Introduction
Irinotecan, a camptothecin analogue that inhibits
topoisomerase I, is a cytotoxic agent used widely for the treatment
of solid tumors, including colorectal (1,2), lung
(3), ovary (4) and gastric (5) cancer. However, treatment with this drug
frequently results in delayed-diarrhea and severe hematological
toxicities, including neutropenia and leukopenia, which can
markedly impact the course of treatment and the quality of life of
patients, and even limit its clinical application (6). Therefore, tolerable and efficient
individualized salvage treatment regimens for irinotecan-treated
cancer patients are required.
One of the isoforms of uridine diphosphate
glucuronosyl transferase (UGT), UGT1A1, is the main enzyme involved
in the metabolism of irinotecan, which converts the active
metabolite of irinotecan (SN-38) to an inactive glucuronide
(SN-38G) (7). Inter-individual
variability in the pharmacokinetics of SN-38 appears to be
associated with severe neutropenia and delayed-diarrhea (8). UGT1A1*28 [-53(TA) 6>7] leads to
decreased glucuronidation of SN-38; thus, genotyping of the
UGT1A1*28 allele may help to predict patient vulnerability to
irinotecan-associated toxicity in early studies (9,10). These
findings led the U.S. Food and Drug Administration to require that
gene-associated information is added to drug product labels in 2005
(11). However, subsequent studies
demonstrated several inconsistencies (12,13),
considering that dose-limiting neutropenia and diarrhea were
dependent on numerous known and unknown factors.
Although the UGT1A1*28 allele is considered to be an
important predictor of irinotecan-associated toxicity, differences
in ethnicity have also been reported (14,15). The
UGT1A1*28 allele frequency in Asian individuals is reduced compared
with that in Caucasian individuals, while severe hematological
toxicity is associated with polymorphisms in UGT1A1*6 in the Asian
population (16). Several previous
Asian studies revealed that severe adverse events were associated
with the homozygosity of the UGT1A1*6 allele (17–19). Thus,
the UGT1A1*6 genotype appears to be another important predictor of
irinotecan-induced adverse events. However, neither large-scale
analysis of the distribution of UGT1A1 polymorphisms, nor
standardized assessment of how UGT1A1 polymorphisms effect
irinotecan treatment has been performed in China.
To understand the clinical significance of these
variants, particularly the more frequent variant of UGT1A1*6, the
frequencies of UGT1A1 variants were examined in 2,093 Chinese
patients from 15 hospitals in Shandong province. The present study
investigated how the coexistence of UGT1A1*6 and UGT1A1*28 may be
able to predict toxicities induced by irinotecan in 105 of the
patients, and searched for other relevant risk factors.
Materials and methods
Genetic testing of patients
A total of 2,093 patients with cancer who underwent
chemotherapy with irinotecan were recruited from 15 hospitals in
Shandong, China (listed in Table I),
regardless of diseases and treatment regimens, and were tested for
the UGT1A1 genotype between May 2011 and September 2015. The median
age of the patients was 58 years (range, 24–89 years). There were
1,414 male and 679 female patients.
 | Table I.Hospitals from which patients were
recruited for the present study. |
Table I.
Hospitals from which patients were
recruited for the present study.
| Hospital name | Location | No. of
patients |
|---|
| Qilu Hospital of
Shandong University | Jinan | 189 |
| Shandong Provincial
Hospital affiliated to Shandong University | Jinan | 261 |
| Shandong Cancer
Hospital | Jinan | 259 |
| Jinan center
Hospital | Jinan | 148 |
| Qingdao Municipal
Hospital | Qingdao | 241 |
| Qingdao center
medical group | Qingdao | 195 |
| Affiliated Hospital
of Qingdao University | Qingdao | 190 |
| Affiliated Hospital
of Jining Medical University | Jining | 65 |
| Weifang people's
Hospital | Weifang | 63 |
| Yantai Yuhuangding
Hospital | Yantai | 50 |
| Linyi people's
Hospital | Linyi | 59 |
| Othersa |
| 373 |
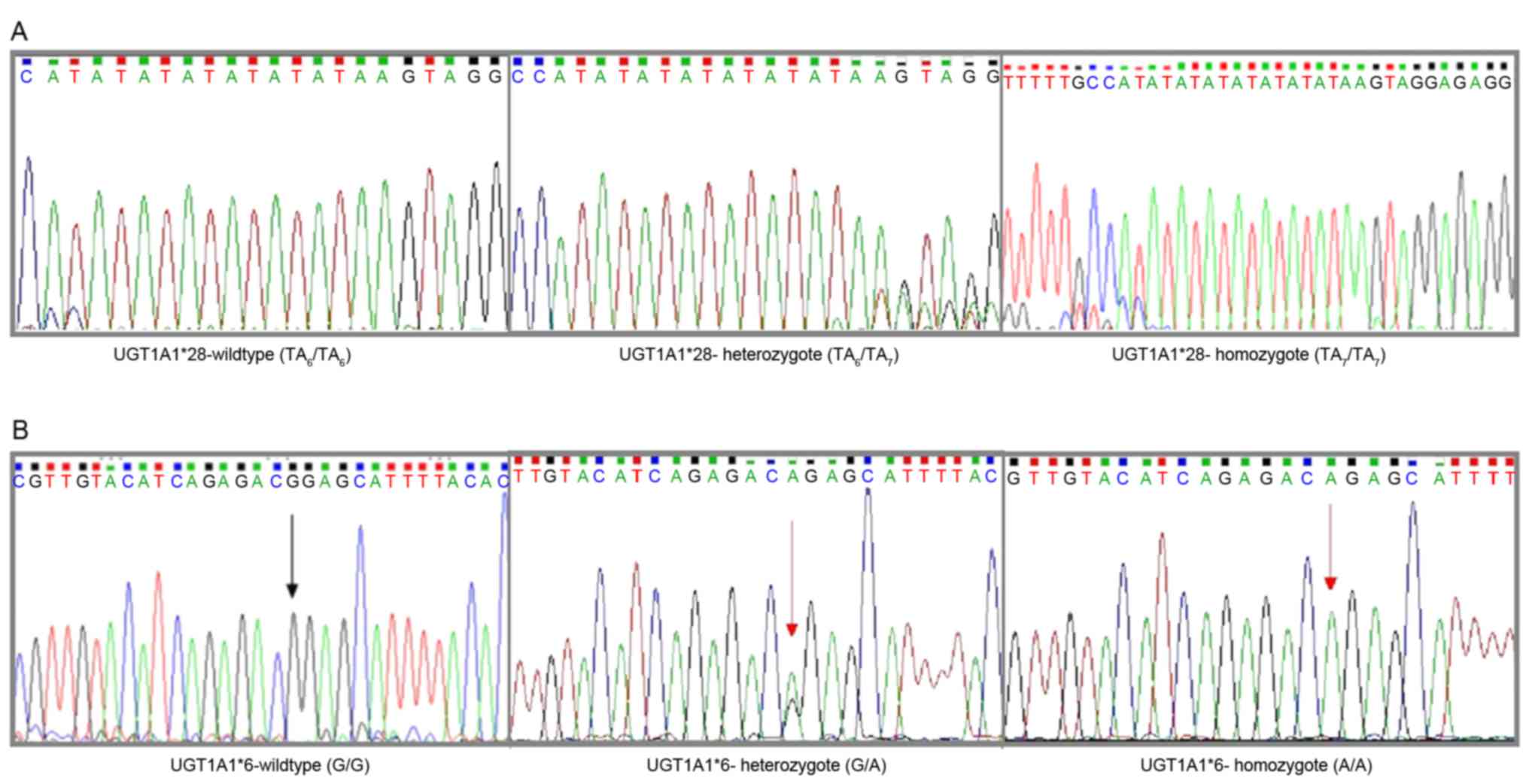
UGT1A1 genotyping assay
Genomic DNA was extracted from 2 ml of peripheral
blood using a QIAamp Blood kit (Qiagen GmbH, Hilden, Germany). One
promoter variant (TA indel) and one exon 1 variant [211G>A
(G71R)] were genotyped. The TA indel was genotyped as previously
described (20). Alleles with 6 and 7
TA repeats are reported as TAn, and genotypes are
assigned based on the number of TA repeats in each allele
(including 6/6, 6/7 and 7/7).
Genotyping for UGT1A1*6 (211G>A) was performed
using the ReverTra Ace qPCR RT kit (cat. no. FSQ-101; Toyobo Co.,
Ltd., Osaka, Japan). The RT reaction and PCR steps were performed
as previously described (21). The
primer sequences obtained from Primer Bank (https://pga.mgh.harvard.edu/primerbank/) were
5′-CTCCACCTTCTTTATCTC-3′ (forward) and 5′-GCATAGCAGAGTCCTTTT-3′
(reverse). Each experiment was repeated three times.
Toxicity studies of irinotecan
Eligibility criteria were as follows: Histologically
confirmed diagnosis of the particular tumor type for which
irinotecan is indicated; a performance status of 70–90 on the
Karnofsky Performance Status (KPS) scale (22); an age of between 22 and 78 years; a
predicted life expectancy of at least 3 months; a wash-out period
of 12 months after previous irinotecan treatment; adequate
base-line organ functions, defined as a total white blood cell
count ≥3.5×109/l, an absolute neutrophil count
≥1.5×109/l, a platelet count ≥100×109/l, a
hemoglobin level ≥90 g/l, creatinine clearance >65 ml/min
(according to the Cockcroft formula), alanine transaminase and
aspartate transaminase levels <2.0 times the upper limit of
normal and a total serum bilirubin level <1.25 times the upper
limit of normal. Exclusion criteria were as follows: Serious
infectious diseases or other severe complications, or any other
medical problems severe enough to prevent compliance with the
protocol. None of the patients were receiving drugs known to
interact with irinotecan or to affect the expression and/or
function of proteins relevant to irinotecan disposition.
The present study was approved by the Ethics
Committees of Qilu Hospital of Shandong University (Shandong,
China). Peripheral blood samples were obtained from the patients
subsequent to obtaining written informed consent.
Treatment
The present study used two different regimens in
this group of patients. Regimen A consisted of irinotecan treatment
alone (300–350 mg/m2 infused for 45 min intravenously
every 3 weeks). Regimen B consisted of irinotecan treatment with
antitumor platinum drugs (irinotecan, 250 mg/m2 iv D1;
DDP, 25 mg/m2 iv D1-D3, every 3 weeks), or with
fluorouracil and derivatives, including 5-fluorouracil (irinotecan,
180 mg/m2 iv D1; LV, 400 mg/m2 iv D1; 5-Fu,
400 mg/m2 iv bolus D1, then 1,200 mg/m2
~46–48 h, every 2 weeks), capecitabine (capecitabine 1,000
mg/m2, po, bid, D1-14; irinotecan, 250 mg/m2
iv D1, every 3 weeks) and S-1 (irinotecan, 180 mg/m2 iv
D1; S-1, 60 mg/m2po, bid, D1-14, every 3 weeks).
Patients underwent chemotherapy cycles until severe toxicity or
disease progression appeared. In addition, targeted drug delivery
may have been combined with these programs.
Pretreatment evaluation and follow
up
The pretreatment evaluation consisted of a complete
medical history, physical and imaging examinations, and a
hematological laboratory examination. Clinical toxicities,
hematological changes and physical condition were monitored prior
to each cycle of chemotherapy. The safety population included all
patients who received at least one dose of study medication and who
had at least one post-baseline safety assessment. The Common
Terminology Criteria for Adverse Events v4.0 (23) was used to evaluate adverse events.
Furthermore, imaging studies using computed tomography or magnetic
resonance imaging were performed prior to the beginning of each
following cycle, as well as for confirmation 4 weeks after the end
of chemotherapy. Clinical evaluations were performed by a
pathologist in a blinded manner with respect to the genetic
results, and clinical data were managed by the study organizer
(XIUWEN WANG, Qilu Hospital of Shandong University).
Statistical analysis
The direct counting method was used to calculate the
allele and genotype frequencies. Hardy-Weinberg equilibrium was
measured using the χ2 test. Normality of the data was
evaluated using the Shapiro-Wilk test. Measurement data were
analyzed using a Student's t-test, and the χ2 test or
Fisher's exact test was used to evaluate the association of the
UGT1A1 genotype with toxicity and other data, one-way analysis of
variance to compare more than two groups, followed by Tukey's test.
In addition, logistic regression models were used to analyze the
risk factors and interference factors. P<0.05 (two-tailed) was
considered to indicate a statistically significant difference. All
statistical analyses were performed using SPSS 17.0 (SPSS, Inc.,
Chicago, IL, USA).
Results
Distribution of UGT1A1
polymorphisms
Between May 2011 and September 2015, 2,093 and 431
(of those 2,093) patients were tested for the UGT1A1*28 and
UGT1A1*6 mutations, respectively (Fig.
1). Allele frequencies are listed in Table II. The χ2 test shows that
the two alleles are consistent with the Hardy-Weinberg equilibrium
(P=0.49 for UGT1A1*28; P=0.12 for UGT1A1*6).
 | Table II.Genotype and allele frequencies. |
Table II.
Genotype and allele frequencies.
| Polymorphism | Frequencies |
|---|
| UGT1A1*28
(−40_-39insTA) |
|
|
Genotype |
|
|
TA6/TA6 | 1601 |
|
TA6/TA7 | 463 |
|
TA7/TA7 | 29 |
|
Allele |
|
|
TA6 | 0.876 |
|
TA7 | 0.124 |
| UGT1A1*6
(211G>A) |
|
|
Genotype |
|
|
GG | 286 |
|
GA | 124 |
|
AA | 21 |
|
Allele |
|
|
G | 0.807 |
|
A | 0.193 |
Role of UGT1A1 in adverse drug
reactions (ADRs) of irinotecan and other high risk factors
Subsequent to evaluation, 105 patients treated in
the Department of Chemotherapy, Qilu Hospital of Shandong
University, were found eligible and included in the final analysis,
although for 4 patients, UGT1A1*6 status was not determined. The
baseline characteristics of the patients are summarized in Table III. There were no significant
differences in gender, age, KPS, metastatic occurrence, primary
tumor site, irinotecan dose intensity or total bilirubin
(TBIL)baseline and hemoglobin (HGB)baseline
between double wild-types and mutants (P>0.05).
 | Table III.Patient characteristics and UGT1A1
status. |
Table III.
Patient characteristics and UGT1A1
status.
| Characteristic | Total (n=103) | Wild-type
(n=53a) | Mutant (n=50) | P-value |
|---|
| Gender, n |
|
|
| 0.554 |
|
Male | 65 | 32 | 33 |
|
|
Female | 38 | 21 | 17 |
|
| Age, years (mean ±
SD) |
| 54.81±10.77 | 57.50±10.81 | 0.209 |
| KPS, n |
|
|
| 0.195 |
|
<90 | 76 | 42 | 34 |
|
|
≥90 | 27 | 11 | 16 |
|
| Metastatic, n |
|
| Miss 1b | 0.636 |
| No | 9 | 4 | 5 |
|
|
Yes | 93 | 49 | 44 |
|
| Primary tumor
sites, n |
|
|
| 0.391 |
|
Lung | 16 | 8 | 8 |
|
|
Colon | 19 | 12 | 7 |
|
|
Rectum | 21 | 7 | 14 |
|
|
Esophagus, stomach | 34 | 19 | 15 |
|
|
Others | 13 | 7 | 6 |
|
| Dose intensity
(mean ± SD) |
| 185.94±37.70 | 182.58±40.00 | 0.661 |
|
TBILbaseline, µmol/l (mean ±
SD) |
| 11.64±8.86 | 13.50±16.78 | 0.478 |
|
HGBbaseline, g/l (mean ±
SD) |
| 118.78±17.78 | 124.02±21.19 | 0.177 |
ADRs
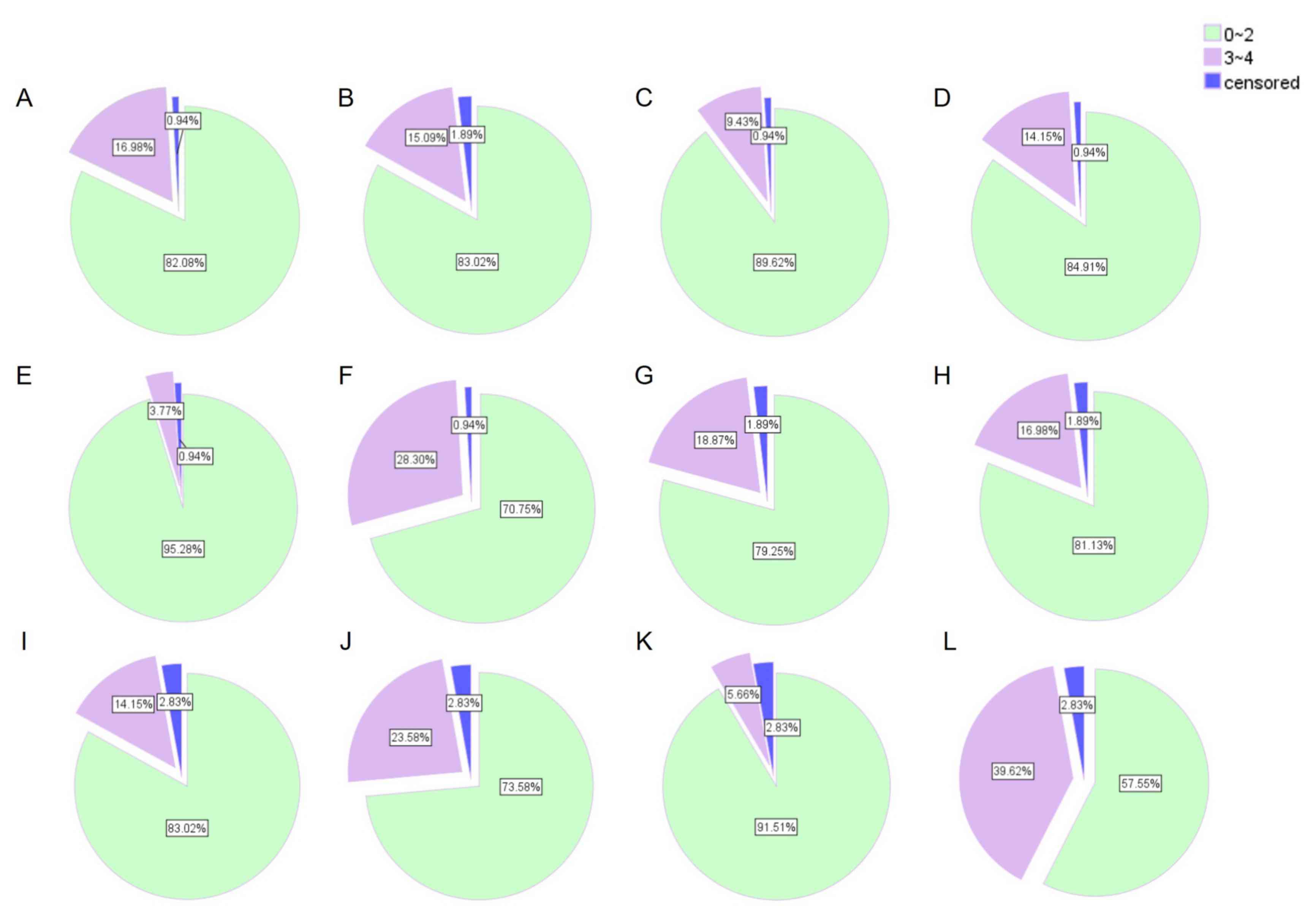
Tolerance to treatment was evaluated at the first
cycle (acute toxicity) and at the end of therapy (cumulative
toxicity) (Fig. 2). Severe acute
toxicity (3–4) of any type was observed in 30 of 105
patients (28.57%), and in 42 of 105 patients (40%) during the
entire course of chemotherapy. The most frequent severe
hematological toxicity was neutropenia, and the predominant
non-hematological toxicities were diarrhea and cholinergic
syndrome.
Association between genotypes and
ADRs
Table IV compares
toxic effects between the TA6/TA6 group and
TA6/TA7 group. The results showed that the
UGT1A1*28 allele was often associated with severe hematological
toxicity, particularly during the first cycle. The frequency of
grade 3–4 leukopenia and neutropenia in the
TA6/TA7 group was higher compared with the
TA6/TA6 group (P<0.05).
 | Table IV.Association between uridine
diphosphate glucuronosyltransferase 1A*28 and irinotecan ADR. |
Table IV.
Association between uridine
diphosphate glucuronosyltransferase 1A*28 and irinotecan ADR.
|
| After the first
cycle | During the entire
course of chemotherapy |
|---|
|
|
|
|
|---|
|
| Grade 3–4, n |
| Grade 1–4, n |
| Grade 3–4, n |
| Grade 1–4, n |
|
|---|
|
|
|
|
|
|
|
|
|
|
|---|
| Adverse event |
TA6/TA6 |
TA6/TA7 | P-value |
TA6/TA6 |
TA6/TA7 | P-value |
TA6/TA6 |
TA6/TA7 | P-value |
TA6/TA6 |
TA6/TA7 | P-value |
|---|
| Total | 86 | 19 |
| 56 | 12 |
| 86 | 19 |
| 56 | 12 |
|
| Hematological |
|
|
|
|
|
|
|
|
|
|
|
|
|
Neutropenia | 9 | 6 | 0.028a | 31 | 7 | 0.948 | 18 | 7 | 0.157 | 50 | 10 | 0.917 |
|
Leukopenia | 4 | 6 | 0.002a | 33 | 8 | 0.763 | 8 | 7 | 0.006a | 50 | 11 | 0.896 |
|
Hemoglobin reduction | 4 | 0 | 1.000 | 27 | 6 | 0.998 | 6 | 0 | 0.590 | 40 | 8 | 0.663 |
|
Non-hematological |
|
|
|
|
|
|
|
|
|
|
|
|
|
Diarrhea | 16 | 2 | 0.517 | 43 | 8 | 0.553 | 17 | 3 | 1.000 | 47 | 9 | 1.000 |
|
Cholinergic syndrome |
|
|
| 13 | 3 | 1.000 |
|
|
| 14 | 4 | 0.738 |
| Total
ADR | 23 | 7 | 0.378 | 65 | 14 | 1.000 | 34 | 8 | 0.896 | 74 | 16 | 0.703 |
Considering the low frequency of genotype A/A, the
A/A and A/G genotypes were designated as the mutant type for
UGT1A1*6 to study the association between UGT1A1*6 and irinotecan
ADRs. It was found that the patients with the mutant type were more
susceptible compared with wild-type patients to severe diarrhea and
total ADR (P=0.030 and P=0.072, respectively, in the first cycle;
P=0.043 and P=0.038, respectively, during the entire course of
chemotherapy). The frequency of grade 3–4 neutropenia in the mutant
group was higher than that in the wild-type group (P=0.003). In
addition, the risk of grade 1–4 leukopenia, neutropenia and
diarrhea was higher in the UGT1A1*6 carriers compared with
wild-type patients (P<0.05; Table
V).
 | Table V.Association between uridine
diphosphate glucuronosyltransferase 1A*6 and irinotecan ADR. |
Table V.
Association between uridine
diphosphate glucuronosyltransferase 1A*6 and irinotecan ADR.
|
| After the first
cycle | During the entire
course of chemotherapy |
|---|
|
|
|
|
|---|
|
| Grade 3–4, n |
| Grade 1–4, n |
| Grade 3–4, n |
| Grade 1–4, n |
|
|---|
|
|
|
|
|
|
|
|
|
|
|---|
| Adverse event | G/G | G/A or A/A | P-value | G/G | G/A or A/A | P-value | G/G | G/A or A/A | P-value | G/G | G/A or A/A | P-value |
|---|
| Total | 67 | 34 |
| 67 | 34 |
| 67 | 34 |
| 67 | 34 |
|
| Hematological |
|
|
|
|
|
|
|
|
|
|
|
|
|
Neutropenia | 9 | 6 | 0.574 | 19 | 19 | 0.007a | 11 | 14 | 0.003a | 34 | 25 | 0.009a |
|
Leukopenia | 5 | 5 | 0.298 | 20 | 21 | 0.002a | 8 | 7 | 0.235 | 33 | 27 | 0.001a |
|
Hemoglobin reduce | 2 | 2 | 0.601 | 21 | 11 | 0.918 | 4 | 2 | 1.000 | 29 | 17 | 0.358 |
|
Non-hematological |
|
|
|
|
|
|
|
|
|
|
|
|
|
Diarrhea | 8 | 10 | 0.030a | 28 | 23 | 0.014a | 9 | 10 | 0.043a | 32 | 23 | 0.038a |
|
Cholinergic syndrome |
|
|
| 10 | 6 | 0.676 |
|
|
| 12 | 6 | 0.974 |
| Total ADR | 16 | 14 | 0.072 | 46 | 32 | 0.004a | 23 | 18 | 0.038a | 55 | 31 | 0.042a |
TBIL and genotype
In the association between TBIL level and UGT1A1
genotype, neither TBILbaseline nor TBILmax
showed any significant difference between groups (P>0.05). In
addition, the TBIL level in the patients did not change
significantly following treatment when analyzing each genotype
separately (P>0.05; data not shown).
Univariate and multivariate analysis
of high-risk variables in irinotecan toxicity
To identify the variables of potential predictive
significance in irinotecan toxicity, univariate and multivariate
analyses were performed using the logistic regression model to
compare the impact of genotypes and other clinical pathological
parameters on the prediction of irinotecan toxicity. Univariate
analysis showed that UGT1A1*28, UGT1A1*6, UGT1A1 status, irinotecan
dose intensity, occurrence of metastasis, HGBbaseline
and KPS were significant high risk factors for diarrhea,
leucopenia, neutropenia, reduced hemoglobin and ADR (Table VI). Multivariate analysis determined
that: i) UGT1A1*6 genotype status and total ADR were independent
predictors of severe diarrhea (P<0.05); ii) patients who carry
the UGT1A1*28 mutant genotype were more susceptible to severe
leucopenia in the first cycle, but the difference was not
statistically significant (P>0.05); iii) the hazard ratio of
severe neutropenia in patients with UGT1A1 mutations was 5.859
times that of wild-type patients; iv) with increasing number of
metastases, the incidence of severe neutropenia and ADRs was also
increased; v) the relative risk in patients with a KPS <90 to
suffer adverse reactions in cycle 1 was 2.837 times greater than
that of patients with a KPS ≥90; vi) irinotecan dose intensity and
HGBbaseline were independent high-risk factors for
leucopenia and reduced hemoglobin, separately (Table VII).
 | Table VI.Univariate logistic regression
analysis for ADRs. |
Table VI.
Univariate logistic regression
analysis for ADRs.
| Factors | Diarrhea | Leucopenia
Cycle1 | Leucopenia | Neutropenia
Cycle1 | Neutropenia | Hemoglobin
reduction | ADR Cycle1 | ADR |
|---|
| UGT1A1*28 |
|
|
|
|
|
|
|
|
| P-value |
| 0.002 | 0.006 | 0.028 |
|
|
|
|
| UGT1A1*6 |
|
|
|
|
|
|
|
|
| P-value | 0.043 |
|
|
| 0.004 |
|
| 0.05 |
| UGT1A1 status |
|
|
|
|
|
|
|
|
| P-value |
| 0.007 | 0.010 |
| 0.001 |
|
|
|
| Dose intensity |
|
|
|
|
|
|
|
|
| P-value |
| 0.008 | 0.033 | 0.042 |
|
|
|
|
| No. of
metastases |
|
|
|
|
|
|
|
|
| P-value |
|
|
|
| 0.003 |
| 0.003 | 0.008 |
|
HGBbaseline |
|
|
|
|
|
|
|
|
| P-value |
|
|
|
|
| 0.000 |
|
|
| KPS |
|
|
|
|
|
|
|
|
| P-value |
|
|
|
|
|
| 0.048 |
|
 | Table VII.High-risk factor logistic regression
analysis. |
Table VII.
High-risk factor logistic regression
analysis.
| Factors | Diarrhea | Leucopenia Cycle
1 | Leucopenia | Neutropenia Cycle
1 | Neutropenia | Hemoglobin
reduction | ADR Cycle 1 | ADR |
|---|
| UGT1A1*28 |
|
|
|
|
|
|
|
|
|
P-value |
| 0.063 |
|
|
|
|
|
|
|
Exp(B) |
| 4.921 |
|
|
|
|
|
|
| 95%
CI |
| 0.916–26.446 |
|
|
|
|
|
|
| UGT1A1*6 |
|
|
|
|
|
|
|
|
|
P-value | 0.048a |
|
|
|
|
|
| 0.045a |
|
Exp(B) | 2.802 |
|
|
|
|
|
| 2.532 |
| 95%
CI | 1.008–7.785 |
|
|
|
|
|
| 1.022–6.276 |
| UGT1A1 status |
|
|
|
|
|
|
|
|
|
P-value |
|
|
|
| 0.018a |
|
|
|
|
Exp(B) |
|
|
|
| 5.859 |
|
|
|
| 95%
CI |
|
|
|
| 1.351–25.421 |
|
|
|
| Dose intensity |
|
|
|
|
|
|
|
|
|
P-value |
| 0.010a | 0.034a | 0.044a |
|
|
|
|
|
Exp(B) |
| 1.025 | 1.016 | 1.014 |
|
|
|
|
| 95%
CI |
| 1.006–1.044 | 1.001–1.032 | 1.000–1.029 |
|
|
|
|
| No. of
metastases |
|
|
|
|
|
|
|
|
|
P-value |
|
|
|
| 0.010a |
| 0.010a | 0.014a |
|
Exp(B) |
|
|
|
| 1.568 |
| 1.464 | 1.449 |
| 95%
CI |
|
|
|
| 1.112–2.212 |
| 1.096–1.956 | 1.078–1.947 |
|
HGBbaseline |
|
|
|
|
|
|
|
|
|
P-value |
|
|
|
|
| 0.003a |
|
|
|
Exp(B) |
|
|
|
|
| 0.902 |
|
|
| 95%
CI |
|
|
|
|
| 0.844–0.965 |
|
|
| KPS |
|
|
|
|
|
|
|
|
|
P-value |
|
|
|
|
|
| 0.035a |
|
|
Exp(B) |
|
|
|
|
|
| 2.837 |
|
| 95%
CI |
|
|
|
|
|
| 1.076–7.478 |
|
Discussion
The genetic association with irinotecan-related
toxicity apparently differs among distinct ethnic populations
(24). There is a considerable
difference in UGT1A1 genetic polymorphisms among genetically
distinct populations; the allele frequency of UGT1A1*28 is higher
in Caucasian and African individuals (0.12–0.27) (25,26)
compared with Asian individuals (~0.12) (27). The UGT1A1*6 allele has been identified
only in the Asian population (0.13–0.15 for UGT1A1*6) (16). To the best of our knowledge, the
present study is the first large-scale study to evaluate the
distribution of the UGT1A1 polymorphism in Shandong Province,
China. The distribution of UGT1A1 genotypes in the present study
was comparable to that of previous studies (16,26–27).
The role of the UGT1A1*28 allele with regard to the
toxicity of irinotecan varies greatly between Asian and Caucasian
individuals (16). In previous
studies, the UGT1A1*28 homozygote has been suggested to associate
with neutropenia only in Caucasian individuals (28–31). A
meta-analysis by Hoskins et al (24) identified that for irinotecan-induced
severe neutropenia, the predictive role of the UGT1A1*28 genotype
increased with an increasing dose of irinotecan. Another
meta-analysis (32) showed an
association between the UGT1A1*28/*28 genotype and an increased
risk of neutropenia, at low doses, as well as at medium or high
doses of irinotecan. However, a more contentious issue is whether
the UGT1A1*28 gene polymorphism can predict severe diarrhea.
Marcuello et al (33) found a
marked association between severe diarrhea (P=0.005) and asthenia
(P=0.03), and patients with the heterozygous and homozygous
UGT1A1*28 genotypes. In a meta-analysis, patients with a
UGT1A1*28/*28 genotype exhibited a higher risk of severe diarrhea
at medium and high irinotecan doses (34). However, Stewart et al (31) and the FOCUS trial (35) challenged the aforementioned
conclusions, supporting that UGT1A1 genotyping is not a useful
prognostic indicator of severe toxicity for patients treated with
this irinotecan dosage and schedule. The present study found a
significant association between the UGT1A1*28 genotype groups and
grade 3–4 hematological toxicity, but not diarrhea, however, this
was not confirmed by logistic analysis.
In view of the distribution of the UGT1A1*6 allele
in different ethnicities, a previous study by Okuyama et al
(36), which focused on Asian
individuals, found that homozygosity for UGT1A1*28 or UGT1A1*6 and
double heterozygosity for UGT1A1*28 and UGT1A1*6 were significantly
associated with severe neutropenia (P<0.001). Sunakawa et
al (37) did not observe any
toxic effects that were associated with the UGT1A1*1/*6 or
UGT1A1*1/*28 genotypes. In the present study, it was found that the
incidence of grade 3–4 diarrhea in patients with mutations (A/A and
G/A) was much higher than that in wild-type (G/G) patients. Severe
diarrhea and ADR was associated with the UGT1A1*6 genotype in a
multiple logistic regression analysis, which also supported this
conclusion.
Marcuello et al (33) found that differences in the mean
levels of bilirubin among the three genotypes were significant pre-
and post-chemotherapy. Bilirubin levels increased significantly
when chemotherapy was initiated in TA6/6 and
TA6/7 patients. Stewart et al (31) showed that patients with
TA7/7 genotype had a statistically greater baseline TBIL
compared with patients with the TA6/6 or
TA6/7 genotype. Baseline bilirubin level has also been
reported to be associated with severe neutropenia (12,38). In
the present study, neither TBILbaseline nor
TBILafter max showed any significant difference between
groups. In addition, the TBIL level in patients did not change
significantly following treatment when analyzing each genotype
separately.
Irinotecan-based genomic studies are no longer
restricted to UGT1A1, but also to other genes involved throughout
the irinotecan-based metabolic process (28,39–41).
Glimelius et al (28)
suggested that the ATP-binding cassette sub-family B member 1 gene
polymorphism (P-glycoprotein) can predict early adverse reactions.
The ATP-binding cassette subfamily C member 2 (multi-drug
resistance protein 2) gene polymorphism was found to be associated
with severe diarrhea (39,40). In addition, UGT1A7*3/*3 is also known
to be associated with early severe neutropenia (4,21).
However, it remains unclear whether the UGT1A1 gene polymorphism
can be used as a predictor in irinotecan-based toxicity, and the
present results provide a novel platform for directing this
research.
The present study has certain limitations, including
the fact that it is hypothesis generated, due to the retrospective
design, and that a relatively small study group was used. Our
hypothesis should be confirmed by prospective studies involving
larger numbers of patients being performed, and these studies
should be aimed at determining whether genotype-adjusted irinotecan
dosages could assist in establishing a well-tolerated dose, as well
as an effective dose for the tumor response in patients with
different genotypes.
In summary, the present data indicated that the
UGT1A1*28 and UGT1A1*6 genotypes were significantly associated with
severe toxicity, which is an additional supplement to previous
studies. Common toxicities can be managed during chemotherapy.
However, the clinical implication appears to be marginal. Together,
the results of the present and previous studies suggest that
genetic testing for the UGT1A1*6 polymorphism may be of use to
predict toxicity in patients with cancer who receive
irinotecan.
Acknowledgements
The present study was supported by the Wu Jieping
Medical Foundation (grant no. 320.6700.1148) and the National
Natural Fund Project (grant no. 81372530).
References
|
1
|
Douillard JY, Cunningham D, Roth AD,
Navarro M, James RD, Karasek P, Jandik P, Iveson T, Carmichael J,
Alakl M, et al: Irinotecan combined with fluorouracil compared with
fluorouracil alone as first-line treatment for metastatic
colorectal cancer: A multicentre randomised trial. Lancet.
355:1041–1047. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tournigand C, André T, Achille E, Lledo G,
Flesh M, Mery-Mignard D, Quinaux E, Couteau C, Buyse M, Ganem G, et
al: FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced
colorectal cancer: A randomized GERCOR study. J Clin Oncol.
22:229–237. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Akie K, Oizumi S, Ogura S, Shinagawa N,
Kikuchi E, Fukumoto S, Harada M, Kinoshita I, Kojima T, Harada T,
et al: Phase II study of irinotecan plus S-1 combination for
previously untreated advanced non-small cell lung cancer: Hokkaido
Lung Cancer Clinical Study Group Trial (HOT) 0601. Oncology.
81:84–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Takakura S, Takano M, Takahashi F, Saito
T, Aoki D, Inaba N, Noda K, Sugiyama T and Ochiai K: Japanese
Gynecologic Oncology Group: Randomized phase II trial of paclitaxel
plus carboplatin therapy versus irinotecan plus cisplatin therapy
as first-line chemotherapy for clear cell adenocarcinoma of the
ovary: A JGOG study. Int J Gynecol Cancer. 20:240–247. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Luo HY, Wang ZQ, Wang FH, Qiu MZ, Teng KY,
Ruan DY, He YJ, Li YH and Xu RH: Phase 2 study of capecitabine and
irinotecan combination chemotherapy (modified XELIRI regimen) in
patients with advanced gastric cancer. Am J Clin Oncol. 34:555–560.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Glimelius B: Benefit-risk assessment of
irinotecan in advanced colorectal cancer. Drug Saf. 28:417–433.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Smith NF, Figg WD and Sparreboom A:
Pharmacogenetics of irinotecan metabolism and transport: An update.
Toxicol In Vitro. 20:163–175. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mathijssen RH, van Alphen RJ, Verweij J,
Loos WJ, Nooter K, Stoter G and Sparreboom A: Clinical
pharmacokinetics and metabolism of irinotecan (CPT-11). Clin Cancer
Res. 7:2182–2194. 2001.PubMed/NCBI
|
|
9
|
Iyer L, Das S, Janisch L, Wen M, Ramírez
J, Karrison T, Fleming GF, Vokes EE, Schilsky RL and Ratain MJ:
UGT1A1*28 polymorphism as a determinant of irinotecan disposition
and toxicity. Pharmacogenomics J. 2:43–47. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Innocenti F and Ratain MJ: ‘Irinogenetics’
and UGT1A: From genotypes to haplotypes. Clin Pharmacol Ther.
75:495–500. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Perera MA, Innocenti F and Ratain MJ:
Pharmacogenetic testing for uridine diphosphate
glucuronosyltransferase 1A1 polymorphisms: Are we there yet?
Pharmacotherapy. 28:755–768. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ramchandani RP, Wang Y, Booth BP, Ibrahim
A, Johnson JR, Rahman A, Mehta M, Innocenti F, Ratain MJ and
Gobburu JV: The role of SN-38 exposure, UGT1A1*28 polymorphism and
baseline bilirubin level in predicting severe irinotecan toxicity.
J Clin Pharmacol. 47:78–86. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Deeken JF, Slack R and Marshall JL:
Irinotecan and uridine diphosphate glucuronosyltransferase 1A1
pharmacogenetics: To test or not to test, that is the question.
Cancer. 113:1502–1510. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ando Y, Saka H, Ando M, Sawa T, Muro K,
Ueoka H, Yokoyama A, Saitoh S, Shimokata K and Hasegawa Y:
Polymorphisms of UDP-glucuronosyltransferase gene and irinotecan
toxicity: A pharmacogenetic analysis. Cancer Res. 60:6921–6926.
2000.PubMed/NCBI
|
|
15
|
Han JY, Lim HS, Shin ES, Yoo YK, Park YH,
Lee JE, Jang IJ, Lee DH and Lee JS: Comprehensive analysis of UGT1A
polymorphisms predictive for pharmacokinetics and treatment outcome
in patients with non-small-cell lung cancer treated with irinotecan
and cisplatin. J Clin Oncol. 24:2237–2244. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jada SR, Lim R, Wong CI, Shu X, Lee SC,
Zhou Q, Goh BC and Chowbay B: Role of UGT1A1*6, UGT1A1*28 and ABCG2
c.421C>A polymorphisms in irinotecan-induced neutropenia in
Asian cancer patients. Cancer Sci. 98:1461–1467. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Onoue M, Terada T, Kobayashi M, Katsura T,
Matsumoto S, Yanagihara K, Nishimura T, Kanai M, Teramukai S,
Shimizu A, et al: UGT1A1*6 polymorphism is most predictive of
severe neutropenia induced by irinotecan in Japanese cancer
patients. Int J Clin Oncol. 14:136–142. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yamamoto N, Takahashi T, Kunikane H,
Masuda N, Eguchi K, Shibuya M, Takeda Y, Isobe H, Ogura T, Yokoyama
A and Watanabe K: Phase I/II pharmacokinetic and pharmacogenomic
study of UGT1A1 polymorphism in elderly patients with advanced
non-small cell lung cancer treated with irinotecan. Clin Pharmacol
Ther. 85:149–154. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lu YY, Huang XE, Wu XY, Cao J, Liu J, Wang
L and Xiang J: Clinical observations on associations between the
UGT1A1 genotype and severe toxicity of irinotecan. Asian Pac J
Cancer Prev. 15:3335–3341. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Te HS, Schiano TD, Das S, Kuan SF,
DasGupta K, Conjeevaram HS and Baker AL: Donor liver uridine
diphosphate (UDP)-glucuronosyltransferase-1A1 deficiency causing
Gilbert's syndrome in liver transplant recipients. Transplantation.
69:1882–1886. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang Y, Shen L, Xu N, Wang JW, Jiao SC,
Liu ZY and Xu JM: UGT1A1 predicts outcome in colorectal cancer
treated with irinotecan and fluorouracil. World J Gastroenterol.
18:6635–6644. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Friendlander AH and Ettinger RL: Karnofsky
performance status scale. Spec Care Dentist. 29:147–148. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
CTCAE, . Cancer Therapy Evaluation
Program: Common Terminology Criteria for Adverse Events. Version
4.0. DCTD, NCI, NIH, DHHS. 2010.
|
|
24
|
Hoskins JM, Goldberg RM, Qu P, Ibrahim JG
and McLeod HL: UGT1A1*28 genotype and irinotecan-induced
neutropenia: Dose matters. J Natl Cancer Inst. 99:1290–1295. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yong WP, Innocenti F and Ratain MJ: The
role of pharmacogenetics in cancer therapeutics. Br J Clin
Pharmacol. 62:35–46. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Premawardhena A, Fisher CA, Liu YT, Verma
IC, de Silva S, Arambepola M, Clegg JB and Weatherall DJ: The
global distribution of length polymorphisms of the promoters of the
glucuronosyltransferase 1 gene (UGT1A1): Hematologic and
evolutionary implications. Blood Cells Mol Dis. 31:98–101. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang A, Xing Q, Qin S, Du J, Wang L, Yu
L, Li X, Xu L, Xu M, Feng G and He L: Intra-ethnic differences in
genetic variants of the UGT-glucuronosyltransferase 1A1 gene in
Chinese populations. Pharmacogenomics J. 7:333–338. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Glimelius B, Garmo H, Berglund A,
Fredriksson LA, Berglund M, Kohnke H, Byström P, Sørbye H and
Wadelius M: Prediction of irinotecan and 5-fluorouracil toxicity
and response in patients with advanced colorectal cancer.
Pharmacogenomics J. 11:61–71. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
McLeod HL, Sargent DJ, Marsh S, Green EM,
King CR, Fuchs CS, Ramanathan RK, Williamson SK, Findlay BP,
Thibodeau SN, et al: Pharmacogenetic predictors of adverse events
and response to chemotherapy in metastatic colorectal cancer:
Results from North American Gastrointestinal Intergroup Trial
N9741. J Clin Oncol. 28:3227–3233. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shulman K, Cohen I, Barnett-Griness O,
Kuten A, Gruber SB, Lejbkowicz F and Rennert G: Clinical
implications of UGT1A1*28 genotype testing in colorectal cancer
patients. Cancer. 117:3156–3162. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Stewart CF, Panetta JC, O'Shaughnessy MA,
Throm SL, Fraga CH, Owens T, Liu T, Billups C, Rodriguez-Galindo C,
Gajjar A, et al: UGT1A1 promoter genotype correlates with SN-38
pharmacokinetics, but not severe toxicity in patients receiving
low-dose irinotecan. J Clin Oncol. 25:2594–2600. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hu ZY, Yu Q, Pei Q and Guo C:
Dose-dependent association between UGT1A1*28 genotype and
irinotecan-induced neutropenia: Low doses also increase risk. Clin
Cancer Res. 16:3832–3842. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Marcuello E, Altés A, Menoyo A, Del Rio E,
Gómez-Pardo M and Baiget M: UGT1A1 gene variations and irinotecan
treatment in patients with metastatic colorectal cancer. Br J
Cancer. 91:678–682. 2004.PubMed/NCBI
|
|
34
|
Hu ZY, Yu Q and Zhao YS: Dose-dependent
association between UGT1A1*28 polymorphism and irinotecan-induced
diarrhoea: A meta-analysis. Eur J Cancer. 46:1856–1865. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Braun MS, Richman SD, Thompson L, Daly CL,
Meade AM, Adlard JW, Allan JM, Parmar MK, Quirke P and Seymour MT:
Association of molecular markers with toxicity outcomes in a
randomized trial of chemotherapy for advanced colorectal cancer:
The FOCUS trial. J Clin Oncol. 27:5519–5528. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Okuyama Y, Hazama S, Nozawa H, Kobayashi
M, Takahashi K, Fujikawa K, Kato T, Nagata N, Kimura H, Oba K, et
al: Prospective phase II study of FOLFIRI for mCRC in Japan,
including the analysis of UGT1A1 28/6 polymorphisms. Jpn J Clin
Oncol. 41:477–482. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sunakawa Y, Ichikawa W, Fujita K,
Nagashima F, Ishida H, Yamashita K, Mizuno K, Miwa K, Kawara K,
Akiyama Y, et al: UGT1A1*1/*28 and *1/*6 genotypes have no effects
on the efficacy and toxicity of FOLFIRI in Japanese patients with
advanced colorectal cancer. Cancer Chemother Pharmacol. 68:279–284.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Meyerhardt JA, Kwok A, Ratain MJ, McGovren
JP and Fuchs CS: Relationship of baseline serum bilirubin to
efficacy and toxicity of single-agent irinotecan in patients with
metastatic colorectal cancer. J Clin Oncol. 22:1439–1446. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Han JY, Lim HS, Shin ES, Yoo YK, Park YH,
Lee JE, Kim HT and Lee JS: Influence of the organic
anion-transporting polypeptide 1B1 (OATP1B1) polymorphisms on
irinotecan-pharmacokinetics and clinical outcome of patients with
advanced non-small cell lung cancer. Lung Cancer. 59:69–75. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
de Jong FA, Scott-Horton TJ, Kroetz DL,
McLeod HL, Friberg LE, Mathijssen RH, Verweij J, Marsh S and
Sparreboom A: Irinotecan-induced diarrhea: Functional significance
of the polymorphic ABCC2 transporter protein. Clin Pharmacol Ther.
81:42–49. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lankisch TO, Schulz C, Zwingers T,
Erichsen TJ, Manns MP, Heinemann V and Strassburg CP: Gilbert's
Syndrome and irinotecan toxicity: Combination with
UDP-glucuronosyltransferase 1A7 variants increases risk. Cancer
Epidemiol Biomarkers Prev. 17:695–701. 2008. View Article : Google Scholar : PubMed/NCBI
|