuclei, and its clinicopathological significance.
Oncol Lett. 7:1954–1958. 2014.PubMed/NCBI
|
|
23
|
Krishnamurti U, Hammers JL, Atem FD,
Storto PD and Silverman JF: Poor prognostic significance of
unamplified chromosome 17 polysomy in invasive breast carcinoma.
Mod Pathol. 22:1044–1048. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Moelans CB and van Diest PJ: CEP17 copy
number increase does not indicate polysomy 17. J Clin Pathol.
67:454–455. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hanna WM, Rüschoff J, Bilous M, Coudry RA,
Dowsett M, Osamura RY, Penault-Llorca F, van de Vijver M and Viale
G: HER2 in situ hybridization in breast cancer: Clinical
implications of polysomy 17 and genetic heterogeneity. Mod Pathol.
27:4–18. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Risio M, Casorzo L, Redana S and
Montemurro F: HER2 gene-amplified breast cancers with monosomy of
chromosome 17 are poorly responsive to trastuzumab-based treatment.
Oncol Rep. 13:305–309. 2005.PubMed/NCBI
|