Introduction
Human papillomavirus (HPV) is a DNA-virus,
associated with epithelial hyperplasias. It is a small
non-enveloped virus that contains a double-stranded, closed
circular DNA genome, associated with histone-like proteins and
protected by a capsid with icosahedron symmetry, formed by two
structural protein types (1). It is
included in the family of papillomaviridae, which actually contains
29 genera formed by 189 genotypes (2). Presently 170 genotypes, classified into
five genera (αPV, βPV, γPV, μPV, νPV), have been accepted to infect
human being and approximately 40 of them, included in the genus
Alphapapillomavirus, are associated with infections of the
ano-genital and oral mucosa (3). One
of the primary interests in HPV is to do with its unique oncogenic
properties; so, genital HPV types usually are categorized according
to their epidemiological association with cervical cancer in low-
and high-risk (LR-HPV and HR-HPV).
Seven of them (HR-HPV: 16, 18, 31, 33, 45, 52, 58)
account for nearly 90% of all cervical cancer cases in the general
population worldwide with little regional variation and
particularly genotypes 16 and 18 together account for the great
majority of cervical carcinomas (70%) in the general population
(4). The HPV actually is estimated to
be the most common sexually transmitted infection in the world
(5) and its prevalence is seen in a
geographic distribution of genotypes (6,7).
Although HPV infection has a high transmission rate,
more than 90% of new HPV infections, including those with high-risk
types, clear or become undetectable within 24 months, and clearance
usually occurs in the first 6 months after infection (8). Only 10% of women fail to clear HPV
infections, resulting in a persistent infection. The main
consequence of persistent infection with HR-HPV is the development
of lesions that may progress to malignancy.
A high prevalence of new HPV infections and
persistent infections are reported in immunocompromised
individuals, including human immunodeficiency virus; human
immunodeficiency virus (HIV)-positive women are at increased risk
of incident HPV infection and reactivation of latent HPV infection,
compared with HIV-negative women (9).
Besides, HIV seropositivity has also been linked to high- and
low-grade cervical squamous intraepithelial lesions (H-SIL and
L-SIL), as well as invasive cervical carcinoma, a recognized
AIDS-defining condition and a common cancer among HIV-positive
women (10).
The aim of our study is to evaluate the prevalence
of HPV infection in HIV infected females vs. HIV-negative women and
its genotypes distribution in Central/Eastern Italy, using a
HPV-DNA detection test, and to identify the best strategies for an
effective HPV-prevention in both these groups of women.
Materials and methods
The authors have collected a representative sample
of HIV-negative (150) and positive (50) women who attended the
outpatients Departments of Gynecology and Obstetrics of University
of Molise (Campobasso, Italy), of Infectious Diseases of University
of Molise (Campobasso, Italy) and of University of Chieti-Pescara
(Chieti, Italy), respectively, from January to July 2015 and who
underwent cervico-vaginal swab collection. Swabs were analysed for
a cytological screening (thinPrep System) and for HPV-DNA by
polymerase-chain-reaction followed by type specific hybridization.
HPV detection and typing was performed by using the Roche Linear
Array HPV genotyping test (Roche Molecular Systems, Inc.,
Branchburg, NJ, USA) and it included probes for 37 HPV types.
Setting
Department of Medicine and Health Sciences,
University of Molise and Department of Medicine and Science of
Aging-Clinic of Infectious Diseases, University ‘G. d'Annunzio’
Chieti-Pescara.
Study population and design
This is a cross-sectional study. The study protocol
received institutional review board (IRB), in accordance with the
Code of Ethics of the Declaration of Helsinki. All females selected
were eligible for participation to the study. Strobe statement and
checklist have been consulted. Exclusion criteria was
pregnancy.
Demographic and behavioral data
Nationality, race and ethnicity were reported. Grade
of instruction, job/profession, marital status, smoke, drugs, use
of alcohol were asked. Age at first sexual intercourse, lifetime
number of sexual partners, sexual behaviour, use of
oral-contraceptives, menopausal state and past history of pregnancy
were reported. Height, weight and body mass index were
evaluated.
Specimen collection and
processing
Swabs were analyzed for a thin-Prep Pap test and for
the presence of HPV-DNA by Linear Array HPV genotyping test, a
qualitative in vitro test for the determination of 37
HPV-DNA genotypes. We considered as low-risk genotypes (LR-HPV): 6,
11, 40, 42, 54, 55, 61, 62, 64, 71, 72, 81, 83, 84, CP6108 (HPV-89)
and as high-risk genotypes (HR-HPV): 16, 18, 26, 31, 33, 35, 39,
45, 51, 52, 53, 56, 58, 59, 66, 67, 68, 69, 70, 73, 82, IS39
(subtype of HPV-82).
Data on HIV infection
Year of diagnosis, viral load (HIV-RNA), CD4 levels,
CD4/CD8 ratio, CD4 nadir, eventual HCV/HBV co-infections,
inflammatory indexes such as erythrocyte sedimentation rate (ESR)
and C-reactive protein (CRP-test), CDC classification and eventual
anti-retroviral therapy were collected. The cut-off point for CD4+
T lymphocytes in 200 cells/mm3 was used to indicate
immunosuppression in the women HIV positive.
Statistical analysis
All females who underwent an adequate swab for HPV
evaluation were included in the final analysis (200/200). HPV
prevalence was estimated within the 95% confidence interval (CI).
CI were calculated by using the SE of log transformation with the
SE of the log prevalence. Statistical analysis samples deemed
positive for high- and low-risk HPV were categorized as single or
multiple infections. To explore the association with age and
overall HPV prevalence, age was categorized into 4 year intervals
(≤25, 26–35, 36–45, ≥46). A post hoc power analysis was done using
the G*Power statistical package for Windows (version 3.1.7;
Microsoft Corporation, Redmond, WA, USA). The data were analyzed by
an EpiInfo7 statistical package for Windows (version 7.1.0.6). For
categorical variables, the Pearson's χ2 test and anova
test were performed to evaluate the significance of differences
between groups. A P<0.05 was considered statistically
significant. A linear regression was performed to evaluate the
relationship between variables.
Results
The study involved 200 women aged 18–66 years (mean,
36.45; SD, 11.12) by which 150 women (group 1) aged 18–63 years
(mean, 34.34; SD, 10.99) were HIV-negative and 50 women (group 2)
aged 23–66 years (mean, 42,80; SD, 8.94) were HIV-positive. The
sample size of 200 was used for the statistical power analysis (in
the ratio of 1 to 3). The alpha level used for this analysis was
P<0.05. The post hoc analyses revealed the statistical power for
this study was 92% for detecting a medium effect. Thus, there was
more than adequate power. The overall HPV prevalence was 33.00%
(95% CI: 27.16–39.41%) with a higher prevalence in group 2, 48%
(95% CI: 33.66–62.58%) vs. group 1, 28% (95% CI: 20.98–35.91%)
(P=0.009) (Table I). Between the two
groups there was a statistically significant difference of HPV
prevalence among women across a board age range representative of
the population (P=0.0027) and there was also a statistically
significant difference of HPV prevalence related to BMI (P=0.0089),
marital status (P=0.0002) and education level (P=0.01) (Table I). There was also a statistically
significant difference of HPV prevalence related to number of
lifetime sex partners (≥3), alcohol and drugs abuse with a
prevalence of 54.12% (95% CI: 42.96–64.98%) (P<0.0001), 72.73%
(95% CI: 39.03–93.98%) (P=0.003) and 91.67% (95% CI: 61.52–99.79%)
(P<0.0001), respectively; but alcohol and drugs abuse was only
observed in group 2. There was also a statistically significant
difference of HPV prevalence related to smoking 51.35% (95% CI:
39.44–63.15%) with a prevalence more statistically significant in
group 2: 75.00% (95% CI: 61.95–84.68%) than in group 1: 40.00% (95%
CI: 28.41–52.82%) (P<0.0001). Particularly the prevalence of
HR-HPV infection in smokers was 39.73% (95% CI: 30.00–50.33%) with
a higher prevalence in group 2 [62.50% (95 CI: 46.65–76.06%)] vs.
group 1 [28.57% (95% CI: 18.09–42.01%)] (P<0.0001). The
frequency of vaccinated women for HPV was very low and it was
observed only in group 1 (5/150): 3.33% (95% CI: 1.39–7.75%). No
association was found between HPV infections and the age at the
first intercourse, menopausal state, pregnancy, cancer familiarity,
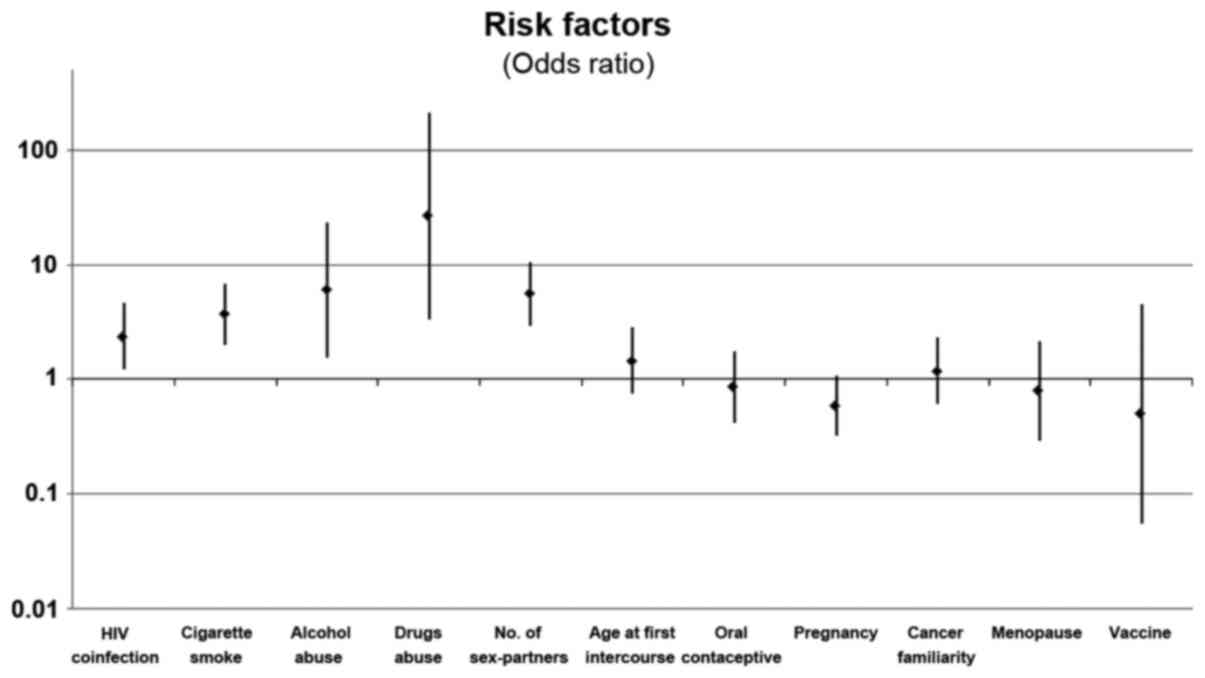
utilization of oral contraceptive. The odds ratio is shown in
Fig. 1.
 | Table I.HPV prevalence and demographic data
between groups. |
Table I.
HPV prevalence and demographic data
between groups.
|
| Group 1 (HIV
negative) | Group 2 (HIV
positive) |
|
|---|
|
|
|
|
|
|---|
|
|
| HPV prevalence |
| HPV prevalence |
|
|---|
|
|
|
|
|
|
|
|---|
| Variable | Sample size | % | (95% CI) | Sample size | % | (95% CI) | P-value |
|---|
| HPV | 150 | 28.00 | (20.98–35.91) | 50 | 48.00 | (33.66–62.58) | 0.009 |
| Age group |
|
|
|
|
|
|
|
| ≤25 | 42 | 35.71 | (23.55–50.04) | 2 | 50.00 | (8.31–91.69) | 0.0027 |
|
26–35 | 39 | 30.77 | (18.88–45.91) | 9 | 66.67 | (41.58–84.89) |
|
|
36–45 | 43 | 30.23 | (18.90–44.62) | 18 | 38.89 | (21.00–60.37) |
|
|
46–55 | 22 | 4.55 | (0.64–26.11) | 18 | 50.00 | (31.00–69.00) |
|
| ≥56 | 4 | 25.00 | (3.59–74.89) | 3 | 33.33 | (4.94–82.78) |
|
| BMI group |
|
|
|
|
|
|
|
|
<18.5 | 14 | 42.86 | (22.37–66.12) | 1 | 0.00 | – | 0.0089 |
|
18.5–24.9 | 99 | 28.28 | (20.59–37.50) | 30 | 53.33 | (38.39–67.71) |
|
|
25–29.9 | 29 | 20.69 | (9.77–38.59) | 13 | 46.15 | (24.57–69.28) |
|
|
>30 | 8 | 25.00 | (6.61–61.09) | 6 | 33.33 | (9.17–71.24) |
|
| Marital status |
|
|
|
|
|
|
|
|
Single | 68 | 42.65 | (32.50–53.46) | 33 | 63.64 | (50.31–75.15) | 0.0002 |
|
Married/engaged | 82 | 15.85 | (9.50–25.27) | 17 | 17.65 | (5.91–42.24) |
|
| Education |
|
|
|
|
|
|
|
|
Graduate | 20 | 25.00 | (11.12–47.04) | 7 | 42.86 | (16.24–74.36) | 0.01 |
| High
school | 82 | 34.15 | (25.24–44.33) | 24 | 54.17 | (37.57–69.89) |
|
| Primary
school | 48 | 18.75 | (10.17–31.99) | 19 | 42.10 | (24.15–62.42) |
|
HPV-typing
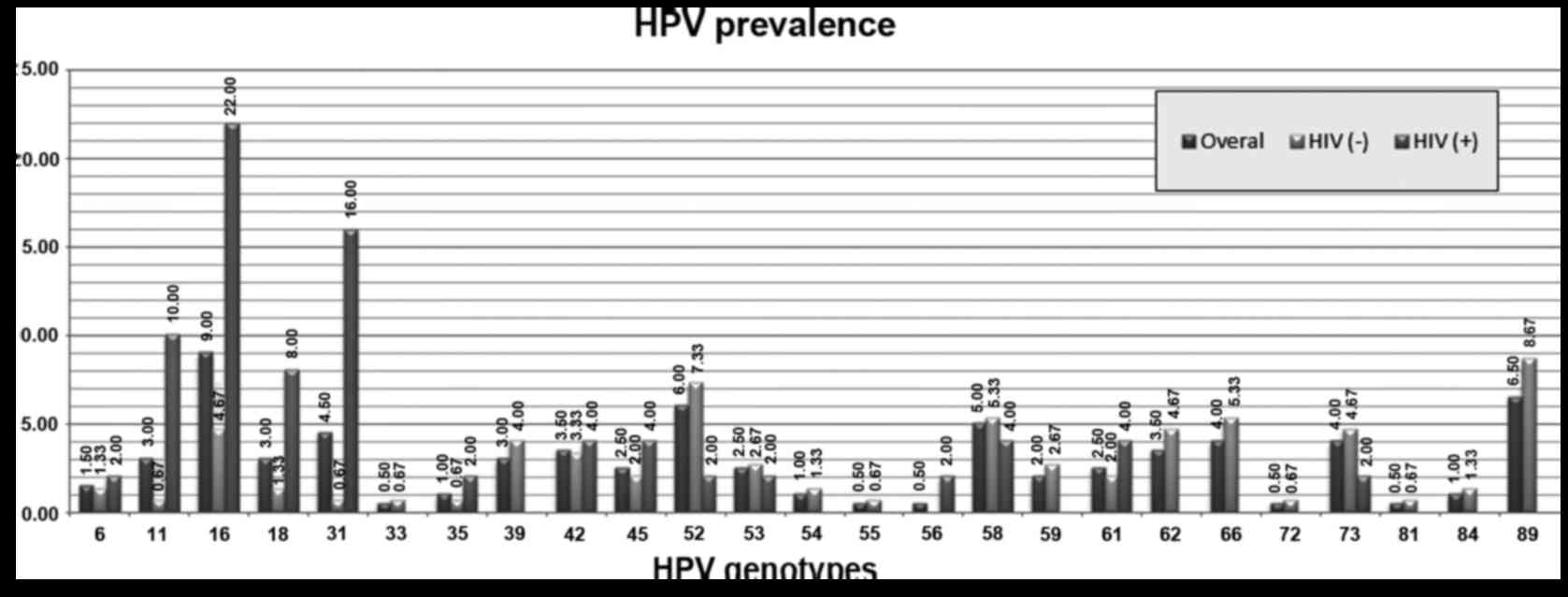
Sixty-six out of two hundred swab resulted positive
to HPV and twenty-five out of thirty-seven different HPV genotypes
were detected (Fig. 2). Multiple HPV
infections (29/66) were observed in 43.94% (95% CI: 33.62–54.81%)
of HPV positive samples with a mean of 4 viruses for woman (range,
2–8). The most frequent genotypes detected were HPV-16, 31, 52, 58,
66, 73 and 89 (Fig. 2). Furthermore,
the prevalence of specific genotypes was different in each group
(Fig. 2) with a statistically
significant higher prevalence of genotypes: 11, 16, 18 and 31 in
group 2 vs. group 1 with a prevalence of 10.00% (95% CI:
3.33–21.81%) (P=0.0007), 22.00% (95% CI: 11.53–35.96%) (P=0.0002),
8.00% (95% CI: 2.22–19.23%) (P=0.016), 16.00% (95% CI: 7.17–29.11%)
(P=0.0001); respectively; An higher prevalence of genotypes: 52, 58
and 73 was detected in the group 1 vs. group 2 with a prevalence of
7.33% (95% CI: 3.72–12.74%) (P=0.17), 5.33% (95% CI: 2.33–10.24%)
(P=0.70), 4.67% (95% CI: 1.90–9.38%) (P=0.40); respectively; but it
was not statistically significant. The overall prevalence of HR-HPV
genotypes was 24% (95% CI: 18.73–30.20%) with a higher prevalence
in HIV-Positive vs. HIV-negative women: 40% (95% CI: 28.41–52.82%)
vs. 18.67% (95% CI: 13.29–25.57%), respectively (P=0.002). The
prevalence of HPV in relation to the presence of HIV infection
(viral load, CD4 level, years from first diagnosis of AIDS and
eventual anti-retroviral therapy) were reported in Table II.
 | Table II.HPV prevalence related to HIV
infection. |
Table II.
HPV prevalence related to HIV
infection.
|
|
| HPV prevalence |
|
|---|
|
|
|
|
|
|---|
| Variable | Sample size | % | (95% CI) | P-value |
|---|
| CD4 |
|
|
|
|
|
<200 | 7 | 57.14 | (28.07–82.00) | 0.05 |
|
201–350 | 4 | 50.00 | (15.48–84.52) |
|
|
351–500 | 15 | 73.33 | (55.81–85.69) |
|
|
>500 | 24 | 29.17 | (15.07–48.87) |
|
| c-ART |
|
|
|
|
|
Yes | 42 | 47.62 | (34.80–60.76) | 0.81 |
| No | 8 | 50.00 | (23.14–76.86) |
|
| Viral load |
|
|
|
|
| <
400 | 14 | 57.14 | (35.87–76.07) | 0.43 |
|
>400 | 36 | 44.44 | (30.74–59.04) |
|
| Years from
diagnosis |
|
|
|
|
|
<5 | 11 | 36.36 | (15.39–64.22) | 0.62 |
|
6–10 | 14 | 22.37 | (22.37–66.12) |
|
|
11–15 | 11 | 40.42 | (40.42–81.86) |
|
|
>15 | 14 | 28.75 | (28.75–71.24) |
|
Discussion
The Central/Eastern Italian regions are, mainly, a
mountainous and agricultural land, so they are relatively isolated
from the rest of country, and without presence of metropolitan
areas; therefore there is a stability in HPV-genotype distribution
(6). In these places, the burden of
prevalence of HPV infection among females was overall greater than
previous estimates in Italy: 33.0 vs. 7–16% (official data of
Italian Superior Health Institute) (11); moreover, our data, according with data
of literature (12–14), showed an higher prevalence of HPV
infection in HIV-seropositive (48.0%) women compared to
HIV-seronegative women (28.0%).
HIV infection seems to be an independent risk factor
for HPV-infection OR: 2.37 (95% CI: 4.59–1.23%). By contrast, the
research into the role of genital HPV in HIV incident infection is
currently limited, whereas it is biologically plausible (10). HPV could predispose to HIV infection
and dissemination through its disruption of the epithelium
integrity and the mucosal immune system by altering the density and
functional activities of epithelial Langerhans cells which capture
infecting pathogens, enabling the recruitment and activation of HIV
target cells such as T-lymphocytes, reducing the expression of
proteins acting in cell adhesion and tumor suppression,
downregulating proteins that promote the infiltration of Langerhans
cells through the epithelium, reducing the production of proteins
involved in antimicrobial activities and upregulating inflammatory
cytokines, which may increase HIV replication (10). HIV infection is often associated with
a greater drugs use (24%), alcohol use (22%) and smoking (48%)
which themselves may play a direct or indirect role in HPV
infection; because alcohol and drugs abuse were only observed in
group 2, it may be a bias and their role in HPV infection may be
influenced by HIV co-infection. Whereas there was a strong
statistically significant difference in HPV prevalence related to
cigarette smoke (P<0.0001) and particularly, tobacco use was
associated with HR-HPV infections. It is possible that smoking
could increases the likelihood of HPV infection trough a local
decrease of immune response in the cervix and an indirect effect
related to metabolism of female hormones (15). The association with HIV infection may
confer an increased susceptibility to the harmful effects of
smoking (16).
Demographic and behavioural factors such as marital
status, education level and number of sexual partners seem to
influence the susceptibility to HPV infection, too. Particularly
the results of multivariate analysis show that number of sexual
partners in a lifetime and single status are independent risk
factors for HPV infections. A statistically significant difference
of HPV prevalence was also observed related to BMI (P=0.0089) and
age groups (P=0.0027) with a lower prevalence of infection in the
group of oldest and/or overweight women. No association was found
between HPV infections and the age at the first intercourse,
menopausal state, pregnancy, cancer familiarity, utilization of
oral contraceptive. This last is in contrast with study of other
authors (17) that found an
association between oral contraceptive and HPV infection. But other
previous researches (18) about the
effect of oral contraceptive use on HPV infections show
inconclusive results, too. Nonetheless, the findings in the present
study do not support the hypothesis that oral contraceptive users
may acquire HPV more often. The HPV-Vaccine seems to have a
protective effect on HPV infection with OR: 0.5 (95% CI:
0.0548–4.5646%) but in this study, the frequency of vaccinated
women for HPV was very low so at the moment it is impossible to
evaluate correctly these data. In fact, the majority of whom were
older than the population targeted for the vaccine. Our results
show that a lower than 500 cell/mm3 CD4 count was
associated with higher HPV infections (P<0.05), thereby
reflecting the inability of HIV-positive women's immune systems to
respond to opportunist infection (14). Other studies suggested that the
frequency of HPV persistence varied inversely with CD4+ count and
found higher HPV prevalence and incidence of oncogenic HPV types in
HIV infected patients, especially those with lower CD4+ counts
(19). These data suggest that the
level of CD4 is important in the pathogenesis of HPV infection in
HIV infected patients (19). No
association was found with stage of HIV disease (CDC stage). In our
study, we found that the stage of immunity (CD4-level) at the time
of the HPV-screening is the most important parameter for detection
of susceptibility to HPV-infection in HIV-positive women;
nevertheless, it is very interesting to point up that the women
with a low level of CD4 are those that only partially respond to
HPV-vaccines (20). The other
parameters that we tested in HIV patients such as viral load, ART
and years from diagnosis is not related to HPV infection risk.
Particularly, ART did not impact HPV detection within 6 months of
therapy initiation, highlighting the importance of continued and
consistent screening, even after ART-initiation and immune
reconstitution (21).
In all these patients it is very important to
perform preventively a primary prevention. The options for the
primary prevention of HPV infections include HPV-vaccination and
other interventions related to sexual health and behaviors,
lifestyle habits (such as smoking) and others yet to be identified.
These last interventions are most important, particularly in
HIV-positive women, where the HPV-vaccination yet must be shown to
be efficacious in HIV-infected individuals (20) particularly in the group with a CD4
count inferior to 500 cell/mm3. Secondly, another
interesting aspect about vaccines issues from our study: the
currently marketed vaccines do not provide total coverage against
HPV infections; in fact, for the great variability of HPV
circulating genotypes, not all most frequently circulating
genotypes are included in 2-or 4-valent HPV vaccines (22–24) such
as genotypes 31, more frequent in HIV-positive women, and 52/58,
more frequent in healthy women. This variability may be
particularly dangerous in HIV-positive women where there is an
increased risk of H-SIL and invasive cervical cancer related also
to other HPV-genotypes different from HPV-16 and −18. But, at
present, a new type of vaccine with 9 genotypes (HPV: 6, 11, 16,
18, 31, 33, 45, 52, 58) is available (9vHPV); it targets 5
additional cancer causing genotypes, which account for about 15% of
cervical cancers and 25% of cervical pre-cancer lesions (25,26), and
preventing over more 95% of persistent HPV31/33/45/52/58 infections
and associated cervical, vulvar, and vaginal disease of any grade
(27). The 9vHPV costs $13 (12.30 €)
more per dose than 4vHPV. The cost of the 9vHPV may be a limiting
factor as well as the socio-political resistance to primary
prevention (25–28). Yet, the 9-valent vaccination strategy
would allow more aggressive changes in screening, and the
incremental health benefits and medical costs averted from
preventing cervical lesions and cervical cancer associated with
HPV-types 31/33/45/52/58 further reducing future costs (26,27). About
the secondary prevention, the cervical-screening is still
recommended in all women, included those vaccinated (25,29).
In conclusion, HPV infection is the most common
sexually transmitted infection in the world and its prevalence is
seen in the geographic distribution of its genotypes (7). The most frequent genotypes detected in
Central/Eastern Italy were HPV-16, HPV-89, HPV-52, HPV-58, HPV-31,
HPV-66 and HPV-73. HPV infection is more common (particularly
high-risk genotypes) and more likely to persist in HIV-positive
women rather than HIV-negative women. The best way to counteract
the HPV infection is the primary prevention that includes
HPV-vaccination and other interventions related to lifestyle
habits, sexual health and behaviors. These last behavioural
interventions are most important, particularly in HIV positive
women, where the HPV-vaccination yet must be shown to be
efficacious in HIV-infected individuals (20), particularly in the group with a low
CD4 count. In fact, the women with a low level of CD4 are those
that only partially respond to HPV-vaccines (20); furthermore, in our study, we confirmed
that the stage of immunity (CD4 level) at the time of the HPV
screening is the most important parameter for detection of
susceptibility to HPV-infection in HIV-positive women. So, it may
be used to check the sub-group of HIV positive women that are more
exposed to HPV infections and to its complications and that have a
partial response to HPV-vaccine. However, for the great variability
of HPV circulating genotypes, currently, the secondary prevention
is still recommended in all women, included those vaccinated, and
particularly in HIV-positive women (25,29).
Nevertheless, at present, a new type of vaccine with 9 genotypes is
available; we believe that, in the near future, it will have a key
role to play in the prevention of HPV infections, indeed it may be
useful for assuring a larger vaccine coverage across the
population, reducing the circulating genotypes in the overall
population and, indirectly, it may also be useful in protecting
those patients that presently respond partially or do not respond
at all to vaccine. So, if larger coverage represents an opportunity
for each woman, so much more so for every HIV-positive woman; it is
mandatory.
Acknowledgements
The authors would like to thank Simon Rees for his
help revising this text before publication.
References
|
1
|
Lazarczyk M, Cassonnet P, Pons C, Jacob Y
and Favre M: The EVER proteins as a natural barrier against
papillomaviruses: A new insight into the pathogenesis of human
papillomavirus infections. Microbiol Mol Biol Rev. 73:348–370.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bernard HU, Burk RD, Chen Z, van Doorslaer
K, zur Hausen H and de Villiers EM: Classification of
papillomaviruses (PVs) based on 189 PV types and proposal of
taxonomic amendments. Virology. 401:70–79. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
de Villiers EM: Cross-roads in the
classification of papillomaviruses. Virology. 445:2–10. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
McKenzie ND, Kobetz EN, Ganjei-Azar P,
Rosa-Cunha I, Potter JE, Morishita A, Lucci JA III, Guettouche T,
Hnatyszyn JH and Koru-Sengul T: HPV in HIV-infected women:
Implications for primary prevention. Front Oncol. 4:1792014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dunne EF, Unger ER, Sternberg M, McQuillan
G, Swan DC, Patel SS and Markowitz LE: Prevalence of HPV infection
among females in the United States. JAMA. 297:813–819. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tartaglia E, Iafusco D, Galderisi A and
Mastrantonio P: Do HPV vaccine genotypes agree with HPV circulating
types? Lancet Infect Dis. 11:585–586. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tartaglia E, Iafusco D, Cocca A, Palomba
S, Rotondi M and Mastrantonio P: HPV at the time of Vaccine: Has
screening reached its goal? Eur J Gynecol Oncol. 33:591–597.
2012.
|
|
8
|
Franco EL, Villa LL, Sobrinho JP, Prado
JM, Rousseau MC, Désy M and Rohan TE: Epidemiology of acquisition
and clearance of cervical human papillomavirus infection in women
from a high-risk area for cervical cancer. J Infect Dis.
180:1415–1423. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mbulawa ZZ, Marais DJ, Johnson LF, Coetzee
D and Williamson AL: Impact of human immunodeficiency virus on the
natural history of human papillomavirus genital infection in South
African men and women. J Infect Dis. 206:15–27. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lissouba P, Van de Perre P and Auvert B:
Association of genital human papillomavirus infection with HIV
acquisition: A systematic review and meta-analysis. Sex Transm
Infect. 89:350–356. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Filia A: Epidemiological aspects of HPV
infection. (last revision Apr 1, 2015). http://www.epicentro.iss.it/problemi/hpv/epid.aspFeb
23–2015
|
|
12
|
Madeddu G, Mameli G, Capobianco G,
Babudieri S, Maida I, Bagella P, Rocca G, Cherchi PL, Sechi LA,
Zanetti S, et al: HPV infection in HIV-positive females: The need
for cervical cancer screening including HPV-DNA detection despite
successful HAART. Eur Rev Med Pharmacol Sci. 18:1277–1285.
2014.PubMed/NCBI
|
|
13
|
Clifford GM, Goncalves MA and Franceschi
S: HPV and HIV Study Group: Human papillomavirus types among women
infected with HIV: A meta-analysis. AIDS. 20:2337–2344. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Camargo M, Soto-De Leon SC, Munoz M,
Sanchez R, Peña-Herrera D, Pineda-Peña AC, Sussmann O, Paez C,
Perez-Prados A, Patarroyo ME and Patarroyo MA: Human papillomavirus
detection in women with and without human immunodeficiency virus
infection in Colombia. BMC Cancer. 14:4512014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Muñoz N, Castellsagué X, de González AB
and Gissmann L: HPV in the etiology of human cancer. Vaccine. 24
Suppl 3:S3/1–10. 2006. View Article : Google Scholar
|
|
16
|
Calvo M, Laguno M, Martínez M and Martínez
E: Effects of tobacco smoking on HIV-infected individuals. AIDS
Rev. 17:47–55. 2015.PubMed/NCBI
|
|
17
|
Cotton SC, Sharp L, Seth R, Masson LF,
Little J, Cruickshank ME, Neal K and Waugh N: TOMBOLA Group:
Lifestyle and socio-demographic factors associated with high-risk
HPV infection in UK women. Br J Cancer. 97:133–139. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Green J, de Gonzalez Berrington A, Smith
JS, Franceschi S, Appleby P, Plummer M and Beral V: Human
papillomavirus infection and use of oral contraceptives. Br J
Cancer. 88:1713–1720. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Silva Ld, Miranda A, Batalha R, Ferreira
L, Santos M and Talhari S: High-risk human papillomavirus and
cervical lesions among women living with HIV/AIDS in Brazilian
Amazon, Brazil. Braz J Infect Dis. 19:557–562. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Denny LA, Franceschi S, de Sanjosé S,
Heard I, Moscicki AB and Palefsky J: Human papillomavirus, human
immunodeficiency virus and immunosuppression. Vaccine. 30 Suppl
5:F168–F174. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rositch AF, Gravitt PE, Tobian AA, Newell
K, Quinn TC, Serwadda D, Ssebbowa P, Kiggundu V, Gray RH and
Reynolds SJ: Frequent detection of HPV before and after initiation
of antiretroviral therapy among HIV/HSV-2 co-infected women in
Uganda. PLoS One. 8:e553832013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hong H, He TF, Ni HX, Zhang S and Xu GZ:
Prevalence and genotype distribution of HPV infection among women
in Ningbo, China. Int J Gynaecol Obstet. 131:96–99. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sammarco ML, Ucciferri C, Tamburro M,
Falasca K, Ripabelli G and Vecchiet J: High prevalence of human
papillomavirus type 58 in HIV infected men who have sex with men: A
preliminary report in Central Italy. J Med Virol. 88:911–914. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu G, Markowitz LE, Hariri S, Panicker G
and Unger ER: Seroprevalence of 9 human papillomavirus types in the
United States, 2005–2006. J Infect Dis. 213:191–198. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Petrosky E, Bocchini JA Jr, Hariri S,
Chesson H, Curtis CR, Saraiya M, Unger ER and Markowitz LE: Centers
for Disease Control and Prevention (CDC): Use of 9-valent human
papillomavirus (HPV) vaccine: Updated HPV vaccination
recommendations of the advisory committee on immunization
practices. MMWR Morb Mortal Wkly Rep. 64:300–304. 2015.PubMed/NCBI
|
|
26
|
Chesson HW, Laprise JF, Brisson M and
Markowitz LE: Impact and cost-effectiveness of 3 doses of 9-Valent
human papillomavirus (HPV) vaccine among US females previously
vaccinated with 4-valent HPV vaccine. J Infect Dis. 213:1694–1700.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Brisson M, Laprise JF, Chesson HW, Drolet
M, Malagón T, Boily MC and Markowitz LE: Health and economic impact
of switching from a 4-valent to a 9-valent HPV vaccination program
in the United States. J Natl Cancer Inst. 108:pii: djv282.
2015.PubMed/NCBI
|
|
28
|
Kojic EM, Rana AI and Cu-Uyin S: Human
papillomavirus vaccination in HIV-infected women: Need for
increased coverage. Expert Rev Vaccines. 15:105–117. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhai L and Tumban E: Gardasil-9: A global
survey of projected efficacy. Antiviral Res. 130:101–109. 2016.
View Article : Google Scholar : PubMed/NCBI
|