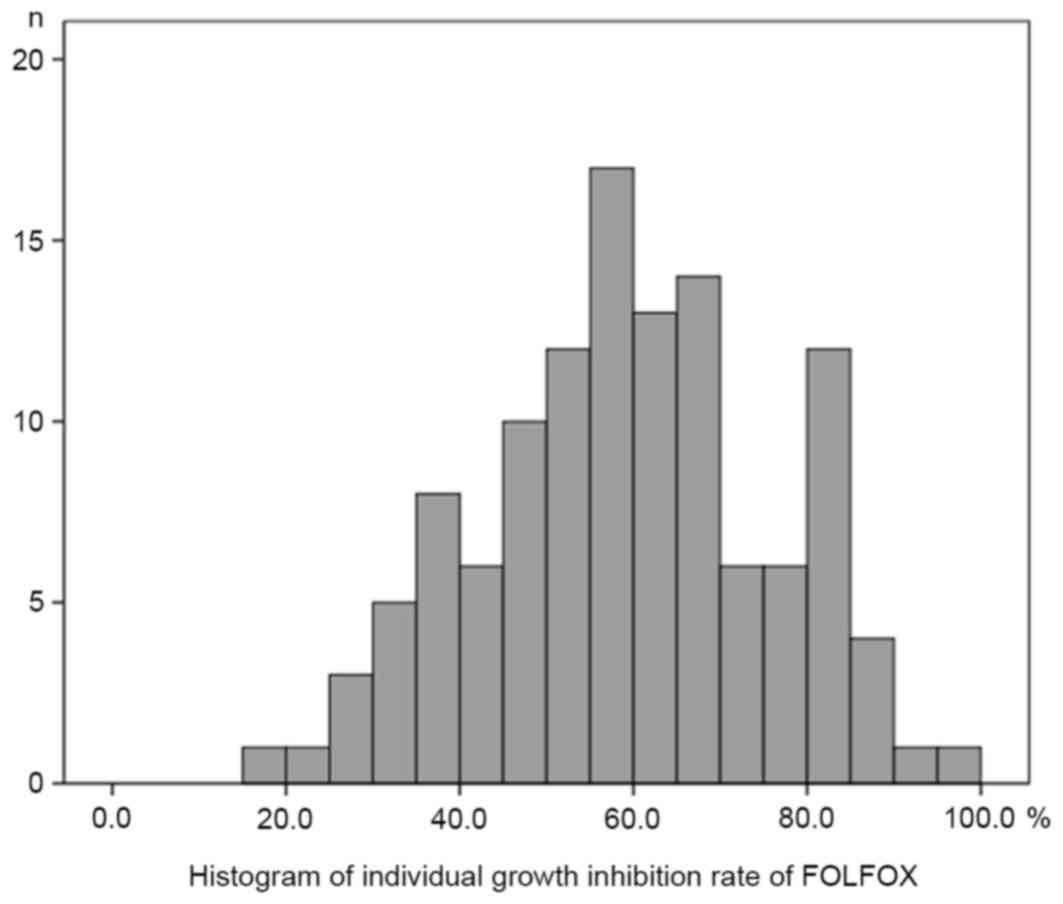
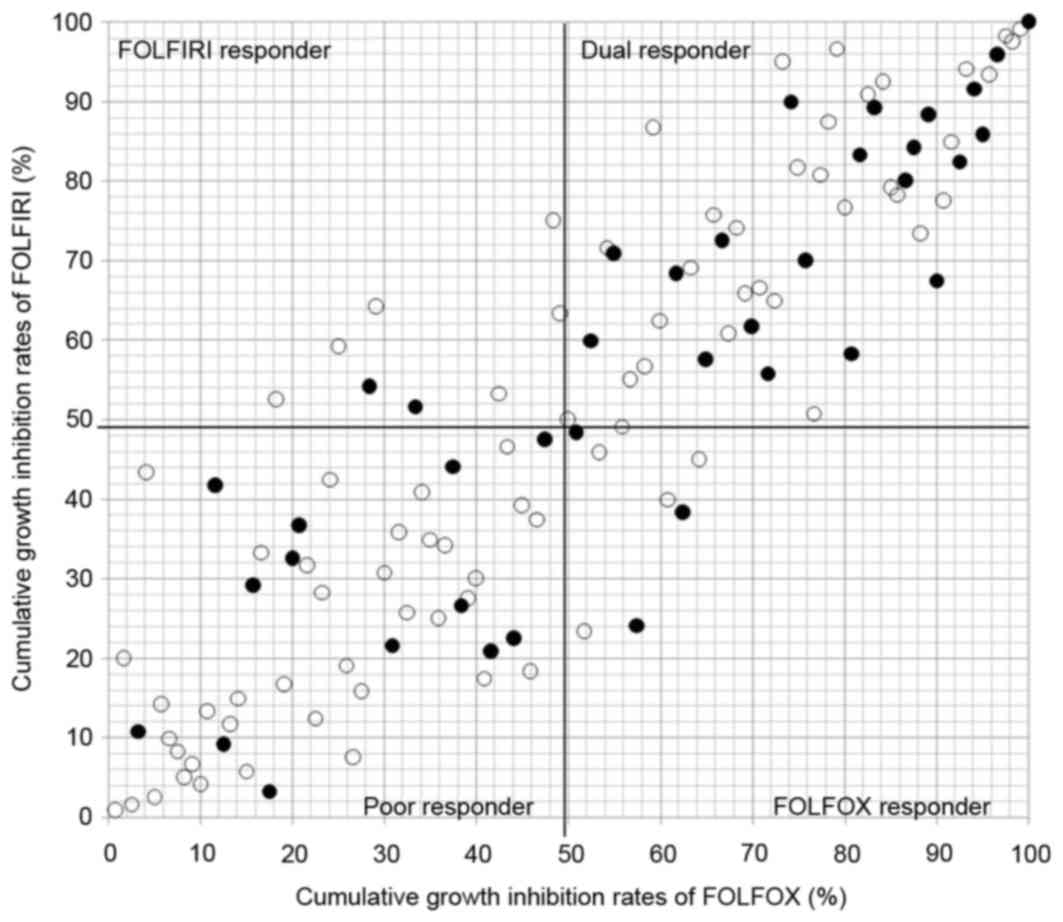
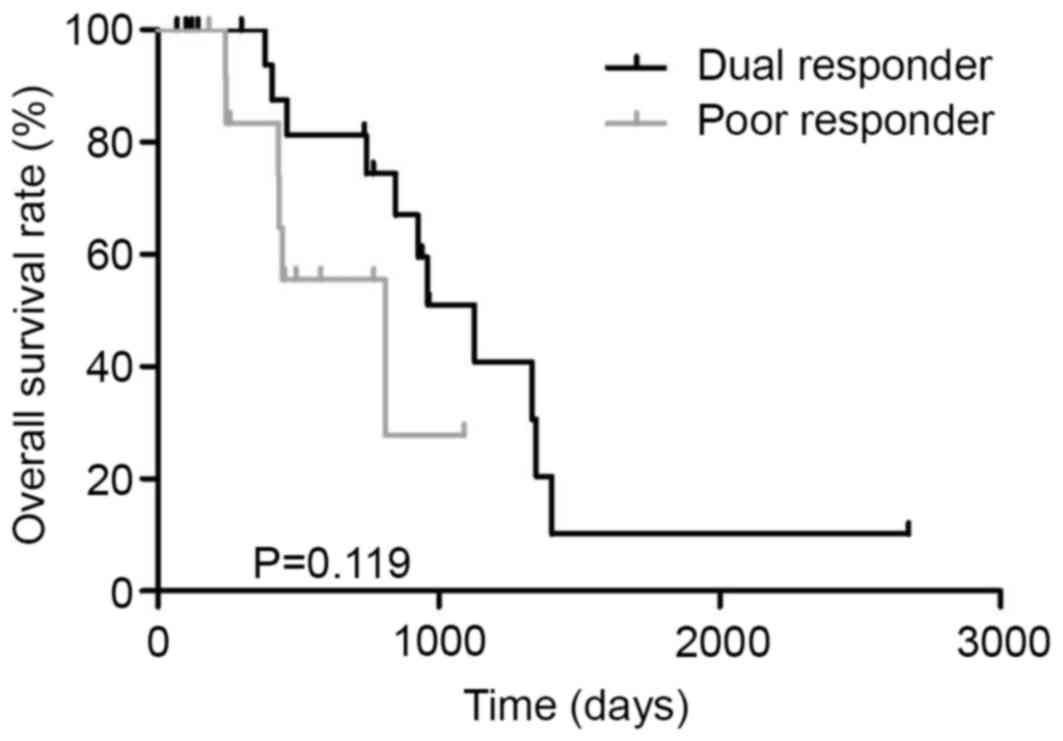
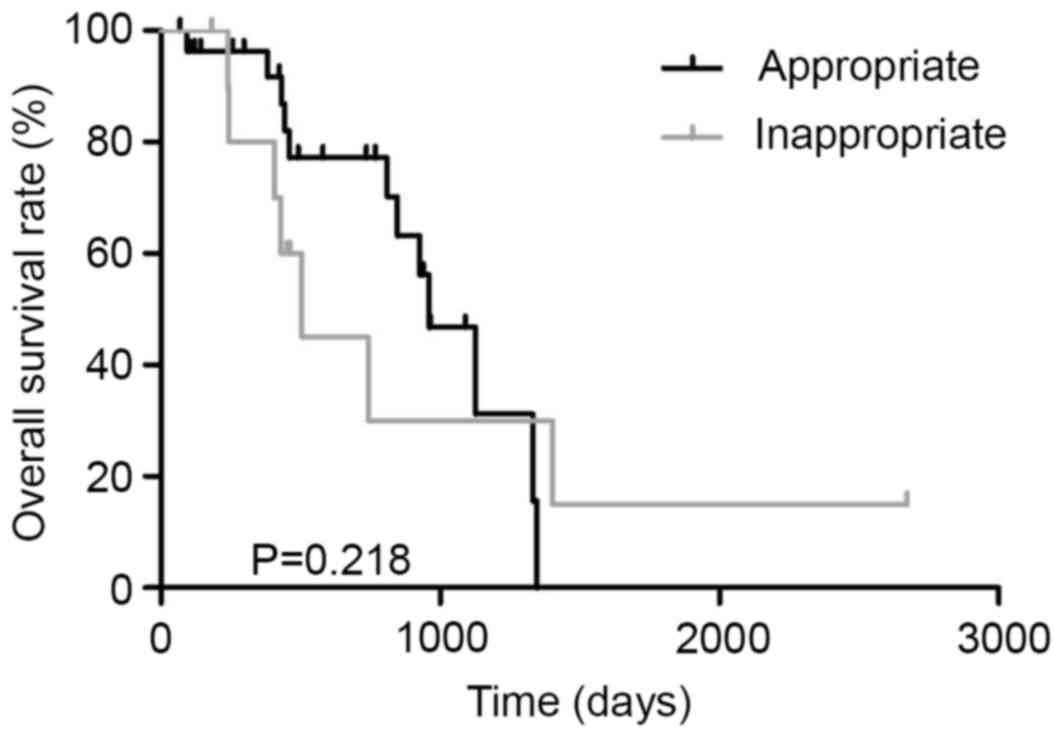
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Adam R, De Gramont A, Figueras J, Guthrie
A, Kokudo N, Kunstlinger F, Loyer E, Poston G, Rougier P,
Rubbia-Brandt L, et al: The oncosurgery approach to managing liver
metastases from colorectal cancer: A multidisciplinary
international consensus. Oncologist. 17:1225–1239. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jones RP, Stättner S, Sutton P, Dunne DF,
McWhirter D, Fenwick SW, Malik HZ and Poston GJ: Controversies in
the oncosurgical management of liver limited stage IV colorectal
cancer. Surg Oncol. 23:53–60. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
NCCN: NCCN Clinical Practice Guidelines in
Oncology. Colon Cancer. Version 1 2017. http://www.nccn.orgFebruary 24–2017
|
|
5
|
Van Cutsem E, Cervantes A, Adam R, Sobrero
A, Van Krieken JH, Aderka D, Aguilar E Aranda, Bardelli A, Benson
A, Bodoky G, et al: ESMO consensus guidelines for the management of
patients with metastatic colorectal cancer. Ann Oncol.
27:1386–1422. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fakih MG: Metastatic colorectal cancer:
Current state and future directions. J Clin Oncol. 33:1809–1824.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Venook A, Niedzwiecki D, Lenz H, Mahoney
M, Innocenti F, O'Neil B, Shaw J, Polite B, Hochster H, Goldberg R,
et al: CALGB/SWOG 80405: Analysis of patients undergoing surgery as
part of treatment strategy. Ann Oncol. 25:1–41. 2014. View Article : Google Scholar
|
|
8
|
Recondo G Jr, Díaz-Cantón E, de la Vega M,
Greco M, Recondo G Sr and Valsecchi ME: Advances and new
perspectives in the treatment of metastatic colon cancer. World J
Gastrointest Oncol. 6:211–224. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tournigand C, André T, Achille E, Lledo G,
Flesh M, Mery-Mignard D, Quinaux E, Couteau C, Buyse M, Ganem G, et
al: FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced
colorectal cancer: A randomized GERCOR study. J Clin Oncol.
22:229–237. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Leung HW, Chan AL, Leung MS and Lu CL:
Systematic review and quality assessment of cost-effectiveness
analysis of pharmaceutical therapies for advanced colorectal
cancer. Ann Pharmacother. 47:506–518. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Drummond MF, Sculpher MJ, Torrance GW,
O'Brien BJ and Stoddart GL: Methods for the economic evaluation of
health care programmes. Oxford University Press; New York, NY:
2005
|
|
13
|
Manca A, Asseburg C, Vergel Y Bravo,
Seymour MT, Meade A, Stephens R, Parmar M and Sculpher MJ: The
cost-effectiveness of different chemotherapy strategies for
patients with poor prognosis advanced colorectal cancer (MRC
FOCUS). Value Health. 15:22–31. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lange A, Prenzler A, Frank M, Kirstein M
and der Schulenburg JM Vogel Aandvon: A systematic review of
cost-effectiveness of monoclonal antibodies for metastatic
colorectal cancer. Eur J Cancer. 50:40–49. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ochiai T, Nishimura K, Watanabe T,
Kitajima M, Hashiguchi T, Nakatani A, Marusasa T, Muraki A, Nagaoka
I and Futagawa S: Leucovorin and fluorouracil plus oxaliplatin or
leucovorin and fluorouracil plus irinotecan as individualized
first-line therapy based on a drug sensitivity test. Exp Ther Med.
1:325–329. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ochiai T, Nishimura K, Watanabe T,
Kitajima M, Nakatani A, Inou T, Washio M, Sakuyama N, Sato T,
Kishine K, et al: Individualized chemotherapy for colorectal cancer
based on the collagen gel droplet-embedded drug sensitivity test.
Oncol Lett. 4:621–624. 2012.PubMed/NCBI
|
|
17
|
Kobayashi H, Tanisaka K, Doi O, Kodama K,
Higashiyama M, Nakagawa H, Miyake M, Taki T, Hara S, Yasutomi M, et
al: An in vitro chemosensitivity test for solid tumors using
collagen gel droplet embedded cultures. Int J Oncol. 11:449–455.
1997.PubMed/NCBI
|
|
18
|
Kobayashi H, Higashiyama M, Minamigawa K,
Tanisaka K, Takano T, Yokouchi H, Kodama K and Hata T: Examination
of in vitro chemosensitivity test using collagen droplet culture
method with colorimetric endpoint quantification. Jpn J Cancer Res.
92:203–210. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
de Gramont A, Figer A, Seymour M, Homerin
M, Hmissi A, Cassidy J, Boni C, Cortes-Funes H, Cervantes A, Freyer
G, et al: Leucovorin and fluorouracil with or without oxaliplatin
as first-line treatment in advanced colorectal cancer. J Clin
Oncol. 18:2938–2947. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Goldberg RM, Sargent DJ, Morton RF, Fuchs
CS, Ramanathan RK, Williamson SK, Findlay BP, Pitot HC and Alberts
SR: A randomized controlled trial of fluorouracil plus leucovorin,
irinotecan and oxaliplatin combinations in patients with previously
untreated metastatic colorectal cancer. J Clin Oncol. 22:23–30.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Douillard JY, Cunningham D, Roth AD,
Navarro M, James RD, Karasek P, Jandik P, Iveson T, Carmichael J,
Alakl M, et al: Irinotecan combined with fluorouracil compared with
fluorouracil alone as first-line treatment for metastatic
colorectal cancer: A multicentre randomized trial. Lancet.
355:1041–1047. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ochiai T, Nishimura K, Noguchi H, Kitajima
M, Tsukada A, Watanabe E, Nagaoka I and Futagawa S: Prognostic
impact of orotate phosphoribosyl transferase among 5-fluorouracil
metabolic enzymes in resectable colorectal cancers treated by oral
5-fluorouracil-based adjuvant chemotherapy. Int J Cancer.
118:3084–3088. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Aki F, Bando Y, Takahashi T, Uehara H,
Numoto S, Ito S, Sasa M and Izumi K: A retrospective study on TS
mRNA expression and prediction of the effects of adjuvant oral
5-fluorouracil in breast cancer. Oncol Lett. 1:981–987.
2010.PubMed/NCBI
|
|
24
|
Komori S, Osada S, Tomita H, Nishio K,
Kumazawa I, Tachibana S, Tsuchiya J and Yoshida K: Predictive value
of orotate phosphoribosyltransferase in colorectal cancer patients
receiving 5-FU-based chemotherapy. Mol Clin Oncol. 1:453–460. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ochiai T, Umeki M, Miyake H, Iida T,
Okumura M, Ohno K, Sakamoto M, Miyoshi N, Takahashi M, Tsumura H,
et al: Impact of 5-fluorouracil metabolizing enzymes on
chemotherapy in patients with resectable colorectal cancer. Oncol
Rep. 32:887–892. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sasako M, Terashima M, Ichikawa W, Ochiai
A, Kitada K, Kurahashi I, Sakuramoto S, Katai H, Sano T and Imamura
H: Impact of the expression of thymidylate synthase and
dihydropyrimidine dehydrogenase genes on survival in stage II/III
gastric cancer. Gastric Cancer. 18:538–548. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kawakami H, Zaanan A and Sinicrope FA:
Implications of mismatch repair-deficient status on management of
early stage colorectal cancer. J Gastrointest Oncol. 6:676–684.
2015.PubMed/NCBI
|
|
28
|
Jung SH, Kim SH and Kim JH: Prognostic
impact of microsatellite instability in colorectal cancer
presenting with mucinous, signet-ring, and poorly differentiated
cells. Ann Coloproctol. 32:58–65. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ashktorab H, Ahuja S, Kannan L, Llor X,
Ellis NA, Xicola RM, Laiyemo AO, Carethers JM, Brim H and Nouraie
M: A meta-analysis of MSI frequency and race in colorectal cancer.
Oncotarget. 23:34546–34557. 2016. View Article : Google Scholar
|
|
30
|
van Kuilenburg AB and Maring JG:
Evaluation of 5-fluorouracil pharmacokinetic models and therapeutic
drug monitoring in cancer patients. Pharmacogenomics. 14:799–811.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Paci A, Veal G, Bardin C, Levêque D,
Widmer N, Beijnen J, Astier A and Chatelut E: Review of therapeutic
drug monitoring ofanticancer drugs part 1-Cytotoxics. Eur J Cancer.
50:2010–2019. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Derissen EJ, Jacobs BA, Huitema AD, Rosing
H, Schellens JH and Beijnen JH: Exploring the intracellular
pharmacokinetics of the 5-fluorouracil nucleotides during
capecitabine treatment. Br J Clin Pharmacol. 81:949–957. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang R, Zhang Y, Zhou H, Zhang P, Yang P,
Tong Q, Lyu Y and Han Y: Individual 5-fluorouracil dose adjustment
via pharmacokinetic monitoring versus conventional
body-area-surface method: A meta-analysis. Ther Drug Monit.
38:79–86. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lee JJ, Beumer JH and Chu E: Therapeutic
drug monitoring of 5-fluorouracil. Cancer Chemother Pharmacol.
78:447–464. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bencsikova B, Bortlicek Z, Halamkova J,
Ostrizkova L, Kiss I, Melichar B, Pavlik T, Dusek L, Valik D,
Vyzula R and Zdrazilova-Dubska L: Efficacy of bevacizumab and
chemotherapy in the first-line treatment of metastatic colorectal
cancer: Broadening KRAS-focused clinical view. BMC Gastroenterol.
15:372015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hecht JR, Cohn A, Dakhil S, Saleh M,
Piperdi B, Cline-Burkhardt M, Tian Y and Go WY: SPIRITT: A
randomized, multicenter, phase II study of panitumumab with FOLFIRI
and bevacizumab with FOLFIRI as second-line treatment in patients
with unresectable wild type KRAS metastatic colorectal cancer. Clin
Colorectal Cancer. 14:72–80. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sartore-Bianchi A, Trusolino L, Martino C,
Bencardino K, Lonardi S, Bergamo F, Zagonel V, Leone F, Depetris I,
Martinelli E, et al: Dual-targeted therapy with trastuzumab and
lapatinib in treatment-refractory, KRAS codon 12/13 wild-type,
HER2-positive metastatic colorectal cancer (HERACLES): A
proof-of-concept, multicentre, open-label, phase 2 trial. Lancet
Oncol. 17:738–746. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Grothey A, Sargent D, Goldberg RM and
Schmoll HJ: Survival of patients with advanced colorectal cancer
improves with the availability of fluorouracil-leucovorin,
irinotecan andoxaliplatin in the course of treatment. J Clin Oncol.
22:1209–1214. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cassidy J, Clarke S, Díaz-Rubio E,
Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS,
Rivera F, et al: Randomized phase III study of capecitabine plus
oxaliplatin compared with fluorouracil/folinic acid plus
oxaliplatin as first-line therapy for metastatic colorectal cancer.
J Clin Oncol. 26:2006–2012. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Douillard JY, Siena S, Cassidy J,
Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham
D, Jassem J, et al: Randomized, phase III trial of panitumumab with
infusional fluorouracil, leucovorin and oxaliplatin (FOLFOX4)
versus FOLFOX4 alone as first-line treatment in patients with
previously untreated metastatic colorectal cancer: The PRIME study.
J Clin Oncol. 28:4697–4705. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bokemeyer C, Bondarenko I, Hartmann, de
Braud F, Schuch G, Zubel A, Celik I, Schlichting M and Koralewski
P: Efficacy according to biomarker status of cetuximab plus
FOLFOX-4 as first-line treatment for metastatic colorectal cancer:
The OPUS study. An Oncol. 22:1535–1546. 2011. View Article : Google Scholar
|
|
42
|
Modest DP, Stintzing S, von Weikersthal
LF, Decker T, Kiani A, Vehling-Kaiser U, Al-Batran SE, Heintges T,
Lerchenmüller C, Kahl C, et al: Impact of subsequent therapies on
outcome of the FIRE-3/AIO KRK0306 trial: First-line therapy with
FOLFIRI plus cetuximab or bevacizumab in patients with KRAS
wild-type tumors in metastatic colorectal cancer. J Clin Oncol.
33:3718–3726. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Haller DG, Rothenberg ML, Wong AO,
Koralewski PM, Miller WH Jr, Bodoky G, Habboubi N, Garay C and
Olivatto LO: Oxaliplatin plus irinotecan compared with irinotecan
alone as second-line treatment after single-agent fluoropyrimidine
therapy for metastatic colorectal carcinoma. J Clin Oncol.
26:4544–4550. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Koopman M, Antonini NF, Douma J, Wals J,
Honkoop AH, Erdkamp FL, de Jong RS, Rodenburg CJ, Vreugdenhil G,
Loosveld OJ, et al: Sequential versus combination chemotherapy with
capecitabine, irinotecan, and oxaliplatin in advanced colorectal
cancer (CAIRO): A phase III randomised controlled trial. Lancet.
370:135–142. 2007. View Article : Google Scholar : PubMed/NCBI
|