Introduction
It is well-known that hepatocellular carcinoma (HCC)
is one of the most common types of malignancy in South-East Asia
(1). For localized tumors, effective
treatments include surgical resection, local ablation therapy,
trans-catheter arterial chemoembolization and liver transplantation
(1–3).
However, HCC is typically diagnosed at the advanced stages in
numerous patients. Although several molecular targeting drugs have
previously been used in a clinical setting, their effects are
limited (4). Therefore, novel
molecular targets are required to manage HCC progression.
HCC is a typical hypervascular tumor, as
demonstrated by dynamic computerized tomography or angiography
(5,6),
and its progression is markedly associated with active
neovascularization (7–9). Angiogenesis is important to tumor
metastasis and growth, as it provides the oxygen, and nutrients for
tumor cells (10,11). Intratumoral microvessel density (IMD),
the most common indicator of tumor angiogenesis, is assessed using
cluster of differentiation (CD)31, CD34 or von Willebrand factor
(vWF) staining (12). It has been
suggested that an increased IMD is a predictor for decreased
disease-free survival (DFS) and overall survival (OS) rates, and
several antiangiogenic agents have begun to be used in the
treatment of HCC (9,13). However, conflicting results have also
identified that a low IMD is a significant unfavorable prognostic
factor of 2-year DFS as well as OS rate (14).
Aquaporins (AQPs) are a family of transmembrane
water channel proteins, which are expressed in numerous types of
fluid-transporting tissue, including glandular epithelia and kidney
tubules, and in non-fluid-transporting tissue, including the
epidermis. There are more than 10 AQPs that have been identified in
mammals (15). Their localization in
the plasma membrane is essential in the regulation of water
transfer (16). The first member to
be identified, AQP1, is a membrane protein that regulates the
permeability of endothelial and epithelial barriers by facilitating
water movement across cell membranes (17). In addition to its basic function,
human AQP1 expression has been revealed to be heterogeneously
expressed in different human tumors (18–23).
Several studies have identified that the upregulation of AQP1
occurs in various malignancies, including in glial tumors (18), breast cancer (19) and colorectal cancer (20). Furthermore, previous studies have
investigated AQP1 expression in the microvessels of multiple
tumors, indicating the potential involvement of AQP1 in tumor
angiogenesis (17,21). Impaired tumor angiogenesis and tumor
migration were identified in AQP1 knockout mice (22). Conversely, AQP1 overexpression is
consistent with bone marrow angiogenesis in patients with active
multiple myeloma, suggesting AQP1 is an indicator of angiogenesis
(23).
However, the role of AQPs in HCC is poorly
characterized. In the present study, the protein expression of AQP1
in HCC tissue samples was investigated, and the clinicopathological
and prognostic value of AQP1 in HCC was analyzed.
Materials and methods
Tissue specimens and clinical
data
Tumor samples and adjacent liver tissues were
collected from 90 patients with HCC who underwent curative surgical
resection without any prior anticancer therapy between May 2007 and
May 2012 at the Centre for Liver Disease in the 458th Hospital of
People's Liberation Army (Guangzhou, China). Patients with
concurrent second primary cancer were excluded. The present study
was approved by the Human Research Ethics Committee of the 458th
Hospital of People's Liberation Army, and written informed consent
was obtained from each patient. OS and DFS were defined as the
interval between dates of surgery and mortality, and between dates
of surgery and recurrence, respectively. Those patients who
developed recurrence were treated with repeated hepatic resection,
trans-catheter arterial embolization or radiofrequency ablation.
Demographical and clinicopathological data consisted of age, sex,
presence of cirrhosis, status of hepatitis B surface antigen
(HBsAg), levels of preoperative α-fetoprotein (AFP), tumor size,
histological grade, Child-Pugh classification, microvascular
invasion and tumor-node-metastasis (TNM) stage (14,24–26).
Serial sections (5 µm thick) were obtained from each tissue block,
and stained with hematoxylin and eosin (H&E; 0.2% hematoxylin
and 1% eosin) using standard pathologic procedures for 1 h.
Briefly, sections were deparaffinized in xylene (2×5 min) and
rehydrated with successive 1-min washes in 100, 96, 80 and 70%
ethanol. Sections were then stained with hematoxylin for 2 min at
room temperature, rinsed with distilled water, rinsed with 0.1%
hydrochloric acid in 50% ethanol, rinsed with tap water for 15 min,
stained with eosin for 1 min at room temperature and rinsed again
with distilled water. The slides were then dehydrated with 95 and
100% ethanol successively followed by xylene (2×5 min), and then
mounted with coverslips. H&E-stained sections were analyzed by
light microscopy (magnification, ×20) using a Leica DM LB2
epifluorescence microscope (Leica Microsystems GmbH, Wetzlar,
Germany). Images that were at the original magnification of ×20 of
H&E staining were acquired with a CCD digital camera (model
7.2; Diagnostic Instruments, Inc., Sterling Heights, MI, USA). The
mean age of the patients was 54.0±10.0 years (standard deviation;
range, 25–73). There were 73 males and 17 females. The average
tumor size was 4.4±1.8 cm (range, 1.3–7.7), with 47 tumors ≤5 cm
and 43 tumors >5 cm. Among the 90 HCC examined in the present
study, 53 exhibited hepatitis B infection. The median follow-up
time was 35.0 months.
Tissue microarray (TMA) construction
and immunohistochemistry (IHC)
Immunohistochemistry images were captured and
analyzed using Image Pro-Plus 4.5 software (Media Cybernetics,
Silver Spring, MA, USA) for integrated optical density
semi-quantitation. Leica DM LB2 epifluorescence microscope (Leica
Microsystems GmbH, Wetzlar, Germany) was used to analyze the images
of IHC at a low magnification (×100) and a high magnification
(×400). Representative sections of HCC or normal liver tissues in
the pre-existing paraffin-embedded tissue blocks were determined
according to the aforementioned H&E staining slides. The TMA
was prepared using a needle to punch a 1.5 mm diameter cylinder in
the representative section of each tissue, and by placing the
cylinders into an array on a recipient paraffin block. Sections
were cut 2-µm thick from the TMA block and mounted on microscope
slides. The TMA consisted of a total of 90 patients with HCC and 90
cases of paraffin-embedded adjacent normal tissue. The
clinicopathological characteristics of patients are summarized in
Table I. The TMA slides were dried
overnight at 37°C, dewaxed in xylene, rehydrated using an alcohol
gradient, and the endogenous peroxidase activity was blocked by
immersing the slides in 0.3% hydrogen peroxide
(H2O2) for 10 min at room temperature.
Antigen retrieval was performed through microwave heating with
sodium citrate buffer (pH 6.0) at 100°C for 30 min. Then,
non-specific binding sites were blocked at room temperature using
the blocking buffer from the Vectastain® Elite ABC kit
(Vector Laboratories, Peterborough, UK) for 45 min. Samples were
then incubated with mouse monoclonal anti-human antibody against
AQP1 (1:500 dilution; cat. no. ab9566; Abcam, Cambridge, MA, USA)
and mouse monoclonal CD34 (1:50 dilution; cat. no. MA1-10202; clone
QB End10; Neomarkers, Inc., Fremont, CA, USA) primary antibodies at
room temperature for 60 min. Following three washes with PBS, the
slides were sequentially incubated with a polymer
peroxidase-labeled rabbit anti-mouse secondary antibody (100
dilution; cat. no. ZDR-5109; ZSGB-BIO, Beijing, China) for 30 min
at room temperature. Then, the slides were stained at 37°C for 1 h
using the 3,3′-diaminobenzidine horseradish peroxidase Color
Development kit (Beyotime Institute of Biotechnology, Haimen,
China). Finally, the sections were counterstained with hematoxylin
for 5 min at room temperature. Known IHC positive slides were used
as a positive control, and anti-AQP1 primary antibody was replaced
with PBS as a negative control.
 | Table I.Clinicopathological characteristics of
patients with hepatocellular carcinoma. |
Table I.
Clinicopathological characteristics of
patients with hepatocellular carcinoma.
| Characteristic | No. of cases (%) |
|---|
| Age, years |
|
| ≤60 | 65 (72.2) |
|
>60 | 25 (27.8) |
| Sex |
|
| Male | 73 (81.1) |
|
Female | 17 (18.9) |
| Cirrhosis |
|
|
Absent | 59 (65.6) |
|
Present | 31 (34.4) |
| Hepatitis B surface
antigen |
|
|
Negative | 36 (40.0) |
|
Positive | 54 (60.0) |
| α-fetoprotein,
ng/ml |
|
|
≤100 | 32 (35.6) |
|
>100 | 58 (64.4) |
| Tumor size, cm |
|
| ≤5 | 47 (52.2) |
|
>5 | 43 (47.8) |
| Histological
grade |
|
| Well
differentiated | 25 (27.8) |
|
Moderately differentiated | 55 (61.1) |
| Poorly
differentiated | 10 (11.1) |
| Child-Pugh
classification |
|
| A | 66 (73.3) |
|
B-C | 24 (26.7) |
| Microvascular
invasion |
|
|
Absent | 65 (72.2) |
|
Present | 25 (27.8) |
| Tumor node
metastasis stage |
|
|
I–II | 50 (55.6) |
|
III–IV | 40 (44.4) |
Evaluation of IMD and AQP1
expression
IMD scores were assessed by immunostaining for CD34
according to Weidner (24).
Subsequent to scanning the immunostained section at a low
magnification (×100), the area within the tumor or adjacent tissues
with the highest number of distinctly highlighted microvessels was
selected as the ‘hot spot’. IMD was defined by the mean value of
vessel number visualized at high magnification (×400) in five
fields within the hot spot. Evaluation of the staining reactions
was strictly confined to the area of highest IMD. For the
sinusoid-like microvessels, which were primarily observed in the
areas with a large trabecular structure and assessed using a
modified method introduced by Tanigawa et al (27), every 40-µm length of lumen was counted
as 1 point. Each stained lumen was regarded as a single countable
microvessel. If there was no lumen, but only a single positive cell
was visible, this cell was also interpreted as representing a
microvessel. Any positive staining of endothelium or mass of
endothelium clearly separated from the surrounding tumor cells and
connective tissue was counted as a microvessel. Immunohistochemical
analysis was performed independently by two investigators (Dr
Li-Min Luo, 458th Hospital of People's Liberation Army and Dr Min
Wei, Southern Medical University). The mean values were accepted if
the two investigators agreed with the values. If the differences
between the observers were >30%, the values were re-estimated
until a consensus was reached. The expression of AQP1 was detected
and assessed using the same method. As the number of microvessels
observed may vary by patient and vascular spots, resulting in an
error in any measurement of AQP1 expression, the AQP1/IMD ratio was
also assessed to avoid this error.
Statistical analysis
Statistical analysis was performed using SPSS
software (version 18.0; SPSS, Inc., Chicago, USA). The associations
between clinical and prognostic variables (patient age, sex,
cirrhosis, HBsAg, AFP, tumor size, histological grade, Child-Pugh
classification, microvascular invasion and TNM stage, and AQP1
expression, IMD and the AQP1/IMD ratio) were determined. Un-paired
Student's t-tests were used to compare values between two groups,
and one-way analysis of variance was performed when ≥3 groups were
present. Correlations were determined by Spearman rank correlation
test. A two-tailed P<0.05 was considered to indicate a
statistically significant difference.
Results
Association between the AQP1
expression/IMD and the clinicopathological factors of the
patients
There were two types of microvessels identified:
Capillary-like microvessels with small, scattered capillaries with
no or a narrow lumen, and sinusoid-like microvessels with
continuous branching and a distinct lumen structure.
Immunohistochemical analysis demonstrated that the AQP1 protein was
markedly expressed in the membrane of microvessels and small
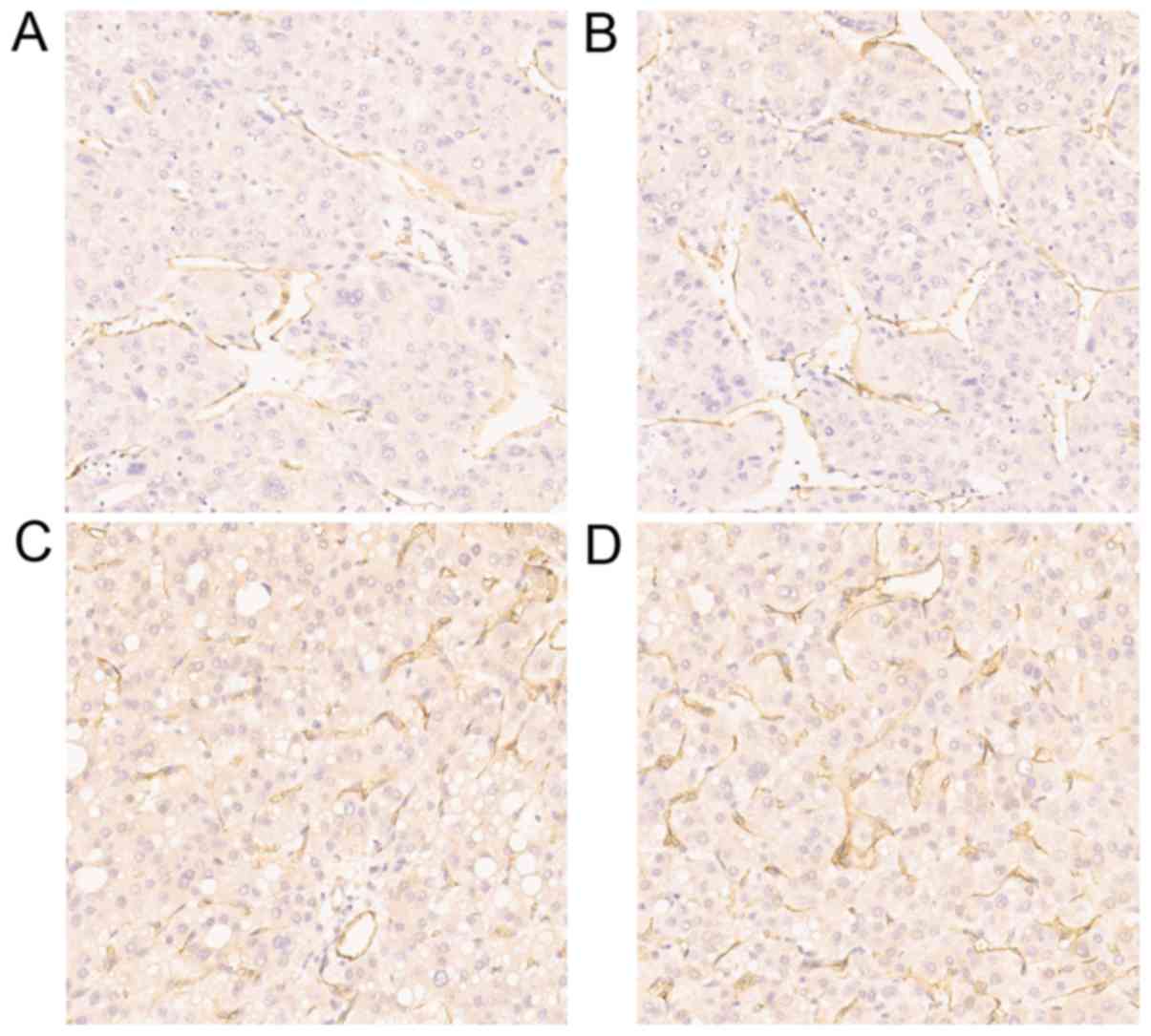
vessels in the majority of HCC samples (Fig. 1A and B), but seldom in the cytoplasm
of tumor cells. The AQP1 expression in microvessels of HCC
presented a significant association with cirrhosis, tumor size,
histological grade, Child-Pugh classification, microvascular
invasion and TNM stage. The expression of AQP1 was significantly
higher in the presence of cirrhosis compared with in absence of
cirrhosis (P=0.048), in tumor sizes >5 cm compared with in tumor
sizes ≤5 cm (P<0.001), in poorly differentiated histological
grades compared with in well or moderately differentiated
histological grade (P=0.001), in Child-Pugh classification B + C
compared with in Child-Pugh classification A (P=0.007), in the
presence of microvascular invasion compared with in absence of
microvascular invasion (P<0.001) and in TNM stage III–IV
compared with in TNM stage I–II (P<0.001) (Table II). However, no significant
variations according to HBsAg and AFP levels were observed
(P>0.05; Table II). CD34 was also
highly expressed in the membrane of microvessels and small vessels
in the majority of HCC samples (Fig. 1C
and D). The IMD score, assessed by CD34 immunostaining, was
significantly associated with tumor size, histological grade,
Child-Pugh classification, microvascular invasion and TNM stage.
IMD scores were higher in tumor sizes >5 cm compared with in
tumor sizes ≤5 cm (P<0.001), in poorly differentiated
histological grade compared with in well and moderately
differentiated histological grades (P=0.002), in Child-Pugh
classification B + C compared with in Child-Pugh classification A
(P=0.019), in the presence of microvascular invasion compared with
in absence of microvascular invasion (P<0.001) and in TNM stage
III–IV compared with in TNM stage I–II (P<0.001) (Table II). However, no significant
differences between IMD score and cirrhosis, HBsAg or AFP were
observed (P>0.05; Table II). A
statistically significant positive correlation was observed between
AQP1 expression and the IMD scores (r=0.227; P<0.001).
 | Table II.Association between AQP1 expression,
IMD, the AQP1/IMD ratio and the clinicopathological features of 90
patients with hepatocellular carcinoma. |
Table II.
Association between AQP1 expression,
IMD, the AQP1/IMD ratio and the clinicopathological features of 90
patients with hepatocellular carcinoma.
| Features | Cases (n) | AQP1 | P-value | IMD | P-value | AQP1/IMD | P-value |
|---|
| Cirrhosis |
|
| 0.048 |
| 0.295 |
| 0.051 |
|
Absent | 59 | 102.9±9.4 |
| 122.0±10.6 |
| 0.84±0.04 |
|
|
Present | 31 | 106.9±8.4 |
| 124.5±10.3 |
| 0.86±0.03 |
|
| Hepatitis B surface
antigen |
|
| 0.189 |
| 0.265 |
| 0.698 |
|
Negative | 36 | 105.8±9.9 |
| 124.4±10.8 |
| 0.85±0.04 |
|
|
Positive | 54 | 103.2±8.6 |
| 121.8±10.3 |
| 0.85±0.04 |
|
| α-fetoprotein,
ng/ml |
|
| 0.372 |
| 0.677 |
| 0.358 |
|
≤25 | 32 | 103.1±8.9 |
| 122.2±10.2 |
| 0.84±0.05 |
|
|
>25 | 58 | 104.9±9.4 |
| 123.2±10.7 |
| 0.85±0.03 |
|
| Tumor size, cm |
|
| <0.001 |
| <0.001 |
| 0.259 |
| ≤5 | 47 | 100.2±6.3 |
| 118.7±7.7 |
| 0.85±0.04 |
|
|
>5 | 43 | 108.7±10.0 |
| 127.3±11.4 |
| 0.85±0.03 |
|
| Histological
grade |
|
| 0.001 |
| 0.002 |
| <0.001 |
| Well
differentiated | 25 | 100.8±7.2 |
| 119.3±8.3 |
| 0.85±0.04 |
|
|
Moderately differentiated | 55 | 104.1±9.1 |
| 122.7±10.7 |
| 0.85±0.04 |
|
| Poorly
differentiated | 10 | 113.7±8.3 |
| 132.7±8.9 |
| 0.86±0.02 |
|
| Child-Pugh
classification |
|
| 0.007 |
| 0.019 |
| 0.366 |
| A | 66 | 102.7±8.8 |
| 121.3±9.6 |
| 0.85±0.04 |
|
|
B-C | 24 | 108.6±9.2 |
| 127.1±11.8 |
| 0.86±0.03 |
|
| Microvascular
invasion |
|
| <0.001 |
| <0.001 |
| 0.506 |
|
Absent | 65 | 101.2±7.0 |
| 110.0±8.3 |
| 0.85±0.04 |
|
|
Present | 25 | 112.3±9.5 |
| 132.9±9.0 |
| 0.84±0.04 |
|
| Tumor node
metastasis stage |
|
| <0.001 |
| <0.001 |
| 0.278 |
|
I–II | 50 | 99.7±5.5 |
| 118.0±7.0 |
| 0.84±0.04 |
|
|
III–IV | 40 | 110.0±9.7 |
| 128.9±10.1 |
| 0.85±0.03 |
|
Analysis of the AQP1/IMD ratio
The AQP1/IMD ratio in cases with poorly
differentiated histological grade was significantly greater
compared with that of cases with well and moderately differentiated
histological grades (P<0.001). However, no significant
differences between AQP1/IMD ratio and cirrhosis, HBsAg, AFP, tumor
size, Child-Pugh classification, microvascular invasion and TNM
stage were observed (P>0.05; Table
II).
Prognostic value of AQP1 expression or
IMD on overall survival and recurrence
Univariate analysis of factors revealed that tumor
size, histological grade, Child-Pugh classification, microvascular
invasion, TNM stage, AQP1 expression and IMD were associated with
5-year DFS, and OS (Table III). The
median 5-year DFS and OS times of patients with low AQP1 expression
were significantly longer compared with that of patients with high
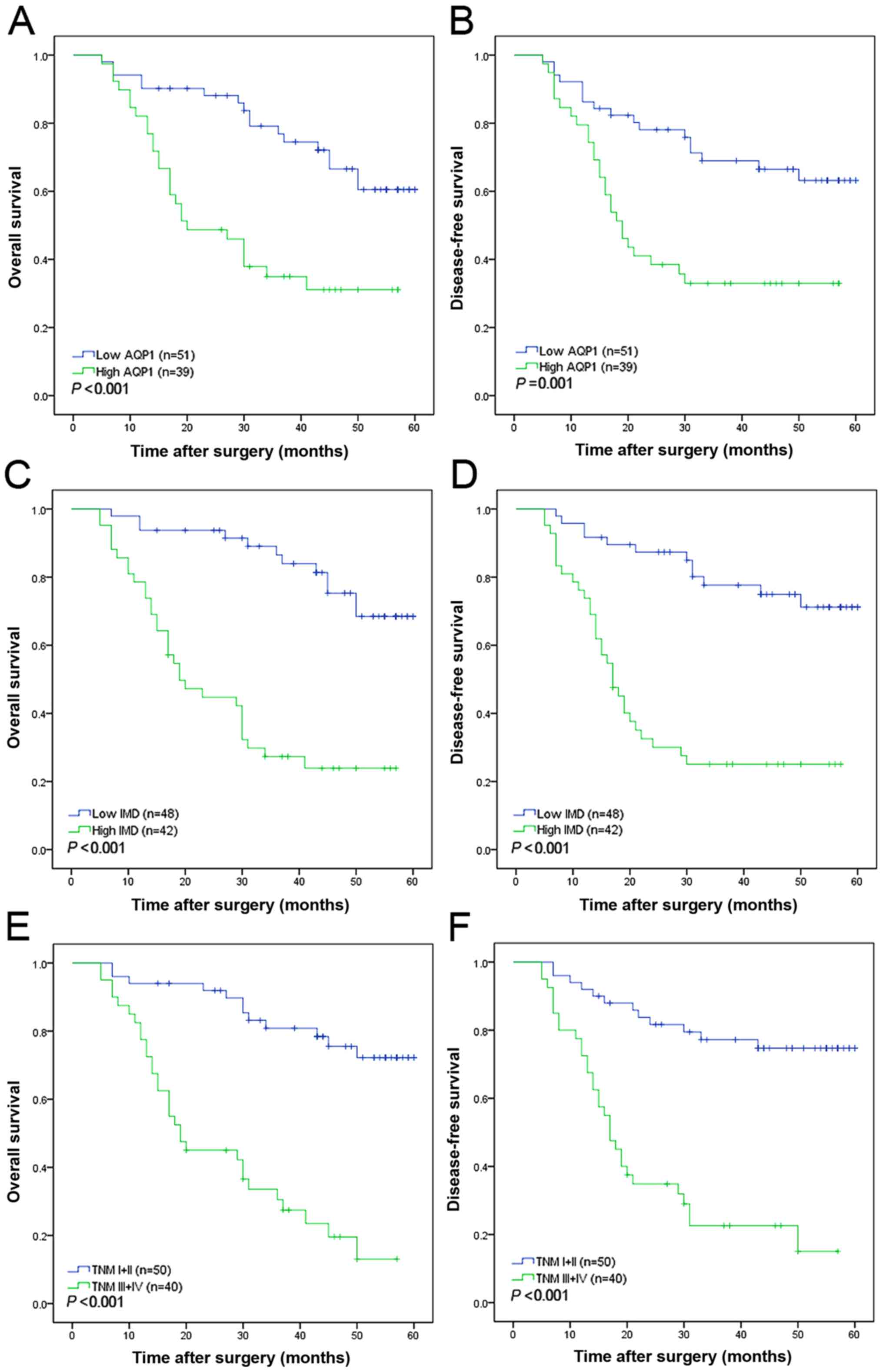
AQP1 expression (P=0.001 for both; Fig.
2A and B; Table III).
Similarly, patients with a high IMD exhibited significantly shorter
5-year DFS and OS times (P<0.001 for both; Fig. 2C and D; Table III). Low AQP1 expression and low IMD
were independent protective factors of 5-year DFS (P=0.001 and
P<0.001, respectively) and OS (P<0.001 and P<0.001,
respectively) (Table III). Notably,
in the multivariate analyses, IMD biomarker was an independent risk
factor of 5-year OS (P=0.042; Fig.
2C) and DFS (P=0.025; Fig. 2D)
(Table III). In addition, TNM stage
was identified as an independent risk factor for OS (P=0.043;
Fig. 2E) and DFS (P=0.049; Fig. 2F) (Table
III).
 | Table III.Univariate and multivariate analysis
of factors associated with survival, and recurrence. |
Table III.
Univariate and multivariate analysis
of factors associated with survival, and recurrence.
|
| Disease free
survival | Overall
survival |
|---|
|
|
|
|
|---|
|
|
| Multivariate |
| Multivariate |
|---|
|
|
|
|
|
|
|---|
| Factor | Univariate
P-value | HR | 95% CI | P-value | Univariate
P-value | HR | 95% CI | P-value |
|---|
| Cirrhosis (absent
vs. present) | 0.071 | 0.911 | 0.393–2.112 | 0.828 | 0.062 | 0.811 | 0.334–1.968 | 0.643 |
| Hepatitis B surface
antigen (negative vs. positive) | 0.744 | 1.924 | 0.887–4.175 | 0.098 | 0.808 | 1.822 | 0.841–3.949 | 0.129 |
| α-fetoprotein (≤25
vs. >25 ng/ml) | 0.347 | 1.248 | 0.567–2.749 | 0.582 | 0.251 | 1.443 | 0.632–3.296 | 0.384 |
| Tumor size (≤5 vs.
>5 cm) | <0.001 | 1.265 | 0.484–3.307 | 0.631 | <0.001 | 1.316 | 0.511–3.389 | 0.570 |
| Histological grade
(well or moderately vs. poorly) | <0.001 | 1.487 | 0.579–3.819 | 0.409 | <0.001 | 1.656 | 0.625–4.385 | 0.310 |
| Child-Pugh (A vs.
B-C) | 0.035 | 1.010 | 0.404–2.526 | 0.983 | 0.018 | 1.084 | 0.418–2.813 | 0.868 |
| Microvascular
invasion (absent vs. present) | <0.001 | 1.638 | 0.766–3.502 | 0.203 | <0.001 | 1.726 | 0.814–3.660 | 0.155 |
| Tumor node
metastasis stage (I–II vs. III–IV) | <0.001 | 2.867 | 1.005–3.502 | 0.049 | <0.001 | 2.921 | 1.033–8.261 | 0.043 |
| AQP1 expression
(low vs. high) | 0.001 | 0.636 | 0.228–1.772 | 0.387 | <0.001 | 0.770 | 0.279–2.127 | 0.615 |
| IMD (low vs.
high) | <0.001 | 3.444 | 1.169–10.149 | 0.025 | <0.001 | 3.074 | 1.039–9.094 | 0.042 |
| AQP1/IMD ratio (low
vs. high) | 0.753 | 0.959 | 0.489–1.880 | 0.903 | 0.899 | 0.999 | 0.502–1.988 | 0.998 |
Discussion
The ability of tumor cells to grow and migrate
requires a sufficient blood supply. A number of malignant tumors
have been identified to induce neovascularization (28,29).
Tanigawa et al (27)
demonstrated an increased microvessel density in malignant HCC and
indicated that IMD was a prognostic factor for HCC. However, the
clinicopathological significance of angiogenesis in HCC remains to
be elucidated (13,14,30). Due
to the diversities in tissue processing and immunostaining
techniques, including the observation for selected vascular hot
spots, antibodies to identify endothelial cells, and the method of
counting the vessels, the results of angiogenesis are not able to
be corroborated easily. Anti-CD34 antibodies have been identified
to be better at identifying endothelial cells compared with
anti-CD31 and anti-vWF antibodies, and with greater sensitivity
(13,14,27). The
anti-CD34 antibody has been suggested to be the most sensitive and
specific marker among the other endothelial markers in HCC
(31). Therefore, IMD score was
assessed using the anti-CD34 antibody in the present study. The
results suggest that IMD may serve an important role in the HCC due
to its association with tumor size, histological grade, Child-Pugh
classification, microvascular invasion and TNM stage, which was in
accordance with Tanigawa et al (27), and Wang et al (32), who hypothesized that IMD is an
independent prognostic factor for HCC.
The number of microvessels in tumors varies in
different patients or hot spots, which may result in differences in
AQP1 expression measurements. For IMD, defined as tumor microvessel
counts, and AQP1 protein, which is primarily expressed in
microvessels, the AQP1/IMD ratio may determine the association
between IMD and AQP1 expression levels in the microvessels of HCC,
and correct subjective and objective errors. In the present study,
AQP1 protein was highly expressed in the membranes of microvessels
and small vessels within the majority of patients with HCC, but was
expressed seldom in the cytoplasm of the tumor cells. The
distribution of AQP1 protein indicated that AQP1 may serve an
important role in transvascular water transport in primary HCC, and
exhibits little effect on water flow in tumor cells.
The expression of AQP1 in HCC tissues was higher
compared with that of adjacent normal liver tissues. These data
indicate the potential role of AQP1 during HCC carcinogenesis. It
is possible that the induction of AQP1 is required in the
development of HCC and serves as an essential driving force for
initiating carcinogenesis. During the cell cycle, as the cell
volume needs to expand rapidly by absorbing water from the
extracellular environment with a minimal volume of energy,
upregulation of AQP1 in microvessels is potentially advantageous
for the growth or survival of tumor cells (33). Furthermore, the result suggests that
HCC, similar to other solid tumors, exhibit high vascular
permeability (34).
Previous studies have demonstrated that AQP1
expression is upregulated in astrocytomas and metastatic carcinomas
(35,36), and AQP1 expression in the microvessels
of neoplastic brain cells was proposed to increase blood-brain
barrier water permeability, resulting in brain tumor edema in
aggressive brain tumors (37).
In addition, the results of the present study
indicated that AQP1 expression in the microvessels of HCC samples
was significantly associated with tumor size, histologic grade,
Child-Pugh classification, microvascular invasion and TNM stage.
The survival analysis results suggested that the AQP1 protein may
be upregulated in the advanced stages of the disease, and may be
involved in the progression and prognosis of HCC.
In the present study, Spearman correlations
demonstrated that there was a positive correlation between IMD and
the expression of AQP1. These results suggest that AQP1 expression
in microvessel endothelial cells of HCC may be associated with
angiogenesis. Additional experiments are required to investigate
whether AQP1 overexpression or knockout in tumor microvessels
affect angiogenesis directly.
Papadopoulos and Verkman (38) demonstrated that the pharmacological
modulation of AQP1 function may provide novel therapeutic
approaches in human disease, including diuretics, and regulators of
intraocular pressure and swelling in the brain, and cornea. In
addition, Ma et al (39)
suggested that topiramate decreases AQP1 protein immunostaining in
lung carcinoma microvessel endothelial cells of mice, and
hypothesized that the suppression of AQP1 expression may be an
important factor for the inhibitory action of topiramate on tumor
metastasis. In conclusion, the results of the present study
indicate that high AQP1 expression may serve an essential role in
HCC carcinogenesis and progression. Additional studies
investigating the molecular mechanisms of AQP1 regulation, and the
association between AQP1 expression and tumor angiogenesis, are
required to verify this novel therapy for HCC.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81672754), the
Natural Science Foundation of Guangdong province (grant no.
2015A030313249) and the ‘Twelfth Five Year Plan’ research project,
Medical Department of General Logistics Department of China (grant
no. CWS11J021).
References
|
1
|
Llovet JM: Updated treatment approach to
hepatocellular carcinoma. J Gastroenterology. 40:225–235. 2005.
View Article : Google Scholar
|
|
2
|
Bruix J, Sala M and Llovet JM:
Chemoembolization for hepatocellular carcinoma. Gastroenterology.
127 5 Suppl 1:S179–S188. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gish RG, Marrero JA and Benson AB: A
multidisciplinary approach to the management of hepatocellular
carcinoma. Gastroenterol Hepatol (NY). 6 3 Suppl 6:1S–16S.
2010.
|
|
4
|
Llovet JM, Ricci S, Mazzaferro V, Hilgard
P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A,
et al: Sorafenib in advanced hepatocellular carcinoma. N Engl J
Med. 359:378–390. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gomaa AI, Khan SA, Leen EL, Waked I and
Taylor-Robinson SD: Diagnosis of hepatocellular carcinoma. World J
Gastroenterol. 15:1301–1314. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ayyappan AP and Jhaveri KS: CT and MRI of
hepatocellular carcinoma: An update. Exp Rev Anticancer Ther.
10:507–519. 2010. View Article : Google Scholar
|
|
7
|
Park YN, Kim YB, Yang KM and Park C:
Increased expression of vascular endothelial growth factor and
angiogenesis in the early stage of multistep hepatocarcinogenesis.
Arch Pathol Lab Med. 124:1061–1065. 2000.PubMed/NCBI
|
|
8
|
Roncalli M, Roz E, Coggi G, Di Rocco MG,
Bossi P, Minola E, Gambacorta M and Borzio M: The vascular profile
of regenerative and dysplastic nodules of the cirrhotic liver:
Implications for diagnosis and classification. Hepatology.
30:1174–1178. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Poon RT, Ng IO, Lau C, Zhu LX, Yu WC, Lo
CM, Fan ST and Wong J: Serum vascular endothelial growth factor
predicts venous invasion in hepatocellular carcinoma: A prospective
study. Ann Surg. 233:227–235. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Carmeliet P and Jain RK: Angiogenesis in
cancer and other diseases. Nature. 407:249–257. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yao DF, Wu XH, Zhu Y, Shi GS, Dong ZZ, Yao
DB, Wu W, Qiu LW and Meng XY: Quantitative analysis of vascular
endothelial growth factor, microvascular density and their
clinicopathologic features in human hepatocellular carcinoma.
Hepatobiliary Pancreat Dis Int. 4:220–226. 2005.PubMed/NCBI
|
|
12
|
Weidner N, Folkman J, Pozza F, Bevilacqua
P, Allred EN, Moore DH, Meli S and Gasparini G: Tumor angiogenesis:
A new significant and independent prognostic indicator in
early-stage breast carcinoma. J Natl Cancer Inst. 84:1875–1887.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sun HC, Tang ZY, Li XM, Zhou YN, Sun BR
and Ma ZC: Microvessel density of hepatocellular carcinoma: Its
relationship with prognosis. J Cancer Res Clin Oncol. 125:419–426.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Murakami K, Kasajima A, Kawagishi N,
Ohuchi N and Sasano H: Microvessel density in hepatocellular
carcinoma: Prognostic significance and review of the previous
published work. Hepatol Res. 45:1185–1194. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhu C, Jiang Z, Bazer FW, Johnson GA,
Burghardt RC and Wu G: Aquaporins in the female reproductive system
of mammals. Front Biosci (Landmark Ed). 1:838–871. 2015.
|
|
16
|
Saadoun S, Papadopoulos MC, Davies DC,
Krishna S and Bell BA: Aquaporin-4 expression is increased in
oedematous human brain tumours. J Neurol Neurosurg Psychiatry.
72:262–265. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mobasheri A, Airley R, Hewitt SM and
Marples D: Heterogeneous expression of the aquaporin 1 (AQP1) water
channel in tumors of the prostate, breast, ovary, colon and lung: A
study using high density multiple human tumor tissue microarrays.
Int J Oncol. 26:1149–1158. 2005.PubMed/NCBI
|
|
18
|
Oshio K, Binder DK, Liang Y, Bollen A,
Feuerstein B, Berger MS and Manley GT: Expression of the
aquaporin-1 water channel in human glial tumors. Neurosurgery.
56:375–381. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mobasheri A and Barrett-Jolley R:
Aquaporin water channels in the mammary gland: From physiology to
pathophysiology and neoplasia. J Mammary Gland Biol Neoplasia.
19:91–102. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yoshida T, Hojo S, Sekine S, Sawada S,
Okumura T, Nagata T, Shimada Y and Tsukada K: Expression of
aquaporin-1 is a poor prognostic factor for stage II and III colon
cancer. Mol Clin Oncol. 1:953–958. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Endo M, Jain RK, Witwer B and Brown D:
Water channel (aquaporin 1) expression and distribution in mammary
carcinomas and glioblastomas. Microvascular Research. 58:89–98.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Saadoun S, Papadopoulos MC, Hara-Chikuma M
and Verkman AS: Impairment of angiogenesis and cell migration by
targeted aquaporin-1 gene disruption. Nature. 434:786–792. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vacca A, Frigeri A, Ribatti D, Nicchia GP,
Nico B, Ria R, Svelto M and Dammacco F: Microvessel overexpression
of aquaporin 1 parallels bone marrow angiogenesis in patients with
active multiple myeloma. Br J Haematol. 113:415–421. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Weidner N: Current pathologic methods for
measuring intratumoral microvessel density within breast carcinoma
and other solid tumors. Breast Cancer Res Treat. 36:169–180. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang C, Bai DS, Huang XY, Shi GM, Ke AW,
Yang LX, Yang XR, Zhou J and Fan J: Prognostic Significance of
Capn4 overexpression in intrahepatic cholangiocarcinoma. PLoS One.
8:e546192013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kiriyama S, Uchiyama K, Ueno M, Ozawa S,
Hayami S, Tani M and Yamaue H: Triple positive tumor markers for
hepatocellular carcinoma are useful predictors of poor survival.
Ann Surg. 254:984–991. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tanigawa N, Lu C, Mitsui T and Miura S:
Quantitation of sinusoid-like vessels in hepatocellular carcinoma:
Its clinical and prognostic significance. Hepatology. 26:1216–1223.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Folkman J, Watson K, Ingber D and Hanahan
D: Induction of angiogenesis during the transition from hyperplasia
to neoplasia. Nature. 339:58–61. 1989. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liotta LA and Stetler-Stevenson WG: Tumor
invasion and metastasis: An imbalance of positive and negative
regulation. Cancer Res. 51 18 Suppl:5054s–5059s. 1991.PubMed/NCBI
|
|
30
|
Chen ZY, Wei W, Guo ZX, Lin JR, Shi M and
Guo RP: Morphologic classification of microvessels in
hepatocellular carcinoma is associated with the prognosis after
resection. J Gastroenterol Hepatol. 26:866–874. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Messerini L, Novelli L and Comin CE:
Microvessel density and clinicopathological characteristics in
hepatitis C virus and hepatitis B virus related hepatocellular
carcinoma. J Clin Pathol. 57:867–871. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang WQ, Liu L, Xu HX, Luo GP, Chen T, Wu
CT, Xu YF, Xu J, Liu C, Zhang B, et al: Intratumoral α-SMA enhances
the prognostic potency of CD34 associated with maintenance of
microvessel integrity in hepatocellular carcinoma and pancreatic
cancer. PLoS One. 8:e711892013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Moon C, Soria JC, Jang SJ, Lee J, Hoque
Obaidul M, Sibony M, Trink B, Chang YS, Sidransky D and Mao L:
Involvement of aquaporins in colorectal carcinogenesis. Oncogene.
22:6699–6703. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yuan F, Leunig M, Huang SK, Berk DA,
Papahadjopoulos D and Jain RK: Microvascular permeability and
interstitial penetration of sterically stabilized (stealth)
liposomes in a human tumor xenograft. Cancer Res. 54:3352–3356.
1994.PubMed/NCBI
|
|
35
|
Saadoun S, Papadopoulos MC, Davies DC,
Bell BA and Krishna S: Increased aquaporin 1 water channel
expression in human brain tumours. Br J Cancer. 87:621–623. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
El Hindy N, Bankfalvi A, Herring A,
Adamzik M, Lambertz N, Zhu Y, Siffert W, Sure U and Sandalcioglu
IE: Correlation of aquaporin-1 water channel protein expression
with tumor angiogenesis in human astrocytoma. Anticancer Res.
33:609–613. 2013.PubMed/NCBI
|
|
37
|
Qin F, Zhang H, Shao Y, Liu X, Yang L,
Huang Y, Fu L, Gu F and Ma Y: Expression of aquaporin1, a water
channel protein, in cytoplasm is negatively correlated with
prognosis of breast cancer patients. Oncotarget. 16:8143–8154.
2016. View Article : Google Scholar
|
|
38
|
Papadopoulos MC and Verkman AS: Potential
utility of aquaporin modulators for therapy of brain disorders.
Prog Brain Res. 170:589–601. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ma B, Xiang Y, Li T, Yu HM and Li XJ:
Inhibitory effect of topiramate on Lewis lung carcinoma metastasis
and its relation with AQP1 water channel. Acta Pharmacol Sin.
25:54–60. 2004.PubMed/NCBI
|