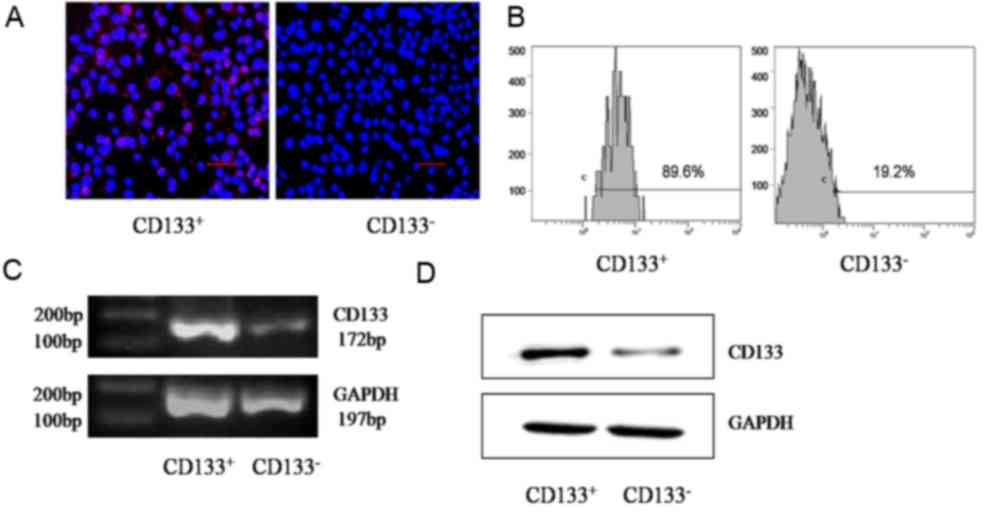
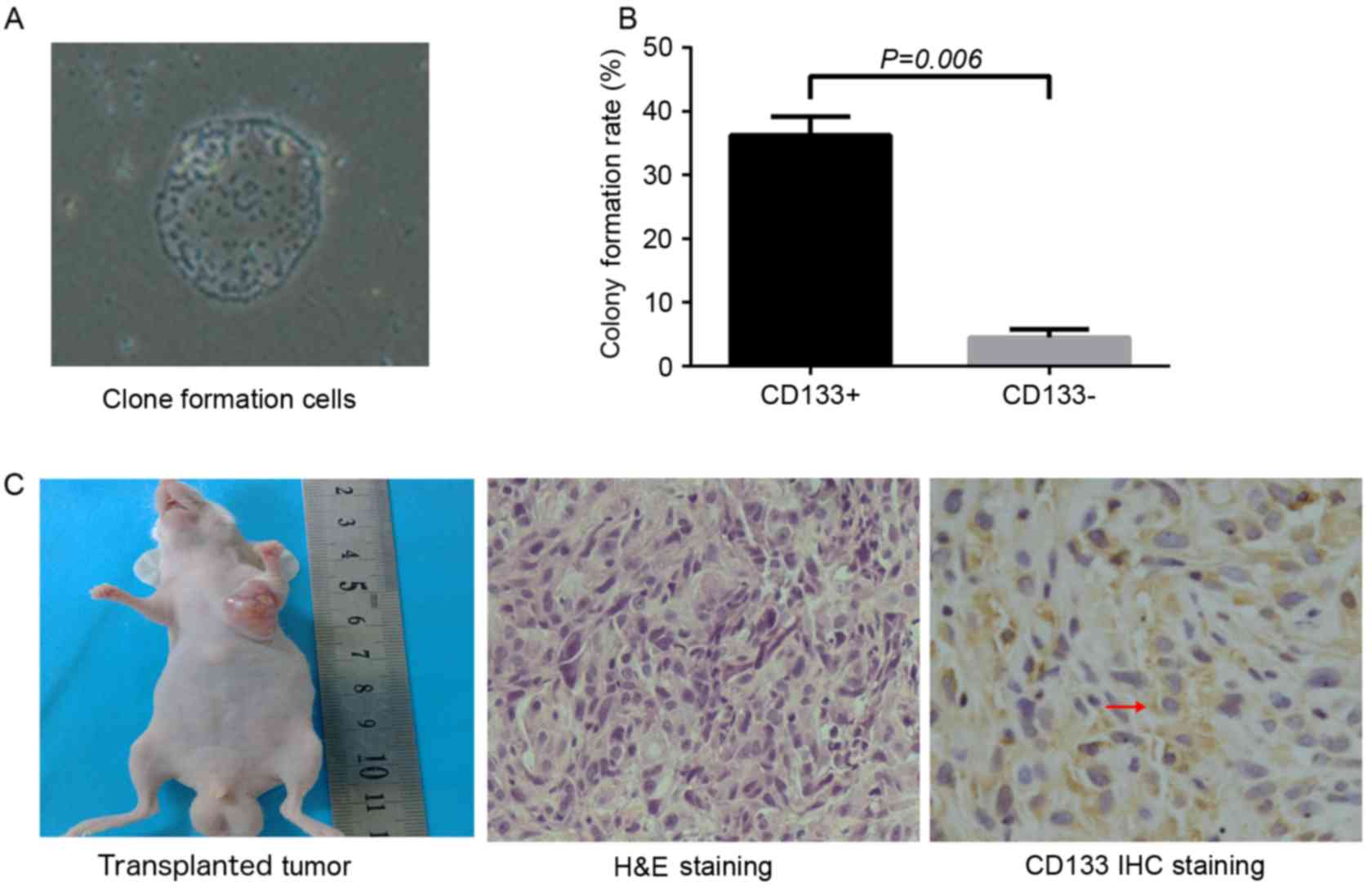
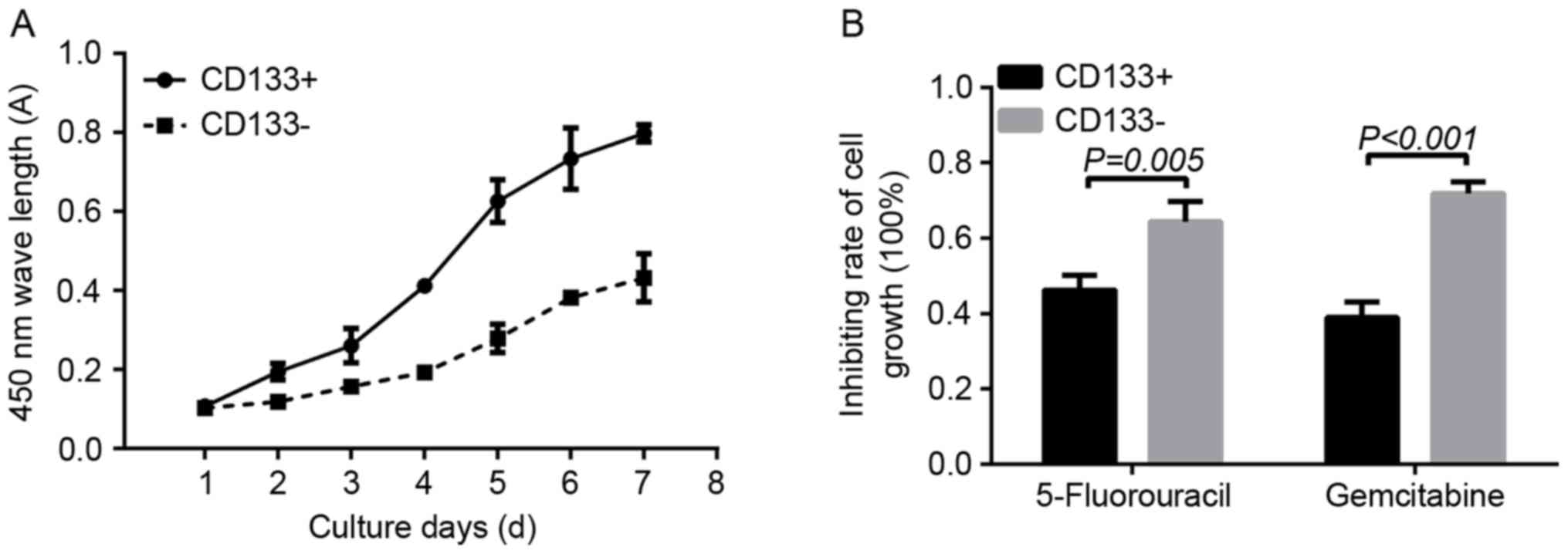
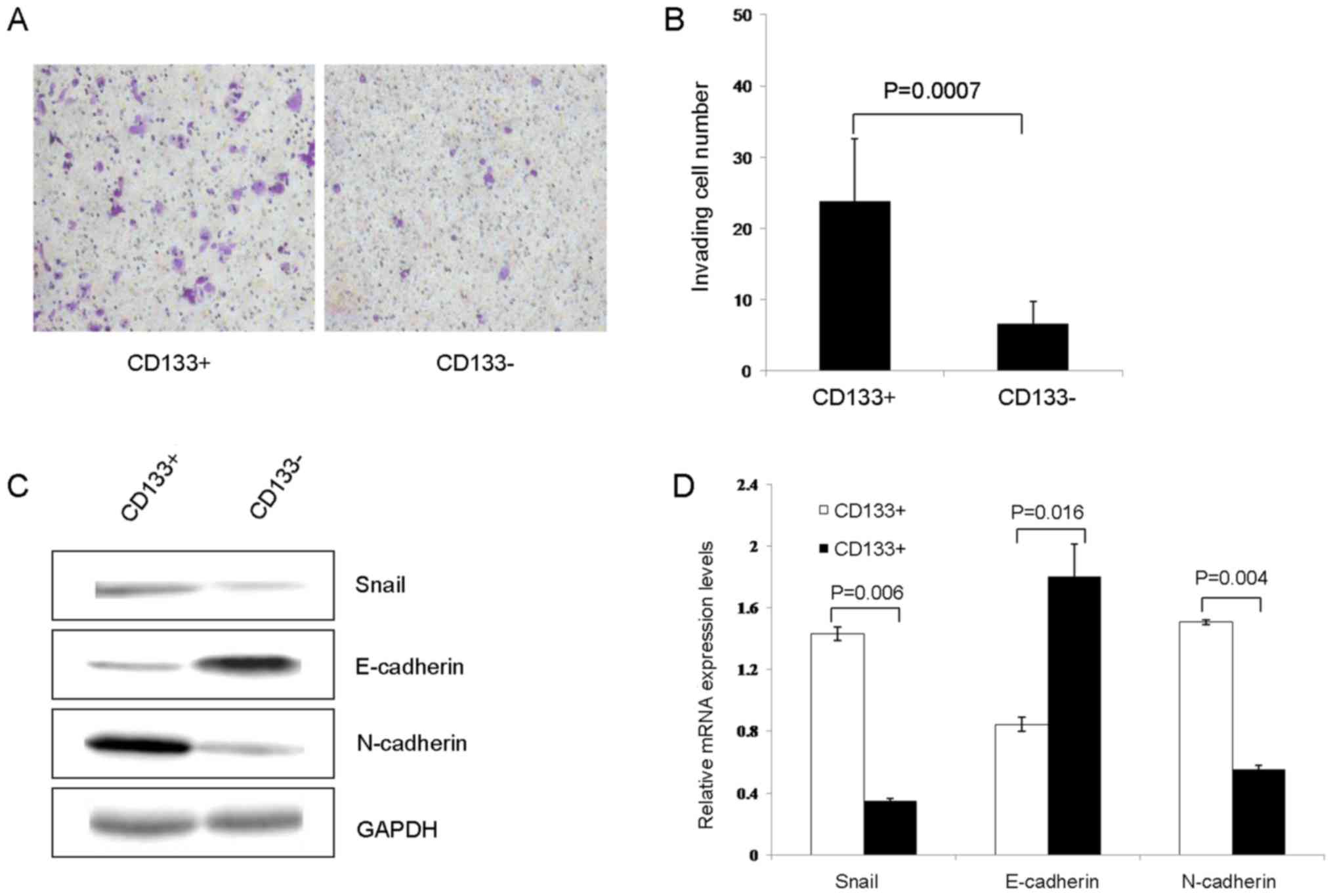
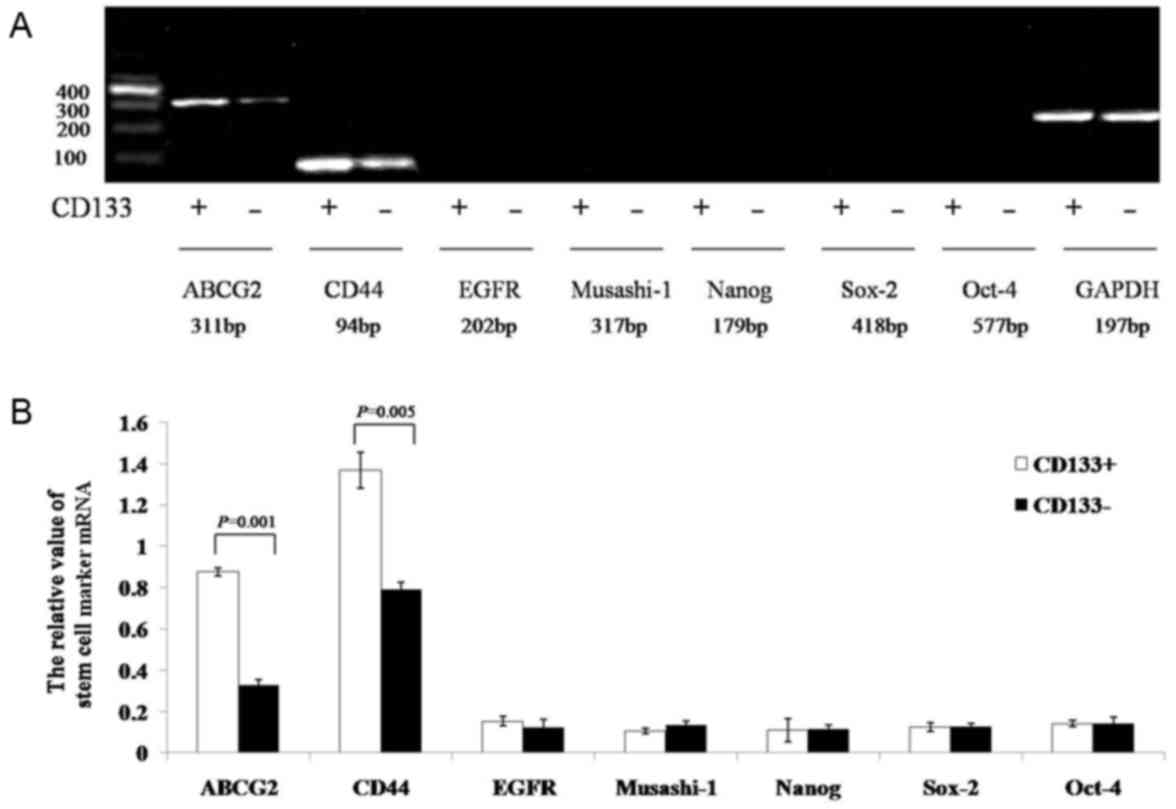
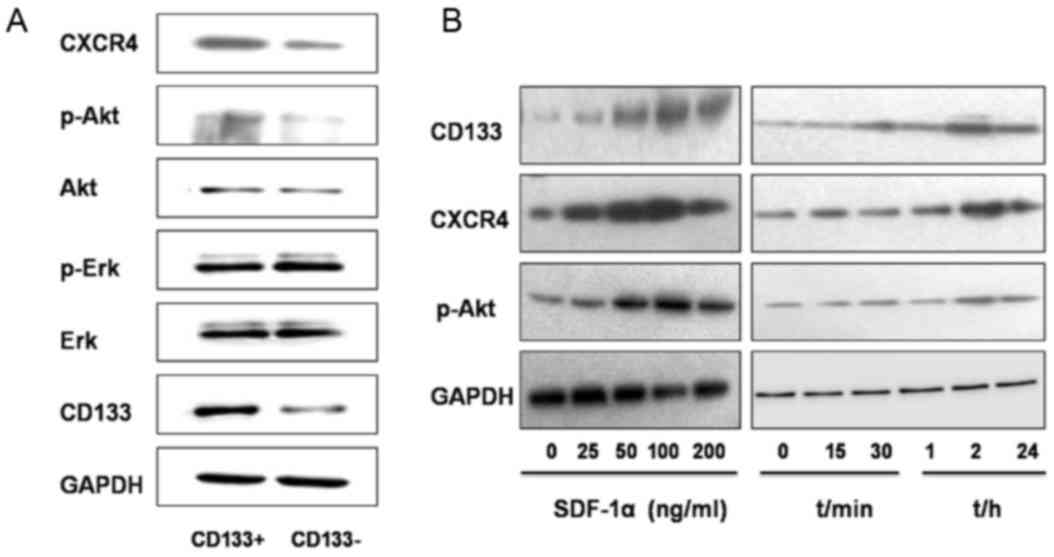
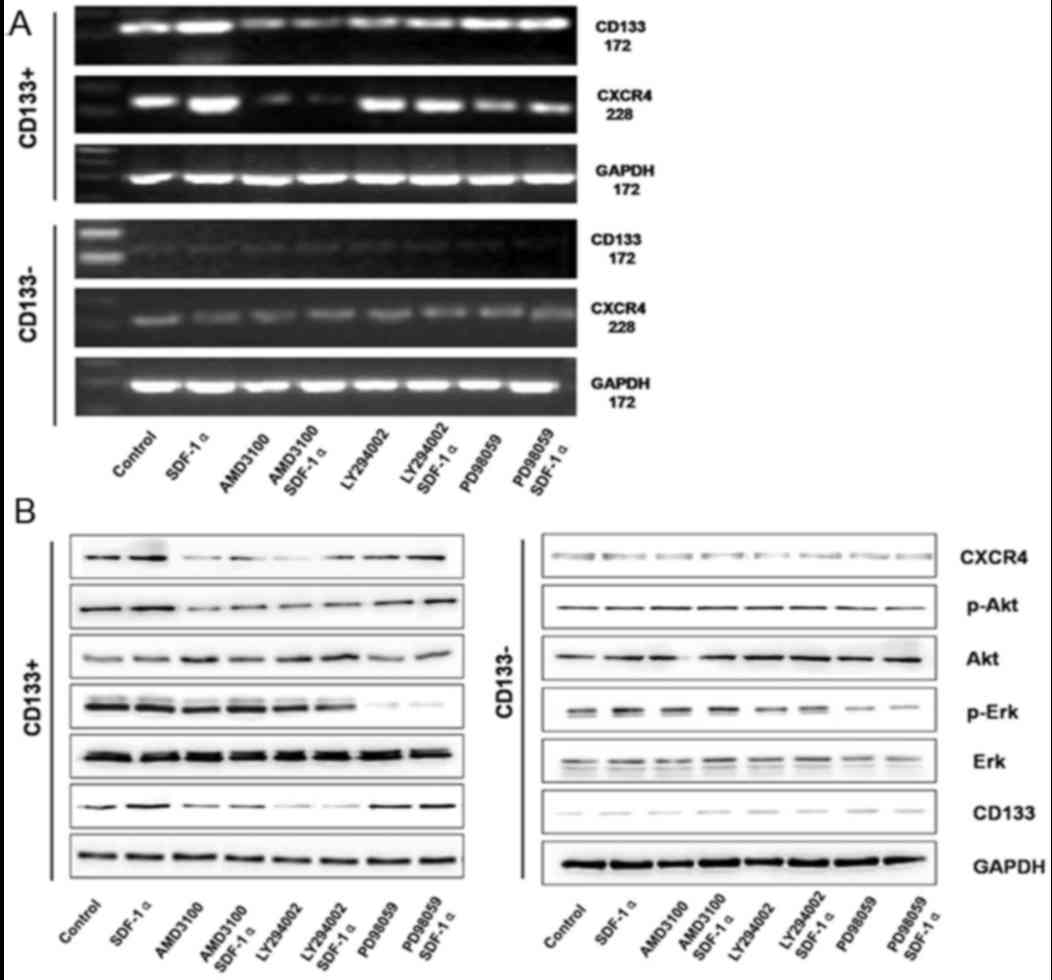
|
1
|
Park HS, Lim JY, Yoon DS, Park JS, Lee DK,
Lee SJ, Choi HJ, Song SY, Lee WJ and Cho JY: Outcome of adjuvant
therapy for gallbladder cancer. Oncology. 79:168–173. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Misra S, Chaturvedi A, Misra NC and Sharma
ID: Carcinoma of the gallbladder. Lancet Oncol. 4:167–176. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Meacham CE and Morrison SJ: Tumour
heterogeneity and cancer cell plasticity. Nature. 501:328–337.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bonnet D and Dick JE: Human acute myeloid
leukemia is organized as a hierarchy that originates from a
primitive hematopoietic cell. Nat Med. 3:730–737. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dalerba P, Dylla SJ, Park IK, Liu R, Wang
X, Cho RW, Hoey T, Gurney A, Huang EH, Simeone DM, et al:
Phenotypic characterization of human colorectal cancer stem cells.
Proc Natl Acad Sci USA. 104:pp. 10158–10163. 2007, View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li C, Heidt DG, Dalerba P, Burant CF,
Zhang L, Adsay V, Wicha M, Clarke MF and Simeone DM: Identification
of pancreatic cancer stem cells. Cancer Res. 67:1030–1037. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bao S, Wu Q, McLendon RE, Hao Y, Shi Q,
Hjelmeland AB, Dewhirst MW, Bigner DD and Rich JN: Glioma stem
cells promote radioresistance by preferential activation of the DNA
damage response. Nature. 444:756–760. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Alvero AB, Chen R, Fu HH, Montagna M,
Schwartz PE, Rutherford T, Silasi DA, Steffensen KD, Waldstrom M,
Visintin I and Mor G: Molecular phenotyping of human ovarian cancer
stem cells unravels the mechanisms for repair and chemoresistance.
Cell Cycle. 8:158–166. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Choy W, Nagasawa DT, Trang A, Thill K,
Spasic M and Yang I: CD133 as a marker for regulation and potential
for targeted therapies in glioblastoma multiforme. Neurosurg Clin N
Am. 23:391–405. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee HJ, You DD, Choi DW, Choi YS, Kim SJ,
Won YS and Moon HJ: Significance of CD133 as a cancer stem cell
markers focusing on the tumorigenicity of pancreatic cancer cell
lines. J Korean Surg Soc. 81:263–270. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang C, Xie J, Guo J, Manning HC, Gore JC
and Guo N: Evaluation of CD44 and CD133 as cancer stem cell markers
for colorectal cancer. Oncol Rep. 28:1301–1308. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sugihara E and Saya H: Complexity of
cancer stem cells. Int J Cancer. 132:1249–1259. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kreso A and Dick JE: Evolution of the
cancer stem cell model. Cell Stem Cell. 14:275–291. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yin BB, Wu SJ, Zong HJ, Ma BJ and Cai D:
Preliminary screening and identification of stem cell-like sphere
clones in a gallbladder cancer cell line GBC-SD. J Zhejiang Univ
Sci B. 12:256–263. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shi CJ, Gao J, Wang M, Wang X, Tian R, Zhu
F, Shen M and Qin RY: CD133(+) gallbladder carcinoma cells exhibit
self-renewal ability and tumorigenicity. World J Gastroenterol.
17:2965–2971. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chatterjee S, Behnam Azad B and Nimmagadda
S: The intricate role of CXCR4 in cancer. Adv Cancer Res.
124:31–82. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sun X, Cheng G, Hao M, Zheng J, Zhou X,
Zhang J, Taichman RS, Pienta KJ and Wang J: CXCL12/CXCR4/CXCR7
chemokine axis and cancer progression. Cancer Metastasis Rev.
29:709–722. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vasko V, Saji M, Hardy E, Kruhlak M, Larin
A, Savchenko V, Miyakawa M, Isozaki O, Murakami H, Tsushima T, et
al: Akt activation and localization correlate with tumour invasion
and oncogene expression in thyroid cancer. J Med Genet. 41:161–170.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Friedl P and Alexander S: Cancer invasion
and the microenvironment: Plasticity and reciprocity. Cell.
147:992–1009. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yu JW, Zhang P, Wu JG, Wu SH, Li XQ, Wang
ST, Lu RQ, Ni XC and Jiang BJ: Expressions and clinical
significances of CD133 protein and CD133 mRNA in primary lesion of
gastric adenocacinoma. J Exp Clin Cancer Res. 29:1412010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lu RQ, Wu JG, Zhou GC, Jiang HG, Yu JW and
Jiang BJ: Sorting of CD133(+) subset cells in human gastric cancer
and the identification of their tumor initiating cell-like
properties. Zhonghua Wei Chang Wai Ke Za Zhi. 15:174–179.
2012.PubMed/NCBI
|
|
22
|
Zhang SS, Han ZP, Jing YY, Tao SF, Li TJ,
Wang H, Wang Y, Li R, Yang Y, Zhao X, et al: CD133(+)CXCR4(+) colon
cancer cells exhibitmetastatic potential and predict poor prognosis
of patients. BMC Med. 10:852012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Magee JA, Piskounova E and Morrison SJ:
Cancer stem cells: Impact, heterogeneity, and uncertainty. Cancer
Cell. 21:283–296. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
O'Brien CA, Pollett A, Gallinger S and
Dick JE: A human colon cancer cell capable of initiating tumour
growth in immunodeficient mice. Nature. 445:106–110. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vander Griend DJ, Karthaus WL, Dalrymple
S, Meeker A, DeMarzo AM and Isaacs JT: The role of CD133 in normal
human prostate stem cells and malignant cancer-initiating cells.
Cancer Res. 68:9703–9711. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Reya T, Morrison SJ, Clarke MF and
Weissman IL: Stem cells, cancer, and cancer stem cells. Nature.
414:105–111. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Singh S, Chitkara D, Mehrazin R, Behrman
SW, Wake RW and Mahato RI: Chemoresistance in prostate cancer cells
is regulated by miRNAs and Hedgehog pathway. PLoS One.
7:e400212012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Thiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Singh A and Settleman J: EMT, cancer stem
cells and drug resistance: An emerging axis of evil in the war on
cancer. Oncogene. 29:4741–4751. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yu J, Vodyanik MA, Smuga-Otto K,
Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA,
Ruotti V, Stewart R, et al: Induced pluripotent stem cell lines
derived from human somatic cells. Science. 318:1917–1920. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu DC, Yang ZL and Jiang S:
Identification of musashi-1 and ALDH1 as carcinogenesis,
progression, and poor-prognosis related biomarkers for gallbladder
adenocarcinoma. Cancer Biomark. 8:113–121. 2010–2011. View Article : Google Scholar
|
|
32
|
Lichtenberger BM, Tan PK, Niederleithner
H, Ferrara N, Petzelbauer P and Sibilia M: Autocrine VEGF signaling
synergizes with EGFR in tumor cells to promote epithelial cancer
development. Cell. 140:268–279. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li CF, Fang FM, Wang JM, Tzeng CC, Tai HC,
Wei YC, Li SH, Lee YT, Wang YH, Yu SC, et al: EGFR nuclear import
in gallbladder carcinoma: Nuclear phosphorylated EGFR upregulates
iNOS expression and confers independent prognostic impact. Ann Surg
Oncol. 19:443–454. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen S, Hou JH, Feng XY, Zhang XS, Zhou
ZW, Yun JP, Chen YB and Cai MY: Clinicopathologic significance of
putative stem cell marker, CD44 and CD133, in human gastric
carcinoma. J Surg Oncol. 107:799–806. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Britton KM, Eyre R, Harvey IJ, Stemke-Hale
K, Browell D, Lennard TW and Meeson AP: Breast cancer, side
population cells and ABCG2 expression. Cancer Lett. 323:97–105.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ping YF, Yao XH, Jiang JY, Zhao LT, Yu SC,
Jiang T, Lin MC, Chen JH, Wang B, Zhang R, et al: The chemokine
CXCL12 and its receptor CXCR4 promote glioma stem cell-mediated
VEGF production and tumour angiogenesis via PI3K/AKT signaling. J
Pathol. 224:344–354. 2011. View Article : Google Scholar : PubMed/NCBI
|