Introduction
Hepatocellular carcinoma (HCC) is one of the most
common types of malignancy and the third leading cause of
cancer-associated mortality globally (1). In China, the incidence of HCC is among
the highest worldwide, with Chinese patients accounting for 55% of
new HCC cases. Chronic infection with hepatitis B virus (HBV) and
hepatitis C virus (HCV) is the major etiology of HCC, which often
arises from a background of viral hepatitis and cirrhosis (2). Lacking specific symptoms at early
stages, the majority of patients with HCC are diagnosed at advanced
stages, with a low 5-year survival rate of 10.1% (3) and a high 5-year recurrence rate of 70%,
even following liver resection (4).
Therefore, early diagnosis, early treatment and early recurrence
detection are extremely important in improving the prognosis of
HCC.
The most common methods used in the early detection
of HCC are analysis of α-fetoprotein (AFP) level and hepatic
ultrasonography. As the most widely used serum biomarker, AFP has a
high specificity of 80–94%; however, its sensitivity is only 41–65%
(5), making it an unsatisfactory
biomarker for the early detection of HCC. To improve the efficiency
of early diagnosis of HCC, novel candidate serum biomarkers have
emerged, including Lens culinaris agglutinin reactive AFP
(6), Des-γ-carboxy prothrombin
(7), α-l-fucosidase (8), glypican-3 (9), squamous cell carcinoma antigen (10), and Golgi protein 73 (GP73) (11). Of these potential HCC serum markers,
GP73 is the most promising.
GP73, a resident type II Golgi membrane protein with
a molecular weight of 73 kDa, is predominantly expressed by biliary
epithelial cells in the normal liver. Aberrant expression of GP73
in hepatocytes is present in chronic liver disease, including viral
and non-viral hepatitis, liver cirrhosis and HCC (12–14).
Additionally, gradually increasing GP73 expression levels in tissue
have been observed throughout the progression from normal liver and
chronic hepatitis to HCC (15).
Importantly, the C-terminal ectodomain of GP73 can be released into
the circulation (16). Since Block
et al (17) first identified
elevated serum GP73 (sGP73) levels in patients with HCC in 2005,
sGP73 has been considered to be a potential biomarker for HCC.
A number of studies have evaluated the early
diagnostic value of sGP73 for HCC. Several studies in which sGP73
was detected using immunoblotting demonstrated that sGP73 levels
were significantly increased in HCC compared with cirrhotic
controls, with a superior sensitivity and specificity to AFP for
HCC diagnosis (11,18). However, as a semiquantitative and
laborious test, immunoblotting is unsuitable for routine clinical
practice. By contrast, enzyme-linked immunosorbent assay (ELISA) is
quantitative and convenient. Although a number of studies have
demonstrated higher levels of sGP73 measured by ELISA in HCC than
in liver cirrhosis (19–21), no significant difference in sGP73
concentrations between HCC and cirrhosis groups was identified by
other studies (22–24), and in a number of studies, the sGP73
level was even lower in HCC than in liver cirrhosis (15,25).
Considering the small sample size of these studies, further
evaluation of ELISA-measured sGP73 in large-scale studies is
required to clarify its diagnostic value in HCC. In addition,
although the overexpression of GP73 in HCC tissue has been reported
to be associated with aggressive behavior and poor overall survival
(OS) time (14), the
clinicopathological effects and prognostic role of sGP73 in HCC
have not yet been characterized.
In the present study, sGP73 levels in 462 patients
with HCC, 186 patients with liver cirrhosis and 83 healthy controls
were measured with ELISA, and the prognostic value of sGP73 in HCC
patients was assessed. It was identified that the diagnostic value
of sGP73 was inferior to AFP for HCC. However, high levels of sGP73
were associated with aggressive clinicopathological characteristics
and poor OS and disease-free survival (DFS) times. Cox multivariate
analysis demonstrated that sGP73 was an independent prognostic
factor for OS and DFS, suggesting that sGP73 has important
prognostic value in HCC.
Materials and methods
Subjects
A total of 731 subjects were enrolled at the Third
Affiliated Hospital of Sun Yat-sen University (Guangzhou, China)
between January 2011 and August 2013, including 462 patients with
HCC (420 males and 42 females, aged 11–84 years), 186 patients with
liver cirrhosis (156 males and 30 females, aged 17–80 years), and
83 healthy controls (59 males and 24 females, aged 22–73 years).
The diagnosis of HCC was made either by histopathology (n=72) or by
two imaging modalities (ultrasound, computed tomography or magnetic
resonance imaging) showing an arterial enhancing lesion, with HBV
and/or HCV infection (n=390). The diagnosis of liver cirrhosis was
based on liver histology or clinical, laboratory and imaging
evidence of hepatic decompensation or portal hypertension. The
clinicopathological characteristics of the HCC patients are
summarized in Table I. In the liver
cirrhosis group, all patients had hepatitis B cirrhosis. This study
was approved by the Ethics Committee of the Third Affiliated
Hospital of Sun Yat-sen University. Informed consent was obtained
from each subject.
 | Table I.Associations between sGP73 levels and
clinicopathological characteristics of 462 patients with
hepatocellular carcinoma. |
Table I.
Associations between sGP73 levels and
clinicopathological characteristics of 462 patients with
hepatocellular carcinoma.
|
|
| sGP73 level, n |
|
|---|
|
|
|
|
|
|---|
|
Characteristics | Total, n | Low | High |
P-valuea |
|---|
| Number of
patients | 462 | 228 | 234 |
|
| Age, years |
|
|
| 0.483 |
|
<50 | 196 | 93 | 103 |
|
|
≥50 | 266 | 135 | 131 |
|
| Sex |
|
|
| 0.228 |
|
Male | 420 | 211 | 209 |
|
|
Female | 42 | 17 | 25 |
|
| ECOG performance
status |
|
|
| <0.001 |
| 0 | 119 | 82 | 37 |
|
| 1 | 284 | 132 | 152 |
|
| 2 | 47 | 12 | 35 |
|
| 3 |
8 |
2 |
6 |
|
| 4 |
4 |
0 |
4 |
|
| BCLC stage |
|
|
| <0.001 |
| 1 | 92 | 65 | 27 |
|
| 2 | 33 | 21 | 12 |
|
| 3 | 310 | 138 | 172 |
|
| 4 | 27 |
4 | 23 |
|
| Tumor number |
|
|
| <0.001 |
| 1 | 214 | 129 | 85 |
|
| 2 | 57 | 35 | 22 |
|
| ≥3 | 191 | 64 | 127 |
|
| Tumor size, cm |
|
|
| <0.001 |
| ≤5 | 188 | 115 | 73 |
|
|
>5 | 274 | 113 | 161 |
|
| Satellite
lesion |
|
|
| <0.001 |
| No | 257 | 156 | 101 |
|
|
Yes | 205 | 72 | 133 |
|
| Vascular
invasion |
|
|
| <0.001 |
| No | 203 | 125 | 78 |
|
|
Yes | 259 | 103 | 156 |
|
| Tumor grade |
|
|
| 0.311 |
| 1 |
3 |
3 |
0 |
|
| 2 | 54 | 34 | 20 |
|
| 3 |
9 | 34 |
2 |
|
|
Unknown | 396 |
|
|
|
| Lymph node
metastasis |
|
|
| 0.009 |
| No | 307 | 165 | 142 |
|
|
Yes | 155 | 63 | 92 |
|
| Distant
metastasis |
|
|
| <0.001 |
| No | 394 | 212 | 182 |
|
|
Yes | 68 | 16 | 52 |
|
| Child-Pugh
class |
|
|
| <0.001 |
| A | 339 | 197 | 142 |
|
| B | 100 | 29 | 71 |
|
| C | 23 |
2 | 21 |
|
| Etiology |
|
|
| 0.200 |
|
HBV | 427 | 213 | 214 |
|
|
HCV |
4 |
3 |
1 |
|
| HBV and
HCV |
2 |
2 |
0 |
|
|
Other | 29 | 11 | 18 |
|
| Cirrhosis
background |
|
|
| 0.99 |
| No | 99 | 48 | 51 |
|
|
Yes | 363 | 178 | 185 |
|
| AFP level,
ng/ml |
|
|
| <0.001 |
|
<400 | 262 | 149 | 113 |
|
|
≥400 | 200 | 79 | 121 |
|
| Number
of patients | 462 | 228 | 234 |
|
Sample collection
A 2-ml blood sample was drawn from each subject,
centrifuged at 4,000 × g for 5 min and then aliquoted. The serum
samples were stored at −80°C until testing. All the blood samples
from the patients with HCC were collected prior to the initiation
of HCC treatment.
Detection of GP73 and AFP
sGP73 was detected using ELSIA kit for Golgi protein
73 (cat. no. SEB668Hu; Cloud-Clone Corp., Houston, TX, USA). Each
serum sample was diluted 20-fold in phosphate-buffered saline, and
tested according to the manufacturer's instructions. Briefly, 100
µl of diluted serum sample was added to the microtiter plate well
pre-coated with an antibody specific to GP73 and incubated for 2 h
at 37°C. Anti-IgG conjugated with biotin (100 µl) was added to each
well and incubated for 1 h at 37°C. Then the plate was washed three
times with washing buffer. Avidin conjugated to horseradish
peroxidase (100 µl) was added to each well and incubated for 30 min
at 37°C. Subsequent to washing five times, 90 µl of
tetramethylbenzidine substrate solution was added to each well and
incubated in the dark for 15 min. The enzyme-substrate reaction was
then terminated by the addition of 50 µl of sulfuric acid solution.
Absorbance was measured at 450 nm in a microplate reader (BioTek
Instruments, Inc., Winooski, VT, USA). The concentration of sGP73
in the samples was determined by comparing the optical density of
the samples to the standard curve.
Serum AFP was tested using a chemiluminescent
immunoassay kit (Roche Diagnostics GmbH, Mannheim, Germany) at the
Clinical Diagnostic Laboratories of the Third Affiliated Hospital
of Sun Yat-Sen University. The upper limit of normal AFP was 8
ng/ml.
Clinical outcome assessment
Laboratory and imaging data were collected every
month in patients with unresectable HCC. For patients who underwent
curative treatment, radical resection or radiofrequency ablation
(RFA), laboratory and imaging examination were conducted every
three months after surgery or RFA. OS time was defined as the
interval between the date of blood sample collection and the date
of mortality from HCC, or the date of last follow-up if patients
were still alive. DFS time was calculated as the interval between
the date of surgery or RFA and the date of local recurrence/distant
metastasis, date of mortality, or the latest date, when
censored.
Statistical analysis
Quantitative values were presented as median
interquartile range (IQR; 25th and 75th percentiles) due to the
abnormal distribution of sGP73. Box-and-whiskers plots were used to
describe sGP73 levels, and the Mann-Whitney U test was employed to
compare the group differences in sGP73 levels. Receiver operating
characteristic (ROC) curves were used to identify the optimal
cutoff value of sGP73 for HCC diagnosis, and the areas under the
curves (AUC) were compared using the Z test. The correlation
between sGP73 and clinicopathological variables in patients with
HCC was assessed by the χ2 test. The Kaplan-Meier method
was employed to estimate the survival rate, and the log-rank test
was used to assess the difference between curves. A Cox
proportional hazards model was utilized to evaluate the prognostic
value of multiple factors on survival rates. P<0.05 was
considered to indicate a statistically significant difference.
Statistical analyses were performed using SPSS version 17.0 (SPSS,
Inc., Chicago, IL, USA) and MedCalc version 9.0 (MedCalc,
Mariakerke, Belgium).
Results
Comparison of sGP73
concentrations
The median concentrations of sGP73 in patients with
HCC, liver cirrhosis and healthy controls were 18.7 (IQR,
5.7–45.6), 18.5 (IQR, 5.31–41.8), and 0 (IQR, 0–8.5) ng/ml,
respectively. As shown in Fig. 1, the
sGP73 levels in the HCC and cirrhosis patients were markedly higher
than those in healthy controls (P<0.001). However, no
significant difference was identified between the HCC and cirrhosis
groups (P=0.632).
Sensitivity and specificity of sGP73
and AFP for HCC diagnosis
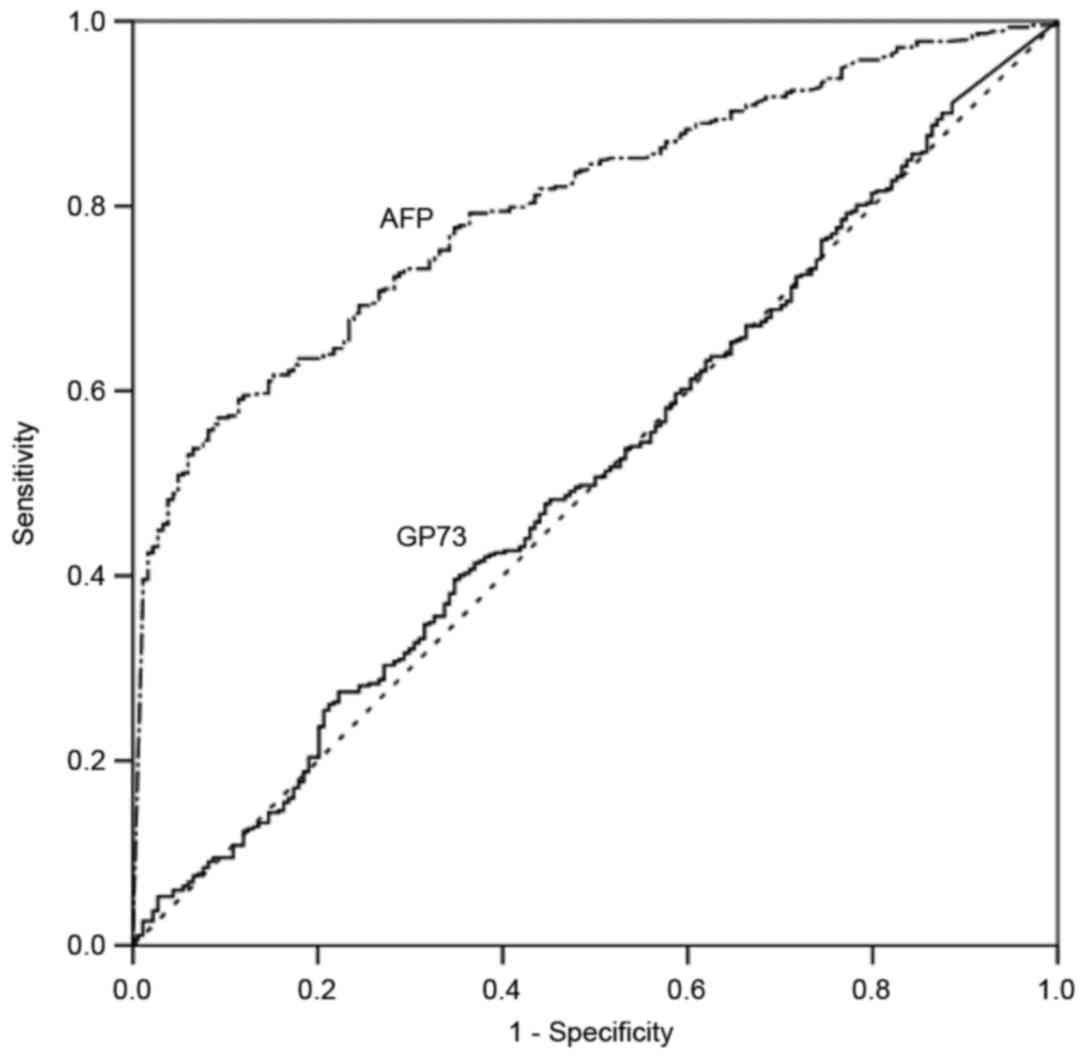
ROC curve analysis was used to define optimal cutoff
values as well as the sensitivity and specificity of sGP73 and AFP
in differentiating HCC and cirrhosis. The AUC for sGP73 was 0.51
[95% confidence interval (CI), 0.46–0.56], with a sensitivity of
27.79% and a specificity of 77.96% at the optimal cut-off point of
43.95 ng/ml. The AUC for AFP was 0.80 (95% CI, 0.77–0.83), with a
sensitivity of 57.36% and a specificity of 90.96% at the optimal
cutoff point of 93.27 ng/ml (Fig. 2).
AFP had a significantly greater AUC than sGP73 for the diagnosis of
HCC (P<0.001).
Serum GP73 levels and HCC
clinicopathological characteristics
To investigate the association between sGP73 levels
and the clinicopathological characteristics of HCC, an optimal
cutoff point of sGP73 for predicting OS was generated by ROC
analysis. A sGP73 concentration >18.74 ng/ml was defined as
high-level and ≤18.74 ng/ml as low-level (AUC, 0.745; 95% CI,
0.695–0.790). According to the cut-off point, high levels of sGP73
were observed in 50.6% (234/462) of patients with HCC.
As shown in Table I,
high levels of sGP73 were significantly correlated with poor
Eastern Cooperative Oncology Group (ECOG) performance status
(P<0.001), advanced Barcelona-Clinic Liver Cancer (BCLC) stage
(P<0.001), multiple lesions (P<0.001), tumor size >5 cm
(P<0.001), satellite lesions (P<0.001), vascular invasion
(P<0.001), lymph node metastasis (P=0.009), distant metastasis
(P<0.001), high Child-Pugh score (P<0.001), and AFP ≥400
ng/ml (P<0.001). Other characteristics, including age, sex,
etiology and liver cirrhosis background, were not significantly
correlated with sGP73 level (all P>0.05).
Serum GP73 levels and HCC
prognosis
Among the 462 HCC patients, complete follow-up
information was available in 313 patients. The median follow-up
time was 16.7 months (range, 0.07–36.13 months). During the
follow-up, 157 patients (50.2%) succumbed to tumor progression.
Among the 128 early-stage HCC patients who received curative
treatment, 48 (37.5%) suffered tumor relapses, and 15 (11.7%)
ultimately succumbed to disease recurrence.
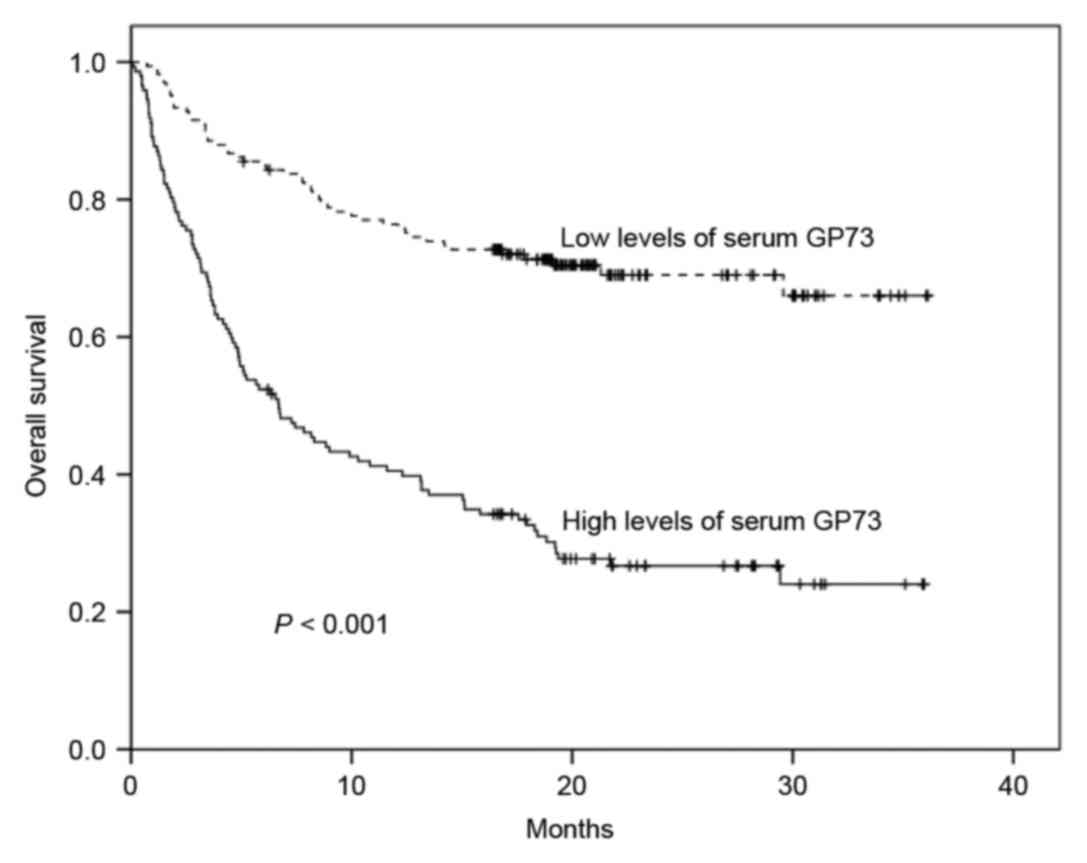
As shown in Fig. 3,
high levels of sGP73 were associated with a significantly reduced
1-year OS rate compared with low levels of sGP73 (40.5 vs. 75.8%;
P<0.001). Poor OS was also associated with poor ECOG performance
status (P<0.001), advanced BCLC stage (P<0.001), multiple
lesions (P<0.001), tumor size >5 cm (P<0.001), satellite
lesions (P<0.001), vascular invasion (P<0.001), lymph node
metastasis (P<0.001), distant metastasis (P<0.001), high
Child-Pugh score (P<0.001), and AFP ≥400 ng/ml (P<0.001)
(Table II). Importantly, Cox
multivariate analysis confirmed that high levels of sGP73
constituted an independent poor prognostic factor for OS [hazard
ratio (HR), 2.004; 95% CI, 1.373–2.925; P<0.001; Table II]. In addition, ECOG performance
status (HR, 1.421; 95% CI, 1.070–1.886; P=0.015), tumor size (HR,
2.400; 95% CI, 1.309–4.400; P=0.005), vascular invasion (HR, 2.397;
95% CI, 1.368–4.199; P=0.002), and Child-Pugh class (HR, 1.920; 95%
CI, 1.426–2.587; P<0.001) were also negative independent
predictors for OS. However, BCLC stage, tumor number, satellite
lesions, lymph node metastasis, distant metastasis and AFP levels
were not independent prognostic factors for OS (Table II).
 | Table II.Univariate and multivariate analyses
of overall survival rate for 313 hepatocellular carcinoma
patients. |
Table II.
Univariate and multivariate analyses
of overall survival rate for 313 hepatocellular carcinoma
patients.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variables | HR | 95% CI |
P-valuea | HR | 95% CI |
P-valueb |
|---|
| Age (<50 vs. ≥50
years) | 1.162 | 0.842–1.605 | 0.361 | – | – | – |
| Sex (female vs.
male) | 1.055 | 0.598–1.862 | 0.853 | – | – | – |
| ECOG performance
status (0 vs. 1 vs. 2 vs. 3 vs. 4) | 2.057 | 1.729–2.447 | <0.001 | 1.421 | 1.070–1.886 | 0.015 |
| BCLC stage (1 vs. 2
vs. 3 vs. 4) | 2.545 | 1.984–3.264 | <0.001 | 0.896 | 0.611–1.313 | 0.512 |
| Tumor number (1 vs.
2 vs. >3) | 2.095 | 1.744–2.517 | <0.001 | 1.276 | 0.944–1.725 | 0.113 |
| Tumor size (<5
vs. ≥5 cm) | 6.582 | 4.321–10.025 | <0.001 | 2.400 | 1.309–4.400 | 0.005 |
| Satellite lesion
(no vs. yes) | 4.985 | 3.536–7.028 | <0.001 | 1.109 | 0.583–2.110 | 0.753 |
| Vascular invasion
(no vs. yes) | 5.751 | 3.919–8.440 | <0.001 | 2.397 | 1.368–4.199 | 0.002 |
| Tumor grade (1 vs.
2 vs. 3) | 0.880 | 0.213–3.634 | 0.860 | – | – | – |
| Lymph node
metastasis (no vs. yes) | 1.880 | 1.364–2.591 | <0.001 | 0.789 | 0.556–1.120 | 0.186 |
| Distant metastasis
(no vs. yes) | 3.378 | 2.312–4.935 | <0.001 | 1.041 | 0.682–1.588 | 0.852 |
| Child-Pugh class (A
vs. B vs. C) | 2.358 | 1.863–2.983 | <0.001 | 1.920 | 1.426–2.587 | <0.001 |
| Etiology (HBV vs.
HCV vs. HBV and HCV vs. other) | 0.660 | 0.394–1.105 | 0.114 | – | – | – |
| Cirrhosis
background (no vs. yes) | 0.841 | 0.570–1.242 | 0.384 | – | – | – |
| Serum AFP (<400
vs. ≥400 ng/ml) | 2.572 | 1.872–3.533 | <0.001 | 1.391 | 0.987–1.959 | 0.059 |
| Serum GP73 (≤18.74
vs. >18.74 ng/ml) | 3.529 | 2.516–4.949 | <0.001 | 2.004 | 1.373–2.925 | <0.001 |
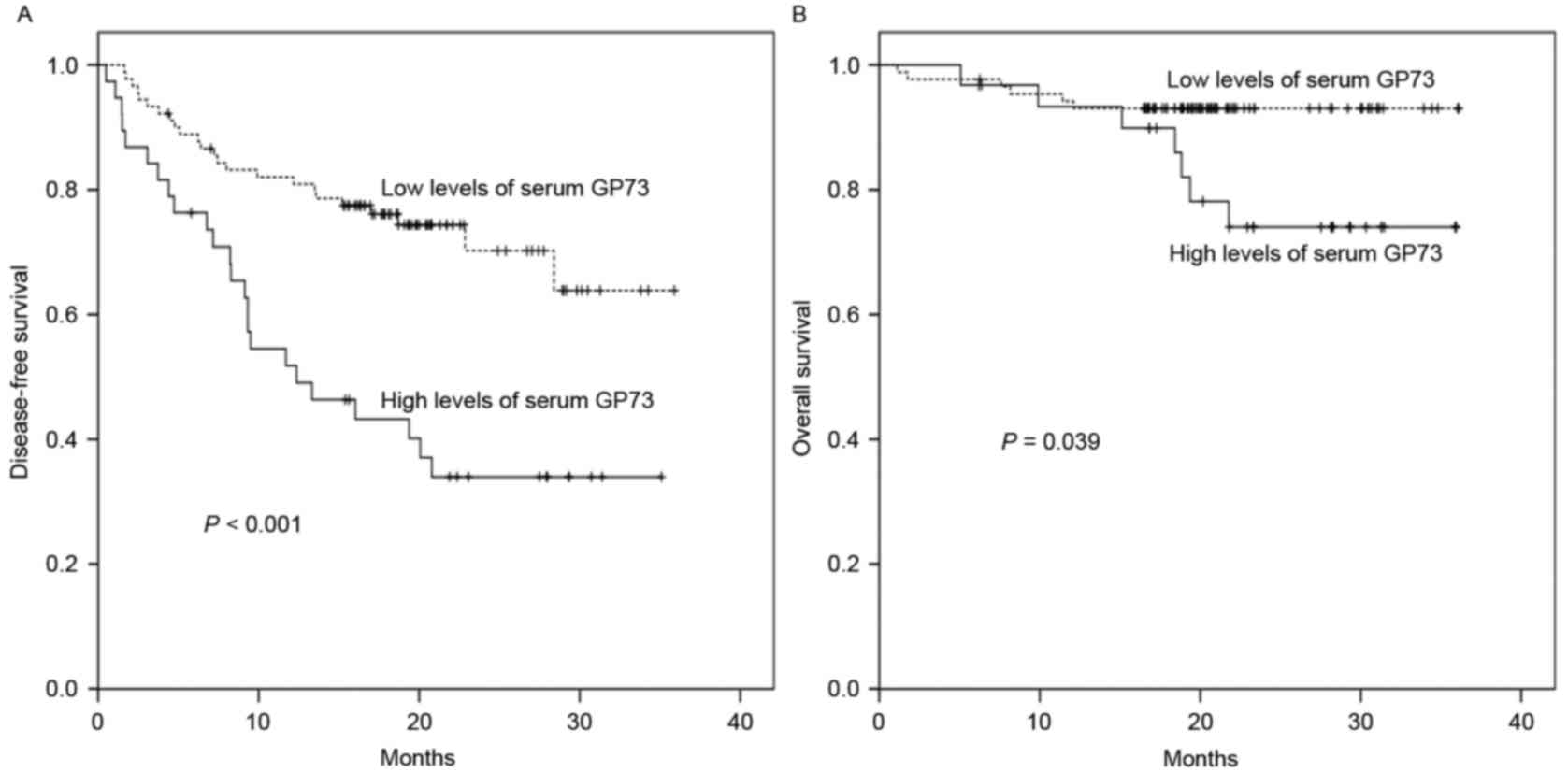
To investigate the predictive value of sGP73 for HCC
recurrence, the follow-up data of 128 HCC patients who underwent
curative treatment was subjected to survival analysis. As shown in
Table III, in the univariate
analysis, poor ECOG performance status (P=0.012), advanced BCLC
stage (P=0.018), multiple lesions (P=0.041), tumor size >5 cm
(P=0.009), satellite lesions (P=0.002), vascular invasion
(P=0.030), and sGP73 (P<0.001; Fig.
4A) were associated with poor DFS. However, only Child-Pugh
class (HR, 3.747; 95% CI, 1.305–10.765; P=0.014) and sGP73 (HR,
4.664; 95% CI, 2.426–8.968; P<0.001) were negative independent
prognostic biomarkers for DFS in the multivariate analysis
(Table III). Similarly, for OS in
this group, tumor size (P=0.019), satellite lesions (P=0.001),
Child-Pugh class (P=0.044), and sGP73 (P=0.039; Fig. 4B) were negative predictors in the
univariate analysis, whereas satellite lesions (HR, 25.202; 95% CI,
1.639–387.455; P=0.021), Child-Pugh class (HR, 3.726; 95% CI,
1.059–13.113; P=0.041), and sGP73 (HR, 4.026; 95% CI, 1.104–14.686;
P=0.035) were independent prognostic factors in the multivariate
analysis (Table III).
 | Table III.Univariate and multivariate analyses
of DFS and OS in 128 hepatocellular carcinoma patients who
underwent curative treatment. |
Table III.
Univariate and multivariate analyses
of DFS and OS in 128 hepatocellular carcinoma patients who
underwent curative treatment.
|
| DFS | OS |
|---|
|
|
|
|
|---|
|
| Univariate
analysis | Multivariate
analysis | Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|
|---|
| Variables | HR | 95% CI |
P-valuea | HR | 95% CI |
P-valueb | HR | 95% CI |
P-valuea | HR | 95% CI |
P-valueb |
|---|
| Age (<50 vs. ≥50
years) | 1.351 | 0.757–2.413 | 0.309 | – | – | – | 3.080 | 0.847–11.195 | 0.088 | 2.354 | 0.550–10.077 | 0.249 |
| Sex (female vs.
male) | 1.346 | 0.326–5.556 | 0.681 | – | – | – | 0.825 | 0.107–6.345 | 0.853 | – | – | – |
| ECOG PS (0 vs. 1
vs. 2 vs. 3 vs. 4) | 2.086 | 1.179–3.693 | 0.012 | 0.923 | 0.320–2.664 | 0.882 | 2.141 | 0.700–6.547 | 0.182 | – | – | – |
| BCLC stage (1 vs. 2
vs. 3 vs. 4) | 1.441 | 1.065–1.949 | 0.018 | 1.457 | 0.781–2.719 | 0.237 | 1.469 | 0.817–2.643 | 0.199 | – | – | – |
| Tumor number (1 vs.
2 vs. >3) | 1.554 | 1.017–2.372 | 0.041 | 1.034 | 0.503–2.124 | 0.928 | 1.881 | 0.911–3.882 | 0.088 | 0.280 | 0.056–1.405 | 0.122 |
| Tumor size (<5
vs. ≥5 cm) | 2.303 | 1.232–4.307 | 0.009 | 1.849 | 0.773–4.422 | 0.167 | 3.724 | 1.241–11.176 | 0.019 | 3.060 | 0.853–10.977 | 0.086 |
| Satellite lesion
(no vs. yes) | 3.134 | 1.500–6.546 | 0.002 | 3.642 | 0.924–14.354 | 0.065 | 6.750 | 2.127–21.415 | 0.001 | 25.202 | 1.639–387.455 | 0.021 |
| Vascular invasion
(no vs. yes) | 2.027 | 1.071–3.836 | 0.030 | 1.399 | 0.591–3.316 | 0.445 | 2.443 | 0.799–7.468 | 0.117 | – | – | – |
| Tumor grade (1 vs.
2 vs. 3) | 0.714 | 0.276–1.846 | 0.488 | – | – | – | 0.532 | 0.104–2.711 | 0.448 | – | – | – |
| Child-Pugh class (A
vs. B vs. C) | 1.707 | 0.909–3.204 | 0.096 | 3.747 | 1.305–10.765 | 0.014 | 2.556 | 0.950–6.878 | 0.044 | 3.726 | 1.059–13.113 | 0.041 |
| Etiology (HBV vs.
HCV vs. HBV and HCV vs. other) | 1.413 | 0.439–4.549 | 0.562 | – | – | – | 0.402 | 0.089–1.815 | 0.236 | – | – | – |
| Cirrhosis
background (no vs. yes) | 2.527 | 0.784–8.147 | 0.121 | – | – | – | 1.737 | 0.225–13.397 | 0.596 | – | – | – |
| Serum AFP levels
(<400 vs. ≥400 ng/ml) | 1.186 | 0.636–2.210 | 0.592 | – | – | – | 1.356 | 0.418–4.406 | 0.612 | – | – | – |
| Serum sGP73 levels
(≤18.74 vs. >18.74 ng/ml) | 2.913 | 1.650–5.140 | <0.001 | 4.664 | 2.426–8.968 | <0.001 | 3.173 | 1.062–9.478 | 0.039 | 4.026 | 1.104–14.686 | 0.035 |
Discussion
In the present study, it was found that sGP73 levels
measured by ELISA were markedly higher in patients with HCC and
liver cirrhosis than in healthy controls, whereas the difference in
sGP73 levels between HCC and liver cirrhosis was not statistically
significant. Both the sensitivity and specificity of sGP73 for the
diagnosis of HCC were inferior to those of AFP. However, high sGP73
levels were significantly associated with aggressive
clinicopathological features and decreased OS and DFS times in HCC.
Multivariate analysis confirmed that sGP73 was an independent
prognostic factor for HCC.
It is not entirely surprising that the diagnostic
value of sGP73 was not detected in the present study. Upregulated
hepatocyte expression of GP73 has previously been reported in the
liver tissues not only of patients with HCC, but also of those with
other liver disease, including liver cirrhosis (12), chronic hepatitis, hepatic atypical
hyperplasia (15), liver cell
adenoma, and focal nodular hyperplasia (23). High tissue levels of GP73 have also
been observed in adenocarcinomas of the prostate, colon and breast,
in renal cell cancer (26), and in
bile duct carcinoma (23), suggesting
that GP73 is not a HCC-specific biomarker. Iftikhar et al
(13) observed that hepatocyte GP73
expression was markedly upregulated in patients with acute
hepatitis, and that the upregulation was reversible upon the
resolution of the acute disease. A similar magnitude of increased
GP73 expression was observed in fully established cirrhosis.
Consistently, a recent study demonstrated that the levels of sGP73
were low in chronic HBV carriers without liver injury, but they
were significantly elevated in patients with prominent hepatic
injury and fibrosis, and the sGP73 levels were positively
correlated with the severity of liver injury, indicating that the
affected hepatocytes released more GP73 into the blood when liver
injury was triggered (27).
Therefore, the increase of sGP73 is a common feature of liver
injury, regardless of the causes.
Although the sGP73 levels in HCC were similar to
those in liver cirrhosis in the present study, a number of studies
reported higher sGP73 levels in HCC than in liver cirrhosis
(19–21), or higher sGP73 levels in liver
cirrhosis than in HCC (15,25). This discrepancy may be partly due to
the heterogeneity of the disease stage of patients with HCC or
liver cirrhosis among different studies. More advanced stages of
HCC or liver cirrhosis reflect more injured hepatocytes, resulting
in higher sGP73 levels in HCC or cirrhosis. Different GP73
antibodies used for the ELISA test may be another reason for this
discrepancy. GP73 ELISA kits are commercially available; however,
the different sensitivities and specificities of the kits provided
by different manufacturers may lead to different results.
Since sGP73 was initially identified, studies have
focused on its role in the early diagnosis of HCC (11,18–21). Few
studies have investigated the association of sGP73 with the
clinicopathological characteristics and prognosis of HCC (15,21). In
the present study, it was identified that high levels of sGP73 were
associated with aggressive tumor behavior and poor DFS and OS
times, and that sGP73 was an independent negative prognostic factor
of HCC, which is consistent with the roles of overexpressed GP73 in
HCC tissue (14). Significantly, with
regard to predicting the risk of relapse and mortality, sGP73 had
comparable HRs (4.664 and 4.026, respectively) with Child-Pugh
class (3.747 and 3.726, respectively), and was superior to BCLC
stage (Table III), suggesting that
sGP73 has an important prognostic value in HCC. In HCC cell lines
and xenograft tumors in mice, GP73 has been shown to promoted
proliferation, migration, invasion and metastasis (28). However, the mechanism by which GP73
promotes tumor progression remains unclear. Recently, a study
demonstrated that GP73 potentiated HCC cell invasion by
upregulating matrix metalloproteinase-13 (29), supporting the role of GP73 in HCC as
suggested by the present study.
In summary, the present study demonstrated that
sGP73 was not a diagnostic marker but rather a prognostic factor
for HCC. High levels of sGP73 were predictive of more aggressive
features of HCC and poorer OS and DFS times; therefore, as a
prognostic marker, sGP73 would be of great benefit for identifying
the patients with a high risk of disease progression or recurrence,
who may benefit from individualized therapy.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81372374 to
Xiang-Yuan Wu), and the Science and Technology Foundation of
Guangdong Province (grant no. 2011B031800014 to Min Dong).
Glossary
Abbreviations
Abbreviations:
|
AFP
|
α-fetoprotein
|
|
AUC
|
areas under curve
|
|
BCLC
|
Barcelona-Clinic Liver Cancer
|
|
DFS
|
disease-free survival
|
|
ECOG
|
Eastern Cooperative Oncology Group
|
|
ELISA
|
enzyme-linked immunosorbent assay
|
|
GP73
|
Golgi protein 73
|
|
HBV
|
hepatitis B virus
|
|
HCC
|
hepatocellular carcinoma
|
|
HCV
|
hepatitis C virus
|
|
OS
|
overall survival
|
|
ROC
|
receiver operating characteristic
|
|
sGP73
|
serum Golgi protein 73
|
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shariff MI, Cox IJ, Gomaa AI, Khan SA,
Gedroyc W and Taylor-Robinson SD: Hepatocellular carcinoma: Current
trends in worldwide epidemiology, risk factors, diagnosis and
therapeutics. Expert Rev Gastroenterol Hepatol. 3:353–367. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zeng H, Zheng R, Guo Y, Zhang S, Zou X,
Wang N, Zhang L, Tang J, Chen J, Wei K, et al: Cancer survival in
China, 2003–2005: A population-based study. Int J Cancer.
136:1921–1930. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ercolani G, Grazi GL, Ravaioli M, Del
Gaudio M, Gardini A, Cescon M, Varotti G, Cetta F and Cavallari A:
Liver resection for hepatocellular carcinoma on cirrhosis:
Univariate and multivariate analysis of risk factors for
intrahepatic recurrence. Ann Surg. 237:536–543. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Debruyne EN and Delanghe JR: Diagnosing
and monitoring hepatocellular carcinoma with alpha-fetoprotein: New
aspects and applications. Clin Chim Acta. 395:19–26. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Aoyagi Y, Suzuki Y, Isemura M, Nomoto M,
Sekine C, Igarashi K and Ichida F: The fucosylation index of
alpha-fetoprotein and its usefulness in the early diagnosis of
hepatocellular carcinoma. Cancer. 61:769–774. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nakagawa T, Seki T, Shiro T, Wakabayashi
M, Imamura M, Itoh T, Tamai T, Nishimura A, Yamashiki N, Matsuzaki
K, et al: Clinicopathologic significance of protein induced vitamin
K absence or antagonist II and alpha-fetoprotein in hepatocellular
carcinoma. Int J Oncol. 14:281–286. 1999.PubMed/NCBI
|
|
8
|
Giardina MG, Matarazzo M, Varriale A,
Morante R, Napoli A and Martino R: Serum alpha-L-fucosidase. A
useful marker in the diagnosis of hepatocellular carcinoma. Cancer.
70:1044–1048. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhu ZW, Friess H, Wang L, Abou-Shady M,
Zimmermann A, Lander AD, Korc M, Kleeff J and Büchler MW: Enhanced
glypican-3 expression differentiates the majority of hepatocellular
carcinomas from benign hepatic disorders. Gut. 48:558–564. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Giannelli G, Marinosci F, Sgarra C, Lupo
L, Dentico P and Antonaci S: Clinical role of tissue and serum
levels of SCCA antigen in hepatocellular carcinoma. Int J Cancer.
116:579–583. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mao Y, Yang H, Xu H, Lu X, Sang X, Du S,
Zhao H, Chen W, Xu Y, Chi T, et al: Golgi protein 73 (GOLPH2) is a
valuable serum marker for hepatocellular carcinoma. Gut.
59:1687–1693. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kladney RD, Cui X, Bulla GA, Brunt EM and
Fimmel CJ: Expression of GP73, a resident Golgi membrane protein,
in viral and nonviral liver disease. Hepatology. 35:1431–1440.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Iftikhar R, Kladney RD, Havlioglu N,
Schmitt-Gräff A, Gusmirovic I, Solomon H, Luxon BA, Bacon BR and
Fimmel CJ: Disease- and cell-specific expression of GP73 in human
liver disease. Am J Gastroenterol. 99:1087–1095. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bao YX, Cao Q, Yang Y, Mao R, Xiao L,
Zhang H, Zhao HR and Wen H: Expression and prognostic significance
of golgiglycoprotein 73 (GP73) with epithelial-mesenchymal
transition (EMT) related molecules in hepatocellular carcinoma
(HCC). Diagn Pathol. 8:1972013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shan SG, Gao YT, Xu YJ, Huang Y, Zhang Q,
Zhai DK, Li JB, Wang FM, Jing X, Du Z and Wang YJ: Gradually
increased Golgi protein 73 expression in the progression of benign
liver diseases to precancerous lesions and hepatocellular carcinoma
correlates with prognosis of patients. Hepatol Res. 43:1199–1210.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bachert C, Fimmel C and Linstedt AD:
Endosomal trafficking and proprotein convertase cleavage of cis
Golgi protein GP73 produces marker for hepatocellular carcinoma.
Traffic. 8:1415–1423. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Block TM, Comunale MA, Lowman M, Steel LF,
Romano PR, Fimmel C, Tennant BC, London WT, Evans AA, Blumberg BS,
et al: Use of targeted glycoproteomics to identify serum
glycoproteins that correlate with liver cancer in woodchucks and
humans. Proc Natl Acad Sci USA. 102:779–784. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Marrero JA, Romano PR, Nikolaeva O, Steel
L, Mehta A, Fimmel CJ, Comunale MA, D'Amelio A, Lok AS and Block
TM: GP73, a resident Golgi glycoprotein, is a novel serum marker
for hepatocellular carcinoma. J Hepatol. 43:1007–1012. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shi Y, Chen J, Li L, Sun Z, Zen L, Xu S,
Zhang Y and Zhang L: A study of diagnostic value of golgi protein
GP73 and its genetic assay in primary hepatic carcinoma. Technol
Cancer Res Treat. 10:287–294. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu WJ, Guo BL, Han YG, Shi L and Ma WS:
Diagnostic value of alpha-fetoprotein-L3 and Golgi protein 73 in
hepatocellular carcinomas with low AFP levels. Tumour Biol.
35:12069–12074. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hou SC, Xiao MB, Ni RZ, Ni WK, Jiang F, Li
XY, Lu CH and Chen BY: Serum GP73 is complementary to AFP and
GGT-II for the diagnosis of hepatocellular carcinoma. Oncol Lett.
6:1152–1158. 2013.PubMed/NCBI
|
|
22
|
Gu Y, Chen W, Zhao Y, Chen L and Peng T:
Quantitative analysis of elevated serum Golgi protein-73 expression
in patients with liver diseases. Ann Clin Biochem. 46:38–43. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Riener MO, Stenner F, Liewen H, Soll C,
Breitenstein S, Pestalozzi BC, Samaras P, Probst-Hensch N,
Hellerbrand C, Müllhaupt B, et al: Golgi phosphoprotein 2 (GOLPH2)
expression in liver tumors and its value as a serum marker in
hepatocellular carcinomas. Hepatology. 49:1602–1609. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bröker ME, Ijzermans JN, Witjes CD, van
Vuuren HJ and de Man RA: The predictive value of Golgi protein 73
in differentiating benign from malignant liver tumors. PLoS One.
9:e1001872014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tian L, Wang Y, Xu D, Gui J, Jia X, Tong
H, Wen X, Dong Z and Tian Y: Serological AFP/Golgi protein 73 could
be a new diagnostic parameter of hepatic diseases. Int J Cancer.
129:1923–1931. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kristiansen G, Fritzsche FR, Wassermann K,
Jäger C, Tölls A, Lein M, Stephan C, Jung K, Pilarsky C, Dietel M
and Moch H: GOLPH2 protein expression as a novel tissue biomarker
for prostate cancer: Implications for tissue-based diagnostics. Br
J Cancer. 99:939–948. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xu Z, Liu L, Pan X, Wei K, Wei M, Liu L,
Yang H and Liu Q: Serum Golgi protein 73 (GP73) is a diagnostic and
prognostic marker of chronic HBV liver disease. Medicine
(Baltimore). 94:e6592015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen X, Wang Y, Tao J, Shi Y, Gai X, Huang
F, Ma Q, Zhou Z, Chen H, Zhang H, et al: mTORC1 Up-Regulates GP73
to promote proliferation and migration of hepatocellular carcinoma
cells and growth of xenograft tumors in mice. Gastroenterology.
149:741–52.e14. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jin D, Tao J, Li D, Wang Y, Li L, Hu Z,
Zhou Z, Chang X, Qu C and Zhang H: Golgi protein 73 activation of
MMP-13 promotes hepatocellular carcinoma cell invasion. Oncotarget.
6:33523–33533. 2015. View Article : Google Scholar : PubMed/NCBI
|