Introduction
Differentiated thyroid carcinoma is a common
malignant tumor of the head and neck, of which papillary carcinoma
and follicular carcinoma are the main pathologic types (1). In clinical practice, differentiated
thyroid carcinoma is always characterized by relative high
malignancy and a slow growth of tumor tissues. Surgical resection
is the major procedure in the treatment of differentiated thyroid
carcinoma, however, the invasion and metastasis of tumor cells are
considered as major contributing factors to the recurrence of tumor
and death of patients (2–4).
An early diagnosis of differentiated thyroid
carcinoma is conducive to the prognosis of patients. Color Doppler
Ultrasonography is a common non-invasive detection method which can
help with early detection of thyroid node and thyroid carcinoma in
clinical practice (5). Human bone
marrow endothelium marker-1 (HBME-1), a common molecular marker of
tumors, has been suggested for its potential use in diagnosis and
prognosing differentiated thyroid carcinoma (6). Currently, there are few studies that
report the expression levels of HBME-1 protein in different types
of differentiated thyroid carcinoma tissues, and their correlation
with ultrasonic manifestation of thyroid. Thereupon, we conducted
immunohistochemistry (IHC) staining to detect the expression of
HBME-1 in nodular goiter, differentiated papillary carcinoma and
differentiated follicular carcinoma tissues, and compared the
differences in HBME-1 expression among the three groups to
investigate the correlation of HBME-1 expression in papillary
carcinoma and follicular carcinoma with ultrasonic manifestations
of thyroid.
Materials and methods
Sample selection
We selected a total of 130 patients with thyroid
diseases who were administered surgical resection at Longnan
Hospital of Daqing City between April, 2014 and April, 2016. The
subjects consisted of 50 patients with nodular goiter as the
control group, 58 patients with papillary thyroid carcinoma as the
papillary carcinoma group and 22 patients with follicular thyroid
carcinoma as the follicular carcinoma group. The control group is
composed of 22 males and 28 females, aged from 25 to 64 years with
an average age of 46.7±2.2 years. The papillary carcinoma group is
composed of 22 males and 36 females, aged from 25 to 69 years with
an average age of 46.9±2.6 years. The follicular carcinoma group is
composed of 10 males and 12 females, aged between 24 and 68 years
with an average of 46.4±2.3 years. The study was approved by the
Ethics Committee of Longnan Hospital of Daqing City and informed
consents were signed by the patients and/or guardians.
Ultrasound examination
In this study, 130 patients received the Color
Doppler Ultrasonography (HI VISION Preirus; Hitachi, Tokyo, Japan)
in which the frequency of the probe was set to 10–13 MHz. During
the examination, in order to fully expose the thyroid, patients
were required to stay in a supine position in order to observe the
size, shape, boundary, calcification, blood flow and cervical lymph
nodes of the nodules.
IHC
The expression of HBME-1 in the nodular goiter,
differentiated papillary thyroid carcinoma and differentiated
follicular thyroid carcinoma were detected using IHC. In this
study, the rabbit anti-human HBME-1 polyclonal antibodies were used
(1:500; cat. no. ab101139; Abcam, Cambridge, UK) The procedure was
conducted in strict accordance with the instructions of rabbit
anti-human HBME-1 polyclonal antibody kit.
Criteria (7) for IHC
assessment were set as follows: Judgment was made according to the
staining strength and area of positive cells, i.e.: i) 0 point for
no color, ii) 1 point for shallow yellow, iii) 2 points for yellow,
and iv) 3 points for medium brown. In each section, we randomly
selected 5 typical regions as the vision field for cell count under
the microscope (×400), and the results were averaged. The
proportion of positive cells was used for scoring according to the
following criteria: 0, 0%; 1, 1–25%; 2, 26–50%; 3, 51–75%; 4,
76–100%. The product of these two scores represented the IHC score,
and the specimen with the IHC score >6 points was considered as
positive.
Statistical analysis
The SPSS 20.0 (IBM, Armonk, NY, USA) software was
used for statistical analysis. Measurement data are presented as
mean ± SD. The Chi-square test was performed for comparison of
measurement data, paired samples t-test was conducted for
comparison of count data, and F-test for comparisons among three or
more groups. p<0.05 suggested that the difference was
statistically significant.
Results
Expression of HBME-1 in differentiated
thyroid carcinoma tissues
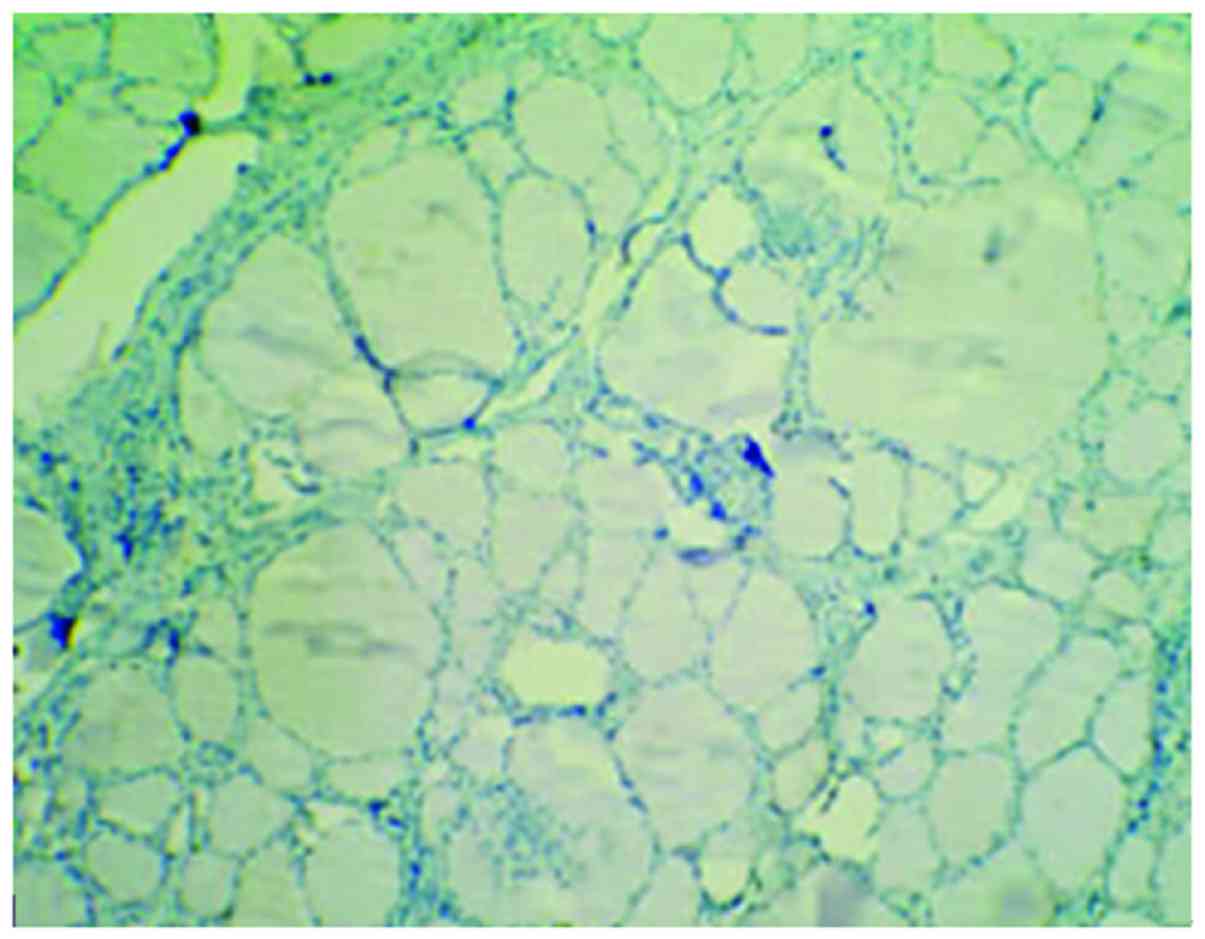
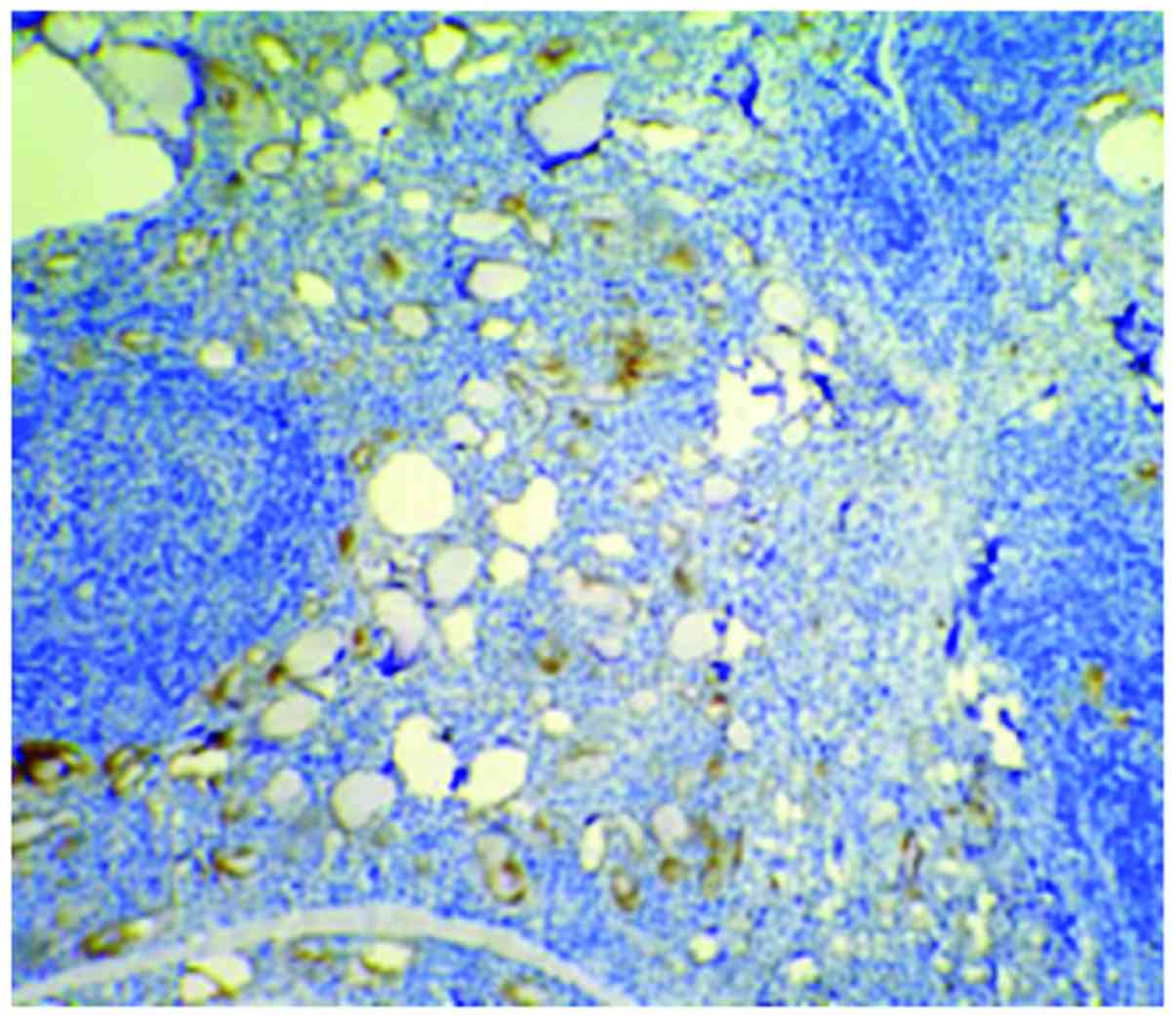
Cells with HBME-1 expression had pale brown
particles (Figs. 1–3). The level of HBME-1 expression and the
average IHC score of HBME-1 expression in the papillary carcinoma
group and the follicular carcinoma group were higher than those in
the nodular goiter group (p<0.05). In the papillary carcinoma
group, the average IHC score in affected tissues was higher than
that in the follicular carcinoma group (p<0.05). There were no
statistically significant differences in comparison of the positive
rate of HBME-1 expression between the papillary carcinoma group and
the follicular carcinoma group (p>0.05) (Table I).
 | Table I.Comparison of HBME-1 expressions among
three groups. |
Table I.
Comparison of HBME-1 expressions among
three groups.
|
|
| HBME-1 expression, n
(%) |
|
|---|
|
|
|
|
|
|---|
| Group | n | Positive | Negative | Average HIS score of
HBME-1 expression (point) |
|---|
| Control group | 50 | 13 (26) | 37 (74) | 3.12±1.65 |
| Papillary carcinoma
group | 58 | 40 (69) | 18 (31) | 8.01±2.74 |
| Follicular carcinoma
group | 22 | 15 (68.2) | 7 (31.8) | 5.56±2.18 |
| F-value |
| 15.562 | 13.815 | 17.447 |
| P-value |
| <0.05 | <0.05 | <0.05 |
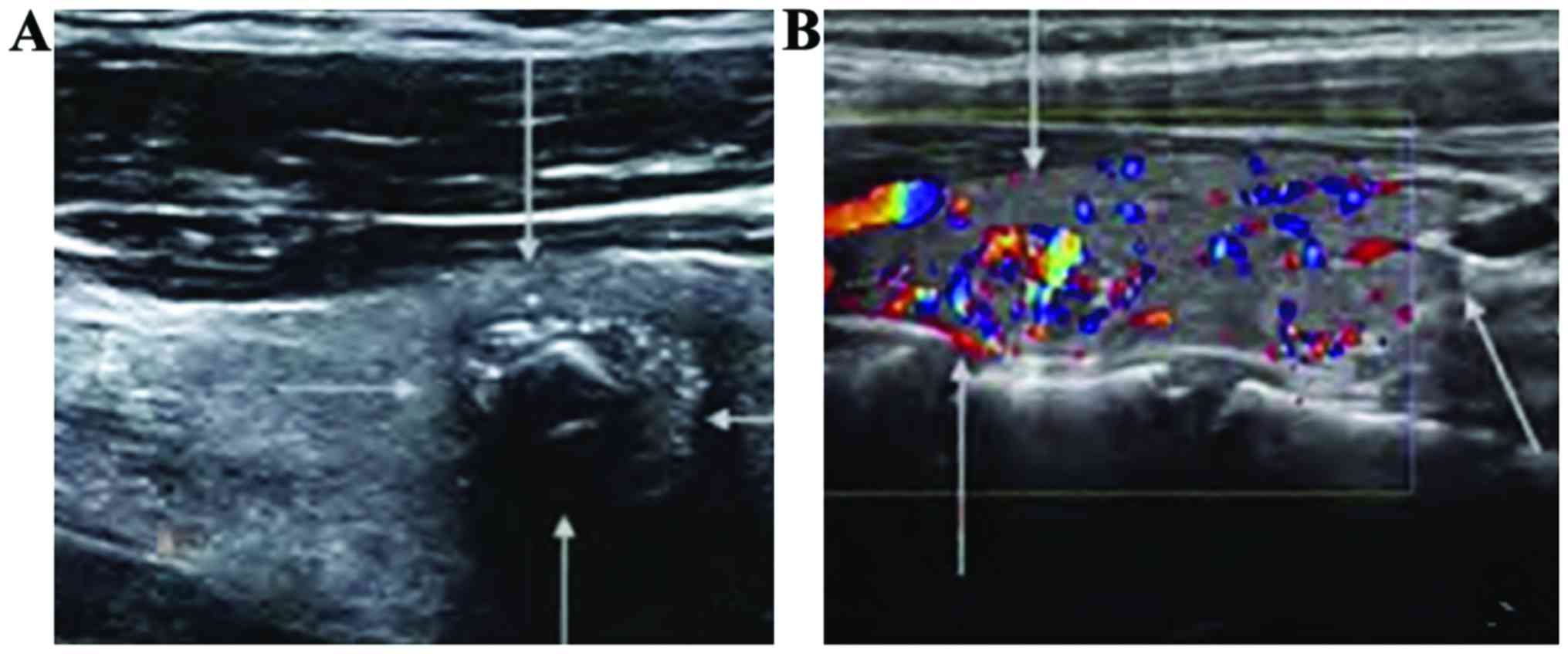
Comparison of ultrasonic
manifestations of thyroid between the papillary carcinoma group and
the follicular carcinoma group
When comparing the diameter, echo, shape, boundary,
calcification and blood flow signal between the papillary carcinoma
group and the follicular carcinoma group, differences were
statistically significant (p<0.05). However, the incidence of
enlargement of cervical lymph nodules was not statistically
significantly different between the two groups (p>0.05)
(Table II and Fig. 4).
 | Table II.Comparison of ultrasonic
manifestations of thyroid between the papillary carcinoma group and
the follicular carcinoma group [n (%)]. |
Table II.
Comparison of ultrasonic
manifestations of thyroid between the papillary carcinoma group and
the follicular carcinoma group [n (%)].
|
|
| Echo | Shape | Boundary | Calcification | Blood flow | Cervical lymph
nodules |
|---|
|
|
|
|
|
|
|
|
|
|---|
| Group | Diameter (cm) | Low echo | Non-low echo | Irregular | Regular | Not clear | Clear | Gross
calcification | Egg shell
calcification | Gravel
calcification | No | Point-strip blood
flow | Abundant | Enlargement | No enlargement |
|---|
| Papillary carcinoma
group (n=58) | 2.17±1.06 | 57 (98.3) | 1 (1.7) | 48 (82.8) | 10 (17.2) | 46 (79.3) | 12(20.8) | 11(19) | 8 (13.8) | 39 (67.2) | 4 (6.9) | 28 (48.3) | 26 (44.8) | 21 (36.2) | 37 (63.8) |
| Follicular carcinoma
group (n=22) | 3.68±1.53 | 16 (72.7) | 6 (27.3) | 12 (54.5) | 10 (45.5) | 12 (54.5) | 10 (45.5) | 9 (40.9) | 9 (40.9) | 4 (18.2) | 6 (27.3) | 5 (22.7) | 11 (50) | 8 (36.4) | 14 (63.6) |
| t/χ2 | 6.942 | 7.873 | 8.951 | 8.005 |
| 10.237 |
|
| 9.658 |
| 0.405 |
| P-value | <0.05 | <0.05 | <0.05 | <0.05 |
| <0.05 |
|
| <0.05 |
| >0.05 |
Correlation of HBME-1 expression in
affected tissues with the ultrasonic manifestation of thyroid in
the papillary carcinoma group and the follicular carcinoma
group
Among patients in the papillary carcinoma group, we
discovered that there were no statistically significant differences
in the comparison of the diameter, echo, shape, boundary,
calcification and enlargement of lymph nodules between the HBME-1
positive patients and HBME-1 negative patients (p>0.05). The
proportion of HBME-1 positive patients with a signal of blood flow
was higher than that in the HBME-1 negative patients (Table III). In patients of the follicular
carcinoma group, the comparison of ultrasonic manifestations of
thyroid between the HBME-1 positive patients and HBME-1 negative
patients presented no statistically significant differences
(p>0.05) (Table IV).
 | Table III.Comparison of the ultrasonic
manifestations of thyroid between the HBME-1 positive patients and
HBME-1 negative patients in the papillary carcinoma group [n
(%)]. |
Table III.
Comparison of the ultrasonic
manifestations of thyroid between the HBME-1 positive patients and
HBME-1 negative patients in the papillary carcinoma group [n
(%)].
|
|
| Echo | Shape | Boundary | Calcification | Blood flow | Cervical lymph
nodules |
|---|
|
|
|
|
|
|
|
|
|
|---|
| Group | Diameter (cm) | Low echo | Non-low echo | Irregular | Regular | Not clear | Clear | Low echo | Non-low echo | Irregular | Regular | Not clear | Clear | Low echo | Non-low echo |
|---|
| HBME-1 positive
patients (n=40) | 1.97±0.85 | 39 (97.5) | 1 (2.5) | 33 (82.5) | 7 (17.5) | 32 (80) | 8 (20) | 9 (22.5) | 7 (17.5) | 24 (50) | 0 (0) | 27 (67.5) | 13 (32.5) | 12 (30) | 28 (70) |
| HBME-1 negative
patients (n=18) | 1.54±0.63 | 18 (100) | 0 (0) | 14 (77.7) | 4 (22.2) | 16 (88.9) | 2 (11.1) | 5 (27.8) | 4 (22.2) | 9 (50) | 4 (22.2) | 4 (22.2) | 10 (55.6) | 5 (27.8) | 13 (72.2) |
| t/χ2 | 5.761 | 6.672 | 7.946 | 9.553 |
| 11.641 |
|
| 9.857 |
| 0.383 |
| P-value | <0.05 | <0.05 | <0.05 | <0.05 |
| <0.05 |
|
| <0.05 |
| >0.05 |
 | Table IV.Comparison of the ultrasonic
manifestations of thyroid between the HBME-1 positive patients and
HBME-1 negative patients in the follicular carcinoma group [n
(%)]. |
Table IV.
Comparison of the ultrasonic
manifestations of thyroid between the HBME-1 positive patients and
HBME-1 negative patients in the follicular carcinoma group [n
(%)].
|
|
| Echo | Shape | Boundary | Calcification | Blood flow | Cervical lymph
nodules |
|---|
|
|
|
|
|
|
|
|
|
|---|
| Group | Diameter (cm) | Low echo | Non-low echo | Irregular | Regular | Not clear | Clear | Low echo | Non-low echo | Irregular | Regular | Not clear | Clear | Low echo | Non-low echo |
|---|
| HBME-1 positive
patients (n=15) | 2.13±0.42 | 8 (53.3) | 7 (46.7) | 8 (53.3) | 7 (46.7) | 8 (53.3) | 7 (46.7) | 6 (40) | 6 (40) | 5 (20) | 3 (20) | 9 (60) | 3 (20) | 3 (20) | 9 (6) |
| HBME-1 negative
patients (n=7) | 2.11±0.37 | 18 (100) | 6 (85.7) | 4 (57.1) | 3 (42.9) | 4 (57.1) | 3 (42.9) | 5 (71.4) | 1 (14.3) | 1 (14.3) | 3 (42.9) | 1 (14.3) | 3 (42.9) | 3(42.9) | 4 (57.1) |
| t/χ2 | 0.282 | 0.321 | 0.467 | 0.008 |
| 0.017 |
|
| 0.034 |
| 0.006 |
| P-value | >0.05 | >0.05 | >0.05 | >0.05 |
| >0.05 |
|
| >0.05 |
| >0.05 |
Discussion
In clinical practice, we always encounter patients
that suffer from the thyroid lesions with atypical symptoms, such
as papillary carcinoma or papillary hyperplasia, which possess some
difficulties in differentiation of these atypical symptoms. For
patients with thyroid micro-carcinoma that have a lesion with a
diameter <0.5 cm, if there are not any characteristic papillary
structures, then the collagen tissues would be distributed in the
follicular structures under the microscope, and, accordingly, a
differential diagnosis would be given to exclude the possibility of
simple hyperplasia of collagen tissue (8–11).
Clinical practice (12–14) has proven that IHC can be used for a
differential diagnosis of papillary thyroid carcinoma. In recent
years, more and more attention has been paid to some tumor markers
with high sensitivity, such as cytokeratin 19 (CK19) and HBME-1.
These markers have been applied in the clinical diagnosis of many
malignant tumors, including thyroid carcinoma (13,15,16).
HBME-1 is an antigen constituent of the microvilli
on the surface of mesothelial cells in humans, and hyaluronic acid
(HA) is the main ingredient of HBME-1 (17). Studies (18,19) have
reported that the high expression of HBME-1 can be detected in
papillary thyroid carcinoma tissues. In this study, our results
show that in the papillary carcinoma group and the follicular
carcinoma group, the level of expression of HBME-1 in affected
tissues and the IHC score of HBME-1 expression were all higher than
those in the control group (p<0.05). In the papillary carcinoma
group, the mean IHC score of HBME-1 expression in affected tissues
was higher than that in the follicular carcinoma group (p<0.05).
There were no statistically significant differences in comparison
to HBME-1 expression in affected tissues between the papillary
carcinoma group and the follicular carcinoma group (p>0.05).
These results suggested that HBME-1 is highly expressed in these
pathologic types (papillary and follicular) of thyroid carcinoma,
and there may be no significant differences in the comparison of
the positive rate of expression between different pathologic types
of thyroid carcinoma. In addition, the IHC score of HBME-1
expression in papillary thyroid carcinoma tissues is significantly
higher than that in the follicular thyroid carcinoma tissues, and
therefore, we can infer that HBME-1 expression in papillary
carcinoma tissues is remarkably higher than that in the follicular
carcinoma tissues.
In recent years, color Doppler ultrasonography has
been widely applied in clinical practice, which effectively
improves the clinical diagnosis of malignant tumors in the thyroid.
In clinical practice, the ultrasonic diagnosis of thyroid for
judgment of the type of thyroid nodule (malignant or benign) is
made through evaluating the shape and boundary of nodule, echo
intensity, calcification and blood flow signals. Generally,
ultrasonic manifestations of thyroid, including low echo, irregular
shape, obscure boundary and micro-calcification, are considered as
potential signs of thyroid carcinoma. In this study, our results
show that between the papillary carcinoma group and the follicular
carcinoma group, differences in the comparison of the nodule
diameter, echo, shape, boundary, calcification and blood flow
signal are statistically significant (p<0.05), however, there
are no statistically significant differences in the comparison of
incidence rate of the enlargement of cervical lymph nodules
(p>0.05). Among patients in papillary carcinoma group, the
difference in the nodule diameter, echo, shape, boundary,
calcification and blood flow signal between the HBME-1-positive
patients and the HBME-1-negative patients were no statistically
significant (p>0.05). On the other hand, in the nodules of
HBME-1-positive patients, the proportion of blood flow signal was
higher than that in the nodules of HBME-1-negative patients. Among
patients in the follicular carcinoma group, there was no
statistically significant difference in the comparison of
ultrasonic manifestation of the thyroid between the HBME-1 positive
patients and the HBME-1 negative patients (p>0.05). Our results
indicate that there is increased blood flow in HBME-1 positive
patients with papillary carcinoma, and there are no statistically
significant differences in the comparison of ultrasonic
manifestations of thyroid between the HBME-1-positive and -negative
patients with follicular carcinoma.
In conclusion, in the tissues of papillary carcinoma
and follicular differentiated thyroid carcinoma, there are
differences in comparison of IHC scores of HBME-1 expression and
some ultrasonic manifestation of thyroid. Among the papillary
thyroid carcinoma patients, the blood flow signal of the HBME-1
positive patients is much higher than that of the HBME-1 negative
patients.
References
|
1
|
Asa SL, Giordano TJ and LiVolsi VA:
Implications of the TCGA genomic characterization of papillary
thyroid carcinoma for thyroid pathology: Does follicular variant
papillary thyroid carcinoma exist? Thyroid. 25:1–2. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mebed AH: Aggressive surgical therapy for
locally invasive differentiated thyroid carcinoma: An experience of
nineteen (19) cases. J Egypt Natl Canc Inst. 19:282–291.
2007.PubMed/NCBI
|
|
3
|
Paschke R, Lincke T, Müller SP, Kreissl
MC, Dralle H and Fassnacht M: The Treatment of Well-Differentiated
Thyroid Carcinoma. Dtsch Arztebl Int. 112:452–458. 2015.PubMed/NCBI
|
|
4
|
Xu D, Wang L, Long B, Ye X, Ge M, Wang K,
Guo L and Li L: Radiofrequency ablation for postsurgical thyroid
removal of differentiated thyroid carcinoma. Am J Transl Res.
8:1876–1885. 2016.PubMed/NCBI
|
|
5
|
Cantisani V, Maceroni P, DAndrea V,
Patrizi G, Di Segni M, De Vito C, Grazhdani H, Isidori AM,
Giannetta E, Redler A, et al: Strain ratio ultrasound elastography
increases the accuracy of colour-Doppler ultrasound in the
evaluation of Thy-3 nodules. A bi-centre university experience. Eur
Radiol. 26:1441–1449. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
de Matos PS, Ferreira AP, de Oliveira
Facuri F, Assumpção LV, Metze K and Ward LS: Usefulness of HBME-1,
cytokeratin 19 and galectin-3 immunostaining in the diagnosis of
thyroid malignancy. Histopathology. 47:391–401. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sadamori H, Yagi T, Iwagaki H, Matsuda H,
Shinoura S, Umeda Y, Ohara N, Yanai H, Ogino T and Tanaka N:
Immunohistochemical staining of liver grafts with a monoclonal
antibody against HCV-Envelope 2 for recurrent hepatitis C after
living donor liver transplantation. J Gastroenterol Hepatol.
24:574–580. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Verslype C, Nevens F, Sinelli N, Clarysse
C, Pirenne J, Depla E, Maertens G, van Pelt J, Desmet V, Fevery J,
et al: Hepatic immunohistochemical staining with a monoclonal
antibody against HCV-E2 to evaluate antiviral therapy and
reinfection of liver grafts in hepatitis C viral infection. J
Hepatol. 38:208–214. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Casey MB, Lohse CM and Lloyd RV:
Distinction between papillary thyroid hyperplasia and papillary
thyroid carcinoma by immunohistochemical staining for cytokeratin
19, galectin-3, and HBME-1. Endocr Pathol. 14:55–60. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee YS, Yun JS, Jeong JJ, Nam KH, Chung WY
and Park CS: Thyroid hemiagenesis associated with thyroid
adenomatous hyperplasia and papillary thyroid carcinoma. Thyroid.
18:381–382. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dong S, Song XS, Chen G and Liu J: Mixed
primary squamous cell carcinoma, follicular carcinoma, and
micropapillary carcinoma of the thyroid gland: A case report. Auris
Nasus Larynx. 43:455–459. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bychkov A, Sampatanukul P, Shuangshoti S
and Keelawat S: TROP-2 immunohistochemistry: A highly accurate
method in the differential diagnosis of papillary thyroid
carcinoma. Pathology. 48:425–433. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Erdogan-Durmus S, Ozcan D, Yarikkaya E,
Kurt A and Arslan A: CD56, HBME-1 and cytokeratin 19 expressions in
papillary thyroid carcinoma and nodular thyroid lesions. J Res Med
Sci. 21:49–54. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen YJ, Zhao RM, Zhao Q, Li BY, Ma QY, Li
X and Chen X: Diagnostic significance of elevated expression of
HBME-1 in papillary thyroid carcinoma. Tumour Biol. 37:8715–8720.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhu X, Sun T, Lu H, Zhou X, Lu Y, Cai X
and Zhu X: Diagnostic significance of CK19, RET, galectin-3 and
HBME-1 expression for papillary thyroid carcinoma. J Clin Pathol.
63:786–789. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu Z, Yu P, Xiong Y, Zeng W, Li X,
Maiaiti Y, Wang S, Song H, Shi L, Liu C, et al: Significance of
CK19, TPO, and HBME-1 expression for diagnosis of papillary thyroid
carcinoma. Int J Clin Exp Med. 8:4369–4374. 2015.PubMed/NCBI
|
|
17
|
El-Mahdy MM, Mabrouk SH, El-Din ZS, Ghazal
FA and Mohamed HH: Diagnostic value of HBME-1 and CK19 expression
in papillary thyroid carcinoma, well-differentiated tumors of
uncertain malignant potential, and benign thyroid nodules. Egypt J
Pathol. 31:68–74. 2011. View Article : Google Scholar
|
|
18
|
Chao TT, Maa HC, Wang CY, Pei D, Liang YJ,
Yang YF, Chou SJ and Chen YL: CIP2A is a poor prognostic factor and
can be a diagnostic marker in papillary thyroid carcinoma. APMIS.
124:1031–1037. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yeşil C, Kandemir O, Haksever H and
Dabakoğlu T: Is BECLIN-1 immunoreactivity more effective than
HBME-1 in diagnosis of papillary thyroid cancer? Acta Chir Belg.
115:299–305. 2015. View Article : Google Scholar : PubMed/NCBI
|