Introduction
Hepatocellular carcinoma (HCC) remains one of the
most common malignancies, and is a leading cause of
cancer-associated mortality due to its high mortality rate
(1). Although previous studies have
demonstrated that surgery is the first choice for HCC treatment,
the diagnostic methods for HCC remain restricted due to hidden
lesions and an increased metastatic rate (2,3). The
5-year survival rate of patients with HCC was 5–9% in the United
States in 2009 (4). Therefore, it is
important to evaluate biomarkers for HCC treatment.
microRNAs (miRNAs/miRs) are endogenous, highly
conserved non-coding RNAs, 20–22 nucleotides in length, that
function in a variety of biological processes at the
transcriptional or post-transcriptional level by targeting the
3′-untranslated regions of genes (5).
Previous evidence has suggested that various miRNAs are involved in
the progression and underlying biology of HCC (6,7). For
example, Tsai et al (8)
indicated that the tumor suppressor miR-122 regulates HCC
metastasis, and Meng et al (9)
suggested that miR-21 expression is abnormal in HCC tissue and is
correlated with HCC growth via regulation of cytochrome b6-f
complex subunit 8, chloroplastic expression. Additionally, miR-34a
is considered to be a tumor suppressor in various types of cancer,
including colon and breast cancer, and to be involved in a variety
of biological processes (9,10). Previous studies have revealed a
significant correlation between miR-34a expression and cancer
metastasis, including in HCC (11,12).
However, few studies refer to the involvement of miR-34a in HCC
metastasis. Li et al (13)
indicated that miR-34a functions as an inhibitor for HCC cell
migration and invasion via the downregulation of c-Met expression.
Although several studies have suggested the involvement of miR-34a
in HCC cell migration or invasion, the basic mechanism remains
unknown.
In the present study, the potential effects of
miR-34a expression on HCC cell migration and invasion were
investigated using Hep3B and Huh7 cells. Comprehensive experimental
methods were used to analyze the potential underlying
mechanism.
Materials and methods
Cell lines and cell culture
The human hepatocellular carcinoma cell lines Hep3B
and Huh7 and the normal hepatocyte cell line THLE-2 were obtained
from the American Type Culture Collection (Manassas, VA, USA). All
cells were cultured in Dulbecco's modified Eagle's medium (DMEM;
Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
containing 10% fetal bovine serum (FBS; Invitrogen; Thermo Fisher
Scientific, Inc.) at 37°C in 5% CO2.
Cell transfection
Cells were plated onto a 60-mm dish, and following
incubation for 24 h, the overexpression vector for miR-34a (sense,
5′-UGGCAGUGUCUUAGCUGGUUGU-3′ and antisense,
5′-AACCAGCUAAGACACUGCCAUU-3′) (Sangon Biotech Co., Ltd., Shanghai,
China) or the scramble control miRNA (sense,
5′-UGUCAGCUUUGGAGCUGGUUGU-3′ and antisense,
5′-AACCUAAGAUGCCACCAGCAUU-3′) was transfected into the Hep3B and
Huh7 cells using Lipofectamine 2000 transfection reagent (Thermo
Fisher Scientific, Inc.). Following transfection for 48 h at 37°C,
cells were prepared for additional analysis.
Cell invasion assay
For cell invasion assay, the Transwell with Matrigel
method was performed as previously described (14). Briefly, following transfection for 48
h, hepatocellular cells (5×105/ml) were re-suspended in
serum-free DMEM containing 0.01% bovine serum albumin (BSA;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) for an additional 24
h. The upper chamber of the Transwell plate was covered with
serum-free DMEM supplemented with 50 mg/l Matrigel, and then
air-dried at 4°C for 15 min. Then, 50 µl fresh serum-free DMEM
medium containing 10 g/l BSA was added, and cultured for 30 min at
37°C. Following removal of this medium, 200 µl cell suspension was
added into the upper chamber of the Transwell plate, and 600 µl
complete DMEM culture supplemented with 10% FBS (Sigma-Aldrich;
Merck KGaA). After 48 h of incubation at 37°C in 5% CO2,
the Transwell plate was washed with PBS buffer to remove the cells
on the upper side of the microporous membrane, followed with
fixation in ice-cold alcohol for 30 min. Non-migratory cells were
removed from the upper surface of the filter with a cotton swab.
Finally, the cells were stained with 0.1% crystal violet for 30 min
at room temperature, and then decolorized with 33% acetic acid. The
absorbance of eluents was observed at an optical density of 570 nm
using a microplate reader (Bio-Rad Laboratories, Inc., Hercules,
CA, USA). Cells transfected with the control vector served as the
negative control.
Cell migration assay
For the cell migration assay, the wound healing
asssay was performed as previously described (15). Hep3B and Huh7 cells (1×105)
transfected with miR-34a overexpression or scramble controls were
seeded in 24-well plates and grown overnight to achieve confluence.
The monolayer cells were scratched using a 20 µl pipette tip to
create the wound. The floating cells were removed by washing twice
with PBS (Sigma-Aldrich; Merck KGaA). Subsequently, serum-free DMEM
was added to permit wound healing. The rate of wound closure was
assessed using images captured after 24 h. Image analysis was
performed using Image-Pro Plus software version 6.0 (Media
Cybernetics, Inc., Rockville, MD, USA).
Quantitative reverse transcription
polymerase chain reaction (RT-qPCR)
Total RNA from the Hep3B or Huh7 cells was collected
48 h after transfection and isolated using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc., USA) as previously
described (16). The samples were
treated with RNase-free DNase I (Promega Corporation, Madison, WI,
USA). Consequently, the concentration and purity for the isolated
RNA were measured with SMA 400 UV-VIS (Merinton Instrument, Ltd.,
Beijing, China). Purified RNA at a density of 0.5 µg/µl with
nuclease-free water was used for cDNA synthesis using the
PrimerScript 1st Strand cDNA Synthesis kit (Invitrogen; Thermo
Fisher Scientific, Inc.), with the following temperature protocol:
30°C for 10 min, 42°C for 50 min, and 95°C for 5 min. The
expressions of targets in the cells were detected in an Eppendorf
Mastercycler (Brinkmann Instruments, Westbury, NY, USA) using the
SYBR ExScript RT-qPCR kit (Takara Bio, Inc., Otsu, Japan). The
total reaction system of 20 µl volume was as follows: 1 µl cDNA
from the above PCR, 10 µl SYBR Premix EX Taq, 1 µl each of the
primers (10 µM), and 7 µl ddH2O. The PCR thermocycler
conditions were as follows: Denaturation at 50°C for 2 min; 95°C
for 10 min; followed by 45 cycles of 95°C for 10 sec and 60°C for 1
min. Melting curve analysis of amplification products was performed
at the end of each PCR to confirm that only one product was
amplified and detected. These data were quantified using the
2−ΔΔCq method (17). GAPDH
was used as the internal control. Primers used for the
amplification of the targets are listed in Table I.
 | Table I.Primers used for target amplification
in the present study. |
Table I.
Primers used for target amplification
in the present study.
| Name | Primer | Sequence (5′-3′) |
|---|
| GAPDH | Sense |
GGGTGGAGCCAAACGGGTC |
|
| Antisense |
GGAGTTGCTGTTGAAGTCGCA |
| SIRT1 | Sense | CAGAGCAT
CACACGCAAGC |
|
| Antisense | CAGGAAACAG
AAACCCCAGC |
| p53 | Sense |
TTCCTCTTCCTGCAGTACTC |
|
| Antisense |
ACCCTGGGCAACCAGCCCTGT |
Western blot analysis
Cells cultured for 48 h were lysed with
radioimmunoprecipitation buffer (Sangon Biotech Co., Ltd.)
containing phenylmethanesulfonyl fluoride (Sigma-Aldrich; Merck
KGaA), and the lysates were then centrifuged at 12,000 × g for 10
min at 4°C. The supernatants were collected, and protein
concentrations were determined using a bicinchoninic protein assay
kit (Pierce; Thermo Fisher Scientific, Inc.). The proteins (30
µg/lane) were separated using SDS-PAGE on a 10% gel, as previously
described (18) followed by transfer
onto a polyvinylidene fluoride membrane (Merck KGaA). The membranes
were blocked using Tris-buffered saline with 0.05% Tween-20 (TBST)
containing 5% non-fat milk for 1 h at room temperature, and then
incubated with rabbit anti-human antibodies [(purchased from Abcam
(Cambridge, MA, USA)] against sirtuin 1 (SIRT1; cat. no. ab12193),
tumor protein 53 (p53; cat. no. ab131442), acetylate-p53 (Ac-p53;
cat. no. ab75754) and GAPDH (cat. no. ab37168; all 1:100) overnight
at 4°C. Subsequently, the membranes were incubated with a
horseradish peroxidase-conjugated goat anti-rabbit IgG secondary
antibody (cat. no. ab205718; 1:5,000; Abcam) for 1 h at room
temperature. Finally, the polyvinylidene fluoride membranes were
washed 3 times with 1X TBST buffer for 10 min each time. The
signals were detected following incubation of the membranes with a
chromogenic substrate using the SuperSignal® West Pico
chemiluminescent western blotting substrate (Pierce; Thermo Fisher
Scientific, Inc.). Analysis was performed using the Image Guage 4.0
program (Fuji, Tokyo, Japan). GAPDH served as the internal
control.
Statistical analysis
All experiments were conducted independently 3
times. All data are presented as the mean ± standard deviation. The
significant difference for the data was calculated using SPSS v19.0
statistical software. P-values were calculated using one-way
analysis of variance followed by Duncan's multiple-range test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Expression of miR-34a in Hep3B and
Huh7 cells
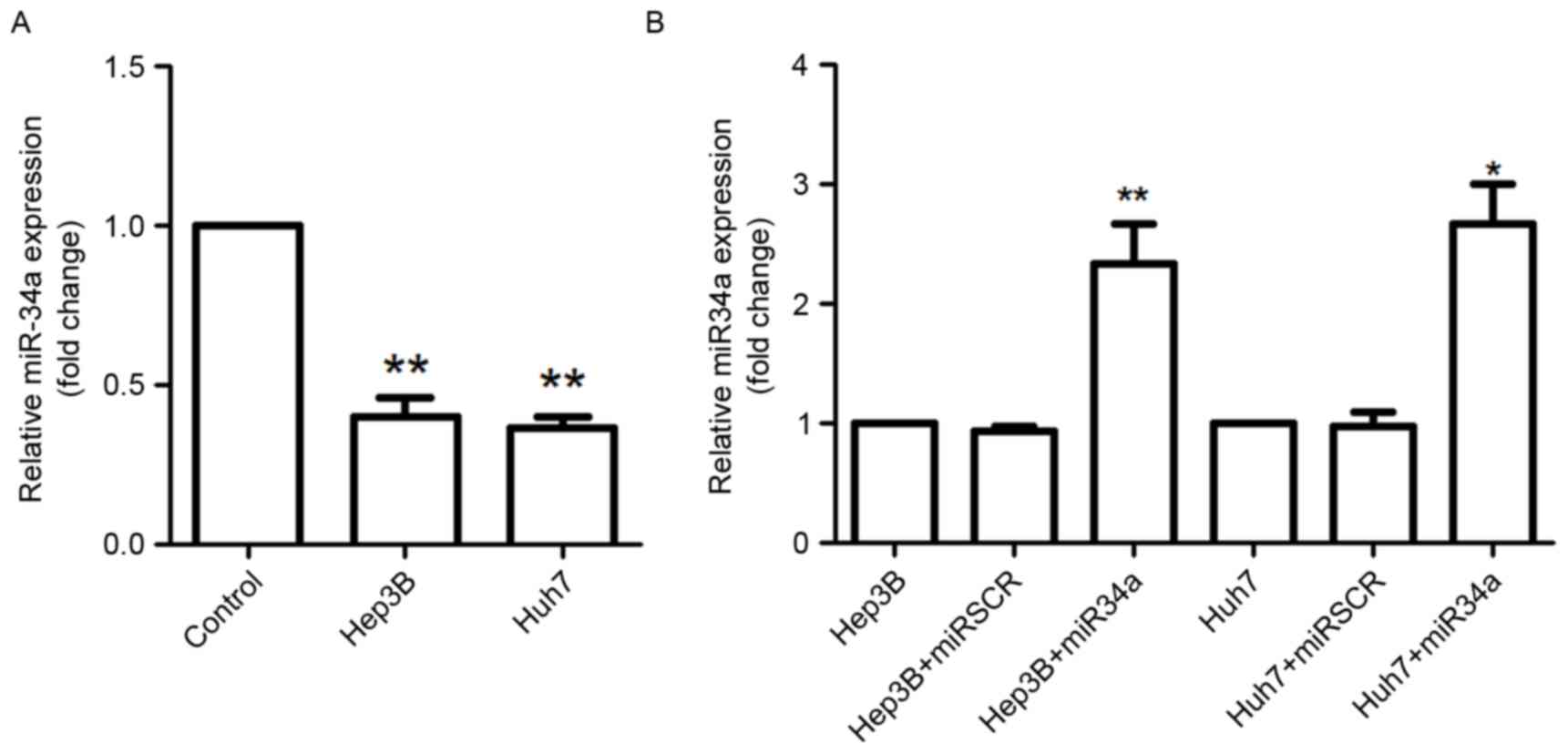
The relative expression levels of miR-34a in THLE-2,
Hep3B and Huh7 cells were assessed using RT-qPCR analysis (Fig. 1). The results demonstrated that
miR-34a expression was significantly decreased in Hep3B and Huh7
cells compared with that in the THLE-2 cells (P<0.01; Fig. 1A). The present study further analyzed
the expression of miR-34a in Hep3B and Huh7 cells following
transfection with miR-34a overexpression vectors, and the results
suggested that miR-34a was highly expressed in these cells by
miR-34a overexpression transfection compared with miR-34a
expression in scramble control cells (Huh7 cells, P<0.05; Hep3B
cells, P<0.01; Fig. 1B).
miR-34a overexpression suppresses
hepatocellular cell migration
When cells were transfected with miR-34a
overexpression vectors, the number of migrated cells was
significantly decreased compared with that for the scramble control
group (P<0.01; Fig. 2). There was
no significant difference between the number of migrated cells for
the negative control group and that for the scramble treated
cells.
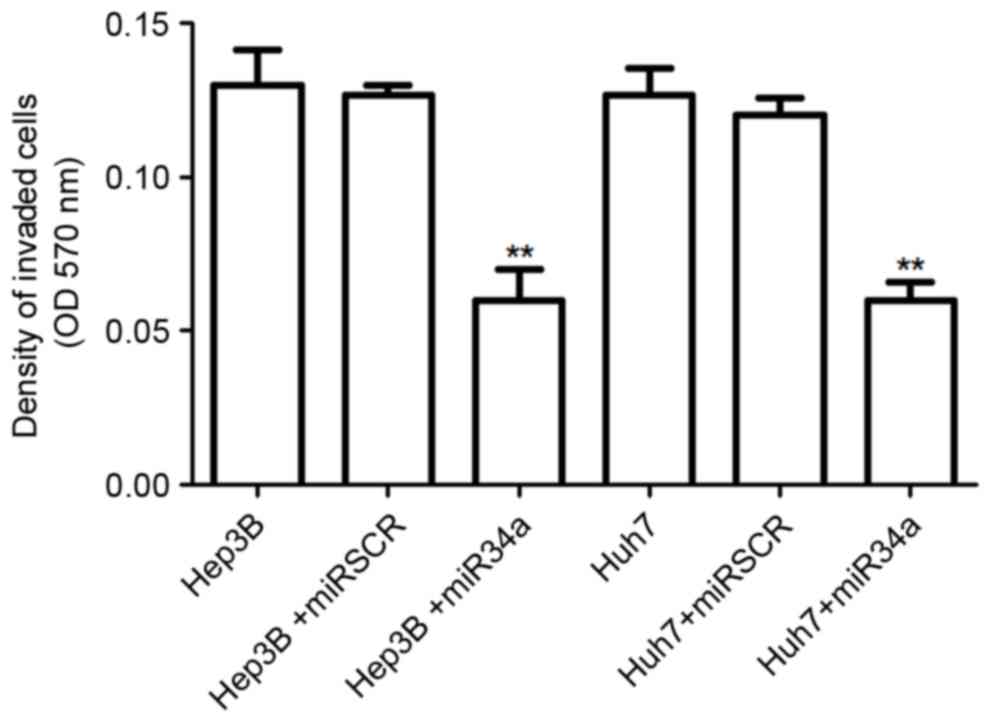
miR-34a overexpression suppresses
hepatocellular cell invasion
The effect of miR-34a expression on hepatocellular
cell invasion was also detected. The results suggested that the
number of invasive cells was significantly decreased by the
overexpression of miR-34a in Hep3B and Huh7 cells compared with
that in the control group (P<0.01; Fig. 3). However, there was no significant
difference in the number of invasive cells between control cells
and the scramble treated cells.
Effects of miR-34a expression on cell
metastasis-associated protein expressions
To further investigate the potential mechanism
underlying the effect of miR-34a on hepatocellular cell migration
and invasion, the expression of proteins including SIRT1, p53 and
Ac-p53 were analyzed (Fig. 4). The
results demonstrated that the SIRT1 mRNA and protein levels were
significantly decreased by the overexpression of miR-34a
(P<0.01; Fig. 4A and B). The
Ac-p53 protein level was significantly increased by the
overexpression of miR-34a in Hep3B and Huh7 cells, compared with
that in control cells (P<0.05; Fig. 4A
and B).
Discussion
Previous studies have demonstrated that miRNAs serve
crucial functions in tumor biology, and miR-34a has been suggested
to be associated with HCC cell migration and invasion (6,19). The
present study analysed the function of miR-34a expression in HCC
cell migration and invasion, and its potential underlying
mechanism. In agreement with previous data (20), the results indicated that miR-34a was
downregulated in the two types of HCC cells. Following transfection
with the miR-34a overexpression vector, the numbers of migrated and
invaded cells were significantly decreased by the overexpression of
miR-34a in Hep3B and Huh7 cells. Additionally, the protein and mRNA
levels of SIRT1 were suppressed, while the protein levels for
Ac-p53 were increased by overexpression of miR-34a.
These results demonstrated that the number of the
migrated and invaded Hep3B or Huh7 cells was significantly reduced
by the overexpression of miR-34a, suggesting that miR-34a
expression was associated with HCC cell migration and invasion.
Cell migration and invasion are important biological processes
associated with tumor metastasis (21). Previous studies have demonstrated that
miR-34a functions as a tumor suppressor in various tumors,
including colon cancer and neuroblastoma (9,22). miR-34a
has been suggested to be involved in the venous metastasis of
Hepatitis B virus-positive HCC (23).
Except for the study conducted by Li et al (13), the involvement of miR-34a expression
in HCC cell migration and invasion has not been fully discussed.
Based on the results of the present study, it was hypothesized that
the overexpression of miR-34a may inhibit HCC metastasis through
suppressing cell migration and invasion.
Concurrently, SIRT1 is an nicotine adenine
dinucleotide-dependent deacetylase that is involved in multiple
biological processes, including DNA damage, apoptosis and
proliferation (24). A previous study
demonstrated that SIRT1 expression is correlated with breast cancer
invasion and metastasis (25), and
Hao et al (26) suggested that
the overexpression of SIRT1 promotes HCC metastasis through the
epithelial mesenchymal transition. In the present study, SIRT1
expression was downregulated by the overexpression of miR-34a,
indicating that miR-34a may suppress HCC metastasis via the
downregulation of SIRT1. Conversely, Lewis et al (27) demonstrated that the absence of p53
promotes HCC metastasis in a mouse model. It has been revealed that
deacetylated p53 is associated with cell growth (28). Ac-p53 is associated with breast cancer
cell migration and invasion through the targeting of the
SMAR1 gene (29). The results
of the present study demonstrated that the overexpression of
miR-34a increased Ac-p53 expression, suggesting that miR-34a may
suppress HCC cell migration and invasion by increasing Ac-p53
expression.
Taken together, the results of the present study
revealed that the overexpression of miR-34a may be a suppressor of
HCC metastasis. The overexpression of miR-34a inhibits Hep3B and
Huh7 cell migration and invasion, and downregulates SIRT1
expression while increasing Ac-p53 expression. The present study
may provide a theoretical basis for studies investigating the
mechanisms of HCC pathogenesis and metastasis, and for the
potential application of miR-34a in HCC metastasis diagnosis.
However, additional studies are needed to explore the basic
underlying mechanisms.
References
|
1
|
Utsunomiya T, Shimada M, Kudo M, Ichida T,
Matsui O, Izumi N, Matsuyama Y, Sakamoto M, Nakashima O, Ku Y, et
al: Nationwide study of 4741 patients with non-B non-C
hepatocellular carcinoma with special reference to the therapeutic
impact. Ann Surg. 259:336–345. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bruix J and Sherman M: American
Association for the Study of Liver Diseases: Management of
hepatocellular carcinoma: An update. Hepatology. 53:1020–1022.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
El-Serag HB, Marrero JA, Rudolph L and
Reddy KR: Diagnosis and treatment of hepatocellular carcinoma.
Gastroenterology. 134:1752–1763. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Martin RC, Scoggins CR and McMasters KM:
Safety and efficacy of microwave ablation of hepatic tumors: A
prospective review of a 5-year experience. Ann Surg Oncol.
17:171–178. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shukla GC, Singh J and Barik S: MicroRNAs:
Processing, maturation, target recognition and regulatory
functions. Mol Cell Pharmacol. 3:83–92. 2011.PubMed/NCBI
|
|
6
|
Ladeiro Y, Couchy G, Balabaud C,
Bioulac-Sage P, Pelletier L, Rebouissou S and Zucman-Rossi J:
MicroRNA profiling in hepatocellular tumors is associated with
clinical features and oncogene/tumor suppressor gene mutations.
Hepatology. 47:1955–1963. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Datta J, Kutay H, Nasser MW, Nuovo GJ,
Wang B, Majumder S, Liu CG, Volinia S, Croce CM, Schmittgen TD, et
al: Methylation mediated silencing of MicroRNA-1 gene and its role
in hepatocellular carcinogenesis. Cancer Res. 68:5049–5058. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tsai WC, Hsu PW, Lai TC, Chau GY, Lin CW,
Chen CM, Lin CD, Liao YL, Wang JL, Chau YP, et al: MicroRNA-122, a
tumor suppressor microRNA that regulates intrahepatic metastasis of
hepatocellular carcinoma. Hepatology. 49:1571–1582. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tazawa H, Tsuchiya N, Izumiya M and
Nakagama H: Tumor-suppressive miR-34a induces senescence-like
growth arrest through modulation of the E2F pathway in human colon
cancer cells. Proc Natl Acad Sci USA. 104:pp. 15472–15477. 2007,
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lodygin D, Tarasov V, Epanchintsev A,
Berking C, Knyazeva T, Körner H, Knyazev P, Diebold J and Hermeking
H: Inactivation of miR-34a by aberrant CpG methylation in multiple
types of cancer. Cell Cycle. 7:2591–2600. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu C, Kelnar K, Liu B, Chen X,
Calhoun-Davis T, Li H, Patrawala L, Yan H, Jeter C, Honorio S, et
al: The microRNA miR-34a inhibits prostate cancer stem cells and
metastasis by directly repressing CD44. Nat Med. 17:211–215. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ji Q, Hao X, Zhang M, Tang W, Yang M, Li
L, Xiang D, Desano JT, Bommer GT, Fan D, et al: MicroRNA miR-34
inhibits human pancreatic cancer tumor-initiating cells. PLoS One.
4:e68162009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li N, Fu H, Tie Y, Hu Z, Kong W, Wu Y and
Zheng X: miR-34a inhibits migration and invasion by down-regulation
of c-Met expression in human hepatocellular carcinoma cells. Cancer
Lett. 275:44–53. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Adini A, Fainaru O, Udagawa T, Connor KM,
Folkman J and D'Amato RJ: Matrigel cytometry: A novel method for
quantifying angiogenesis in vivo. J Immunol Methods. 342:78–81.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li Q, Ding C, Chen C, Zhang Z, Xiao H, Xie
F, Lei L, Chen Y, Mao B, Jiang M, et al: miR-224 promotion of cell
migration and invasion by targeting Homeobox D 10 gene in human
hepatocellular carcinoma. J Gastroenterol Hepatol. 29:835–842.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hummon AB, Lim SR, Difilippantonio MJ and
Ried T: Isolation and solubilization of proteins after TRIzol
extraction of RNA and DNA from patient material following prolonged
storage. Biotechniques. 42(467–470): 4722007.
|
|
17
|
Ish-Shalom S and Lichter A: Analysis of
fungal gene expression by real time quantitative PCR. Methods Mol
Biol. 638:103–114. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang Y, Kuramitsu Y, Takashima M, Yokoyama
Y, Iizuka N, Tamesa T, Sakaida I, Oka M and Nakamura K:
Identification of four isoforms of aldolase B down-regulated in
hepatocellular carcinoma tissues by means of two-dimensional
Western blotting. In Vivo. 25:881–886. 2011.PubMed/NCBI
|
|
19
|
Li N, Fu H, Tie Y, Hu Z, Kong W, Wu Y and
Zheng X: miR-34a inhibits migration and invasion by down-regulation
of c-Met expression in human hepatocellular carcinoma cells. Cancer
Lett. 275:44–53. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pogribny IP, Starlard-Davenport A,
Tryndyak VP, Han T, Ross SA, Rusyn I and Beland FA: Difference in
expression of hepatic microRNAs miR-29c, miR34a, miR-155, and
miR-200b is associated with strain-specific susceptibility to
dietary nonalcoholic steatohepatitis in mice. Lab Invest.
90:1437–1446. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Condeelis J and Pollard JW: Macrophages:
Obligate partners for tumor cell migration, invasion, and
metastasis. Cell. 124:263–266. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Welch C, Chen Y and Stallings RL:
MicroRNA-34a functions as a potential tumor suppressor by inducing
apoptosis in neuroblastoma cells. Oncogene. 26:5017–5022. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang P, Li QJ, Feng Y, Zhang Y, Markowitz
GJ, Ning S, Deng Y, Zhao J, Jiang S, Yuan Y, et al:
TGF-β-miR-34a-CCL22 signaling-induced Treg cell recruitment
promotes venous metastases of HBV-positive hepatocellular
carcinoma. Cancer Cell. 22:291–303. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Peng L, Yuan Z, Li Y, Ling H, Izumi V,
Fang B, Fukasawa K, Koomen J, Chen J and Seto E: Ubiquitinated
sirtuin 1 (SIRT1) function is modulated during DNA damage-induced
cell death and survival. J Biol Chem. 290:8904–8912. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chung YR, Kim H, Park SY, Park IA, Jang
JJ, Choe JY, Jung YY, Im SA, Moon HG, Lee KH, et al: Distinctive
role of SIRT1 expression on tumor invasion and metastasis in breast
cancer by molecular subtype. Hum Pathol. 46:1027–1035. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hao C, Zhu PX, Yang X, Han ZP, Jiang JH,
Zong C, Zhang XG, Liu WT, Zhao QD, Fan TT, et al: Overexpression of
SIRT1 promotes metastasis through epithelial-mesenchymal transition
in hepatocellular carcinoma. BMC Cancer. 14:9782014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Levine AJ, Bargonetti J, Bond GL, Hoh J,
Onel K, Overholtzer M, Stoffel AK, Walsh CA and Jin S: The p53
network. Protein Rev. 2:1–23. 2005. View Article : Google Scholar
|
|
28
|
Prives C and Manley JL: Why is p53
acetylated? Cell. 107:815–818. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Singh K, Mogare D, Giridharagopalan RO,
Gogiraju R, Pande G and Chattopadhyay S: p53 target gene SMAR1 is
dysregulated in breast cancer: Its role in cancer cell migration
and invasion. PLoS One. 2:e6602007. View Article : Google Scholar : PubMed/NCBI
|