Introduction
Recently, in the treatment of advanced low rectal
cancer, preoperative chemoradiotherapy (CRT) has been widely
accepted as a standard therapy. Previous studies have shown that
preoperative CRT contributes to tumor down-staging and decreases
locoregional recurrence post-surgery (1). Although the clinical significance of the
CRT response has not been fully elucidated, several reports
described that it may represent a predictor of clinical outcome,
including tumor recurrence and patient survival. However, patients
show different responses to CRT, with some cases showing little or
no response (2). Therefore,
prediction of the CRT response is warranted to avoid unnecessary
treatment and adverse events such as radiation dermatitis,
hematologic toxicity, and enteritis.
The cancer stem cell (CSC) theory, proposed by
Hamburger et al, states that tumor cells are not only
heterogeneous but also show a hierarchy, and CSCs, comprising a
small part of tumors, are responsible for this heterogeneity due to
their self-renewal and proliferation abilities (3). CSCs have been reported to be associated
with tumor progression and recurrence. Although conventional
cytotoxic therapies, such as chemotherapy or radiotherapy, target
rapidly dividing cells, CSCs are estimated to be resistant to those
therapies, as they divide more slowly (4,5). Finding a
specific marker to identify CSCs is important, and prior studies
have reported several putative CSC markers for colorectal cancer
(CRC), including leucine-rich repeat-containing G protein-coupled
receptor 5 (LGR5) and cluster of differentiation-133 (CD133)
(6,7).
LGR5 is a glycoprotein hormone receptor with a seven
transmembrane domain. Carmon et al reported that R-spondin
proteins function as ligands of LGR5 and that LGR5 is a target of
the Wnt/β-catenin signaling pathway, which is important for the
maintenance of the colonic crypt (8).
Barker et al demonstrated that LGR5 localized at the crypt
base in the small intestine, and that LGR5-positive cells could
generate multiple cell lineages of intestinal epithelium (9). Thus, LGR5 is considered a stem cell
marker in the small intestine and colon. Of note, LGR5 is detected
in many different tumors, including CRC (10–13). In
CRC, previous studies, including two meta-analyses, demonstrated
that LGR5 expression was associated with tumor progression such as
tumor depth, lymphovascular invasion, lymph node metastasis,
advanced tumor stage, and poor overall survival (OS) (14–16).
Moreover, prior studies have evaluated LGR5 expression in rectal
cancer patients treated with preoperative CRT, and reported
associations with worse sensitivity to CRT and poor patient
prognosis (17).
CD133 is a five transmembrane glycoprotein that is
widely detected in many tumors, including colon cancers (18–20). Prior
studies have reported that CD133-positive colon cancer cells have
self-renewal and proliferation abilities (7). In CRC, CD133 has also been reported to
be associated with tumor depth, lymphovascular invasion, lymph node
metastasis, and advanced tumor stage (21). Further, two meta-analyses demonstrated
that CD133 expression correlated with worse OS (22,23). The
correlations between CD133 expression and response to CRT have also
been evaluated, with CD133 shown to associate with CRT resistance
and poor patient prognosis (24–26).
However, these previous reports evaluated the
clinical significance of LGR5 and CD133 expressions separately. To
our knowledge, the significance of combined LGR5 and
CD133expression remains unclarified. Therefore, herein, we
evaluated both LGR5 and CD133 expressions immunohistochemically in
low rectal cancer patients treated with preoperative CRT using
serial sections, and analyzed the relationships between those
expressions and clinicopathological features and patient
prognosis.
Materials and methods
Patients and specimens
Sixty one consecutive patients underwent curative
resection after CRT for advanced low rectal cancer in the
Department of Surgical Oncology, University of Tokyo Hospital
between March 2001 and October 2009. All patients were diagnosed as
low rectal cancer, and the tumor depth was estimated to be deeper
than the muscularis propria. Tumor depth, nodal status, and
presence of distant metastases were determined by computed
tomography and magnetic resonance imaging. They received a total
radiation dose of 50.4 Gy (1.8 Gy in 28 fractions) and concomitant
chemotherapy (oral administration of tegafur-uracil 300 mg/m2/day
and leucovorin 75 mg/day). Total mesorectal excision with lymph
node dissection was performed following an interval of 6–8 weeks
post-CRT. All patients underwent regular follow-up examinations
post-surgery. Tumor markers were examined every 3 months, and
abdominal and chest computed tomography was performed every 6
months. Total colonoscopy was performed annually. Since we targeted
the residual cancer tissue, 5 cases with no residual cancer cells
after CRT were excluded. We analyzed 56 surgically resected
specimens after CRT by immunohistochemistry.
All specimens were fixed in 10% formalin and
embedded in paraffin. The histopathological findings were confirmed
by the Department of Pathology, University of Tokyo. Data were
collected from the patients' medical records. The TNM
classification was determined according to the Union for
International Cancer Control, 7th edition. The post-CRT
histological tumor regression grade was evaluated according to the
Japanese Classification of Colorectal Carcinoma, 8th edition (Grade
0: No necrosis or regressive change, 1a: >66.6% vital residual
tumor cells, 1b: 33.3–66.6% vital residual tumor cells, 2:
<33.3% vital residual tumor cells, 3: No vital residual tumor
cells).
This study was approved by the ethics committee of
the University of Tokyo on July 29, 2014 [No. 10476-(1)] and written informed consent was obtained
from all patients.
LGR5 and CD133 Immunohistochemical
staining
The tumor specimens were immunohistochemically
stained, as described below. The sections were deparaffinized in
xylene, hydrated through a graded series of ethanol, and endogenous
peroxidase was blocked. Heat-induced antigen retrieval was
performed by incubation in sodium citrate buffer using an autoclave
and non-specific proteins were blocked with 5% bovine serum
albumin. Primary anti-LGR5 rabbit (Clone EPR3065Y, Epitomics,
Burlingame, CA, USA) and anti-CD133 mouse monoclonal antibodies
(Clone AC133, Miltenyi Biotec, Auburn, CA, USA) were added at
dilutions of 1:100 and the sections were incubated overnight at
4°C. They were incubated with the Dako Envision kit (Dako,
Carpinteria, CA, USA) following the manufacturer's recommendations.
The reactivity was visualized in 2% 3,3′-diaminobenzidine
tetrahydrochloride and 50 mM tris-buffer containing 0.3% hydrogen
peroxidase. The crypt base of the normal colon mucosa and renal
tubules were used as the positive controls for LGR5 and CD133,
respectively. As a negative control, the antibody was replaced with
PBS.
Evaluation of LGR5 and CD133
immunostaining
We defined positive expression of LGR5 as >50%
positive cancer cells out of all cancer cells, since Saigusa et
al also evaluated LGR5 expression in rectal cancer tissue after
CRT using same cut-off value to clarify clinical significance of
LGR5 (17). Positive expression of
CD133 was defined as >5% positive cancer cells out of all cancer
cells (27), since we previously
showed CD133 expression in rectal cancer after CRT was associated
with CRT response (22), and
speculated the cut-off value to be appropriate. The sections were
observed by a surgeon trained in pathology and a skillful
pathologist, independently in a blinded fashion (S.K. and T.M.).
Any discrepancies were resolved by discussion. Subsequently, we
analyzed the correlations between the expressions of LGR5 and CD133
and clinicopathological factors and patient prognosis.
Statistical analysis
The correlations between LGR5 and CD133 expressions
and clinicopathological factors were evaluated by the chi-squared
test, Fisher's exact test, or unpaired t-test, as appropriate. OS
and disease-free survival (DFS) were analyzed by the Kaplan-Meier
method. P-values <0.05 were considered statistically
significant. All analyses were performed using JMP 11.0 software
(SAS Institute Inc., Cary, NC, USA).
Results
Clinicopathological findings
The clinicopathological factors are listed in
Table I. The median patient age was
61 (range, 33–78) years, and 37 patients (66.1%) were male. After
preoperative CRT, 21 (37.5%) and 35 (62.5%) patients had T1-2 and
T3-4 tumors, respectively. Thirty-nine patients (69.6%) showed
papillary carcinoma or well-differentiated adenocarcinoma
histology. Nine (16.1%) and six (10.7%) patients had lymph node and
distant metastases (3 liver, 1 lung, 1 brain, and 1 paraaortic
lymph node metastases), respectively. Based on the response to CRT
classification, 17 (30.4%), 18 (32.1%), and 21 (37.5%) patients
were categorized as Grades 1a, 1b, and 2, respectively. The median
follow-up period was 5.8 (range, 0.8–10.9) years.
 | Table I.Characteristics of rectal cancer
patients. |
Table I.
Characteristics of rectal cancer
patients.
| Characteristic | No. of patients
(%) |
|---|
| Gender |
|
|
Male | 37 (66.1) |
|
Female | 19 (33.9) |
| Mean age ± SD
(years) | 61.1±9.9 |
| pT
stagea |
|
|
T1-2 | 21 (37.5) |
|
T3-4 | 35 (62.5) |
| Histological
type |
|
| Pap,
Well | 39 (69.6) |
| Mod,
Por, Muc | 17 (30.4) |
| Lymphatic
invasion |
|
|
Present | 5 (8.9) |
|
Absent | 51 (91.1) |
| Vascular
invasion |
|
|
Present | 31 (55.4) |
|
Absent | 25 (44.6) |
| Lymph node
metastasis |
|
|
Present | 9 (16.1) |
|
Absent | 47 (83.9) |
| Distant
metastasis |
|
|
Present | 6 (10.7) |
|
Absent | 50 (89.3) |
| TNM
Stagea |
|
|
I–II | 44 (78.6) |
|
III–IV | 12 (21.4) |
| Tumor regression
gradeb |
|
| 1a | 17 (30.4) |
| 1b | 18 (32.1) |
| 2 | 21 (37.5) |
LGR5 and CD133 expressions
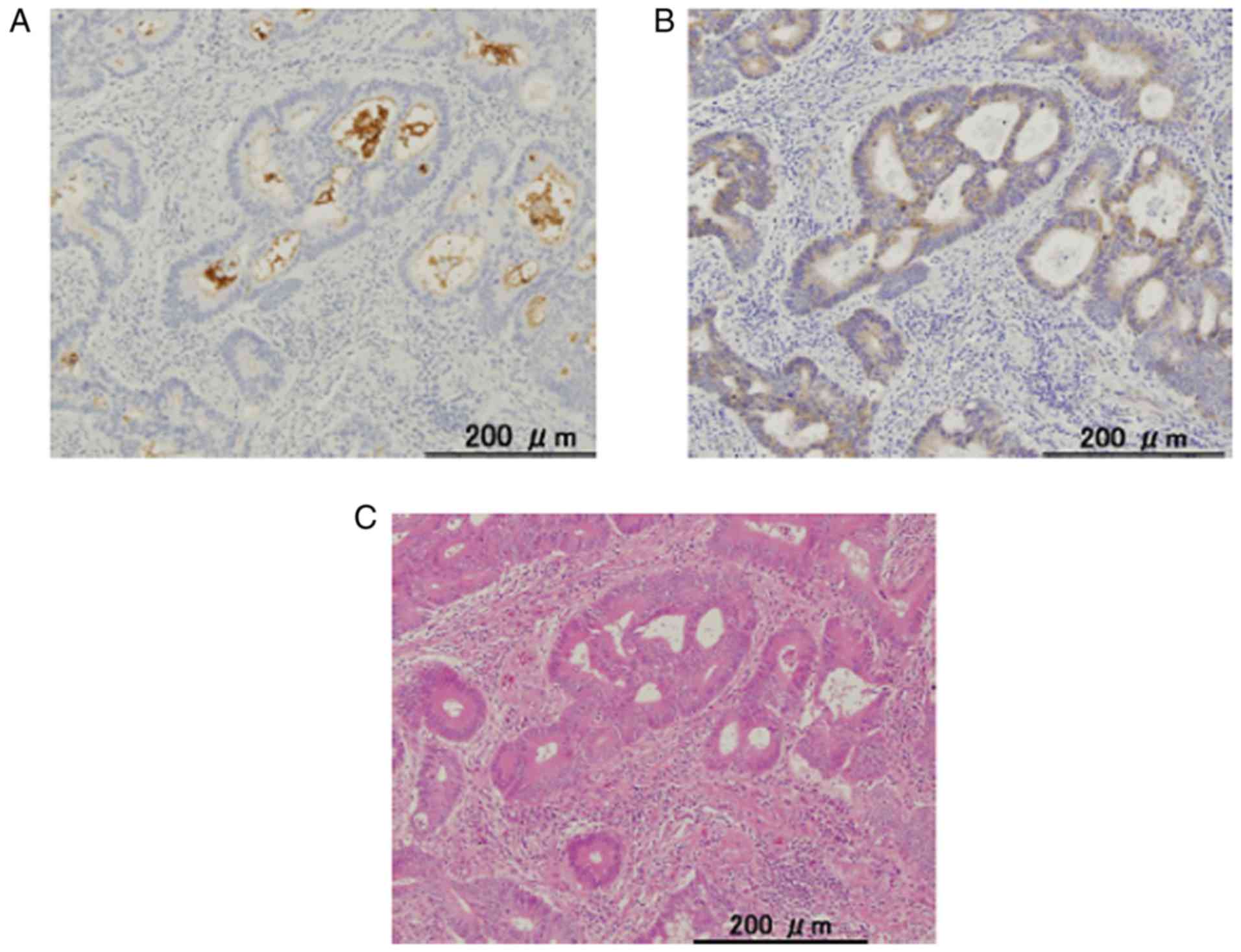
LGR5 expression was detected in the cytoplasm while
CD133 expression was detected at the cell membrane and the
intraglandular cellular debris in some tumor glands (Fig. 1). The staining pattern was mostly
similar to that previously reported. As a result of the
immunohistochemistry scoring, 36 (64.3%) and 29 (51.8%) patients
were categorized as LGR5-positive and CD133-positive, respectively.
Twenty-one patients (37.5%) showed positive expressions of both
markers.
Relationships between LGR5 expression
and clinicopathological features
LGR5 expression significantly correlated with
lymphatic invasion (P=0.03), lymph node metastasis (P<0.01), and
TNM stage (P<0.01) (Table II). No
significant difference in OS or DFS was found [LGR5 positive vs.
negative: 5-year OS 85.0% vs. 85.0% (P=0.86), 5-year DFS 63.9% vs.
70.0% (P=0.67), respectively].
 | Table II.LGR5 expression and
clinicopathological features. |
Table II.
LGR5 expression and
clinicopathological features.
|
| LGR5+ (n=36) | LGR5- (n=20) |
|
|---|
|
|
|
|
|
|---|
| Characteristic | n | % | n | % | P-value |
|---|
| Gender |
|
|
|
|
|
|
Male | 25 | 69.4 | 12 | 60.0 | 0.48 |
|
Female | 11 | 37.9 | 8 | 29.6 |
|
| Mean age ± SD
(years) | 60.2±11.1 | 62.8±7.1 | 0.51 |
| pT stage |
|
|
|
|
|
|
T1-2 | 15 | 42.0 | 6 | 30.0 | 0.38 |
|
T3-4 | 21 | 58.0 | 14 | 70.0 |
|
| Histological
type |
|
|
|
|
|
| Pap,
Well | 24 | 66.7 | 15 | 75.0 | 0.51 |
| Mod,
Por, Muc | 12 | 33.3 | 5 | 25.0 |
|
| Lymphatic
invasion |
|
|
|
|
|
|
Present | 5 | 13.9 | 0 | 0.0 | 0.03 |
|
Absent | 31 | 86.1 | 20 | 100.0 |
|
| Vascular
invasion |
|
|
|
|
|
|
Present | 19 | 52.8 | 12 | 60.0 | 0.60 |
|
Absent | 17 | 47.2 | 8 | 40.0 |
|
| Lymph node
metastasis |
|
|
|
|
|
|
Present | 9 | 25.0 | 0 | 0.0 | <0.01 |
|
Absent | 27 | 75.0 | 20 | 100.0 |
|
| Distant
metastasis |
|
|
|
|
|
|
Present | 5 | 13.9 | 1 | 5.0 | 0.28 |
|
Absent | 31 | 86.1 | 19 | 95.0 |
|
| TNM Stage |
|
|
|
|
|
|
I–II | 24 | 66.7 | 20 | 100 | <0.01 |
|
III–IV | 12 | 33.3 | 0 | 0 |
|
| Tumor regression
gradea |
|
|
|
|
|
| 1a | 11 | 30.6 | 6 | 30.0 | 0.94 |
| 1b | 11 | 30.6 | 7 | 35.0 |
|
| 2 | 14 | 38.9 | 7 | 35.0 |
|
Relationship between CD133 expression
and clinicopathological features
CD133 expression significantly correlated with
vascular invasion (P<0.01) and the tumor regression grade
(P<0.01) (Table III). No
significant difference in OS or DFS was found [CD133 positive vs.
negative, 5-year OS 80.9% vs. 88.9% (P=0.40), 5-year DFS 58.6% vs.
73.9% (P=0.19), respectively].
 | Table III.CD133 expression and
clinicopathological features. |
Table III.
CD133 expression and
clinicopathological features.
|
| CD133+ (n=29) | CD133- (n=27) |
|
|---|
|
|
|
|
|
|---|
| Characteristic | n | % | n | % | P-value |
|---|
| Gender |
|
|
|
|
|
|
Male | 18 | 62.1 | 19 | 70.4 | 0.51 |
|
Female | 11 | 37.9 | 8 | 28.9 |
|
| Mean age ± SD
(years) | 61.9±10.2 | 60.3±9.6 | 0.48 |
| pT stage |
|
|
|
|
|
|
T1-2 | 8 | 27.6 | 13 | 48.1 | 0.11 |
|
T3-4 | 21 | 72.4 | 14 | 51.9 |
|
| Histological
type |
|
|
|
|
|
| Pap,
Well | 19 | 65.5 | 20 | 74.1 | 0.49 |
| Mod,
Por, Muc | 10 | 34.5 | 7 | 25.9 |
|
| Lymphatic
invasion |
|
|
|
|
|
|
Present | 4 | 13.8 | 1 |
3.7 | 0.17 |
|
Absent | 25 | 86.2 | 26 | 96.3 |
|
| Vascular
invasion |
|
|
|
|
|
|
Present | 21 | 72.4 | 10 | 37.0 | <0.01 |
|
Absent | 8 | 27.6 | 17 | 63.0 |
|
| Lymph node
metastasis |
|
|
|
|
|
|
Present | 6 | 20.7 | 3 | 11.2 | 0.32 |
|
Absent | 23 | 79.3 | 24 | 88.9 |
|
| Distant
metastasis |
|
|
|
|
|
|
Present | 4 | 13.8 | 2 |
7.4 | 0.44 |
|
Absent | 25 | 86.2 | 25 | 92.6 |
|
| TNM Stage |
|
|
|
|
|
|
I–II | 20 | 69 | 24 | 88.9 |
0.06 |
|
III–IV | 9 | 31 | 3 | 11.1 |
|
| Tumor regression
gradea |
|
|
|
|
|
| 1a | 14 | 48.3 | 3 | 11.1 | <0.01 |
| 1b | 11 | 37.9 | 7 | 25.9 |
|
| 2 | 4 | 13.8 | 17 | 63.0 |
|
Relationships between combined LGR5
and CD133 expression and clinicopathological features
No significant correlation was found between the
expressions of the two markers (Table
IV). The patients were divided into groups of positive
expression of both markers and of all other patients, and the
relationships between combined expression and the
clinicopathological features were analyzed (Table V). Expression of both LGR5 and CD133
significantly correlated with lymphatic invasion (P=0.04), the
tumor regression grade (P<0.01), and TNM stage (P<0.01). No
significant difference in OS or DFS was found [LGR5 and CD133
positive vs. LGR5 or CD133 negative: 5-year OS 78.1% vs. 85.0%
(P=0.53), 5-year DFS 57.1% vs. 71.3% (P=0.25), respectively].
 | Table IV.Correlation between LGR5 and CD133
expression. |
Table IV.
Correlation between LGR5 and CD133
expression.
|
| CD133
expression |
|
|---|
|
|
|
|
|---|
| LGR5
expression | +(%) | − (%) | P-value |
|---|
| + | 21 (37.5) | 15 (26.8) | 0.19 |
| − | 8 (14.3) | 12 (21.4) |
|
 | Table V.LGR5 and CD133 expression and
clinicopathological features. |
Table V.
LGR5 and CD133 expression and
clinicopathological features.
|
| LGR5+ and CD133+
(n=21) | LGR- or CD133-
(n=35) |
|
|---|
|
|
|
|
|
|---|
| Characteristic | n | % | n | % | P-value |
|---|
| Gender |
|
|
|
|
|
|
Male | 14 | 66.7 | 23 | 65.7 | 0.94 |
|
Female | 7 | 33.3 | 12 | 34.3 |
|
| Mean age ± SD
(years) | 61.4±11.0 | 61.0±9.2 | 0.82 |
| pT stage |
|
|
|
|
|
|
T1-2 | 6 | 28.6 | 15 | 42.9 | 0.28 |
|
T3-4 | 15 | 71.4 | 20 | 57.1 |
|
| Histological
type |
|
|
|
|
|
| Pap,
Well | 12 | 57.1 | 27 | 77.1 | 0.12 |
| Mod,
Por, Muc | 9 | 42.9 | 8 | 22.9 |
|
| Lymphatic
invasion |
|
|
|
|
|
|
Present | 4 | 19.0 | 1 | 2.9 |
0.04 |
|
Absent | 17 | 81.0 | 34 | 97.1 |
|
| Vascular
invasion |
|
|
|
|
|
|
Present | 15 | 71.4 | 16 | 45.7 |
0.06 |
|
Absent | 6 | 28.6 | 19 | 54.3 |
|
| Lymph node
metastasis |
|
|
|
|
|
|
Present | 6 | 28.6 | 3 | 8.6 |
0.05 |
|
Absent | 15 | 71.4 | 32 | 91.4 |
|
| Distant
metastasis |
|
|
|
|
|
|
Present | 4 | 19.0 | 2 | 5.7 | 0.13 |
|
Absent | 17 | 81.0 | 33 | 94.3 |
|
| TNM Stage |
|
|
|
|
|
|
I–II | 12 | 57.1 | 32 | 91.4 | <0.01 |
|
III–IV | 9 | 42.9 | 3 | 8.6 |
|
| Tumor regression
gradea |
|
|
|
|
|
| 1a | 11 | 52.4 | 6 | 17.1 | <0.01 |
| 1b | 7 | 33.3 | 11 | 31.4 |
|
| 2 | 3 | 14.3 | 18 | 51.4 |
|
Discussion
Herein, we examined the expressions of LGR5 and
CD133 in low rectal cancer patients treated with preoperative CRT
immunohistochemically, using serial sections. LGR5 expression was
associated with lymphatic invasion, lymph node metastasis, and TNM
stage, while CD133 expression was associated with vascular
invasion. Although CD133 expression correlated with the response to
CRT, LGR5 expression did not. Moreover, the co-expression of LGR5
and CD133 significantly correlated with lymphatic invasion, the
tumor regression grade, and TNM stage. No significant correlation
with OS or DFS was found.
We evaluated the expressions of LGR5 and CD133 in
low rectal cancer using surgically resected specimens after CRT,
since the assessment of very small biopsy samples obtained pre-CRT
is difficult. There are several reports evaluating the expressions
of these markers, separately, post-CRT. Saigusa et al
(28) investigated LGR5 expression in
rectal cancer patients treated with preoperative CRT and revealed a
correlation with patient prognosis, while no correlation with any
clinicopathological factor or prognosis was observed in CRC
patients treated without preoperative therapy. They also described
that CSCs were relatively increasing after CRT, because CSCs are
resistant to CRT, as compared to non-CSCs (28). Kawamoto et al (25) evaluated CD133 expression in rectal
cancer patients treated with preoperative CRT and described that,
while CD133 expression in pre-CRT specimens before CRT did not
associate with prognosis, those obtained post-CRT associated with
DFS.
Regarding clinicopathological factors, prior studies
in CRC patients have demonstrated correlations between LGR5 and
CD133 expressions and tumor depth, lymph node metastasis, lymphatic
invasion, vascular invasion, and advanced tumor stage (14–16,21,23,24).
However, the precise mechanisms of these correlations are still
under investigation. Hirsch et al showed that silencing of
LGR5 reduced proliferation, migration, and colony formation in CRC
cell lines (29). The correlation
between LGR5 and tumor proliferation in thyroid cancers has also
been reported (12). Carmon et
al (8) demonstrated that LGR5
enhanced cell proliferation through Wnt/β-catenin signaling
(8), and LGR5 has been reported to
promote epithelial-mesenchymal transition through activation of
Wnt/β-catenin signaling in breast cancer (13). Moreover, Xi et al (10) showed a correlation between LGR5
expression and matrix metalloproteinase-2 in gastric cancer, and
declared that LGR5 played an important role in tumor invasion and
metastasis through matrix metalloproteinase-2, which degrades type
IV collagen of the extracellular matrix and basal membrane. With
respect to CD133, Chao et al (30) reported that CD133+ colon cancer cells
had an enhanced ability to interact with neighboring fibroblasts,
indicating that CD133+ cells are more invasive than CD133-cells. In
lung and pancreatic cancers, CD133 expression has been shown to
relate to vasculogenesis (27) and to
be an important factor for epithelial-mesenchymal transition
(31). Our findings were consistent
with those of these previous studies.
Regarding the response to CRT, Saigusa et al
reported that patients with high LGR5 expression had a poor
pathological response to CRT (17).
Hongo et al (24) reported
that high CD133 expression correlated with worse response to CRT.
Although the mechanisms remain unclear, there are several reports
indicating resistance to chemotherapy and radiotherapy of CSCs.
LGR5 expression has been reported to increase after radiation in
CRC cell lines (28), and
LGR5-positive CRC cells are reportedly resistant to chemotherapy
through the ATP-binding cassette family (32). Moreover, Bao et al (5) demonstrated that CD133-positive glioma
cells showed reduced sensitivity to radiation through activation of
DNA damage repair. In CRC cell lines, CD133 expression has been
reported to increase after irradiation (33), and CD133-positive CRC cells show
reduced sensitivity to chemotherapy (34).
Herein, we could not show the correlation between
LGR5 expression and the response to CRT, although CD133 expression
was associated with a poor response. This can be attributed to
several factors. First, the role of LGR5 in chemotherapy resistance
is controversial, although Planutis et al (35) reported that LGR5-expressing CRC cells
were more sensitive to anticancer drugs. Second, single irradiation
in vitro is different from multiple courses of irradiation
in clinical settings. Third, CRT regimens are not identical with
respect to the concomitant chemotherapy and frequency of
radiotherapy. Finally, our study had a relatively small sample
size, which may have affected the statistical power. Regarding the
patient prognosis, prior studies, including several meta-analyses,
have demonstrated that these markers are poor prognostic factors
(15,16,22,23).
However, we could not show these correlations due to the small
sample size.
Several putative cancer stem cell markers, including
LGR5 and CD133, have been reported, but their clinical significance
is not fully understood. Because of the heterogeneity of CRC, a
single marker for CSCs may not be sufficient for precise
evaluation. To our knowledge, there is no previous report
evaluating both LGR5 and CD133 expression in specimens of CRC
patients. In CRC cell lines, Kobayashi et al reported that
LGR5+/CD133+ and LGR5-/CD133+ cells formed colonies, while almost
all LGR5-/CD133-cells could not; their results moreover showed that
the colony formation of LGR5+/CD133+ cells was higher than that of
LGR5-/CD133+ cells (36). Therefore,
we suppose that the subgroup with both LGR5 and CD133 expression
are at high risk of malignancy. Accordingly, patients with both
LGR5 and CD133 expression had significantly more lymphatic
invasion, worse tumor regression grade and more advanced TNM stage,
and tended to have more vascular invasion and lymph node
metastasis. Although more correlations between the combined
expression and clinicopathological factors were found than in the
analysis of the single markers, further studies are necessary.
Our data should be interpreted with caution, as this
study has several limitations. First, this was a retrospective
study with a relatively small patient number. Second, we
investigated protein expression using immunohistochemistry, but did
not investigate the gene expression. Third, there are different
methods of evaluating positive immunohistochemical staining of LGR5
and CD133. Therefore, prospective studies with a larger number of
patients and the same methodology are needed.
We here demonstrated that the expression of LGR5 was
associated with lymphatic invasion, lymph node metastasis, and TNM
stage, while CD133 associated with the response to CRT in low
rectal cancer. Moreover, combined expression of LGR5 and CD133 may
be a more useful marker than the evaluation of a single marker.
References
|
1
|
Bosset JF, Collette L, Calais G, Mineur L,
Maingon P, Radosevic-Jelic L, Daban A, Bardet E, Beny A and Ollier
JC: EORTC Radiotherapy Group Trial 22921: Chemotherapy with
preoperative radiotherapy in rectal cancer. N Engl J Med.
355:1114–1123. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tomono A, Yamashita K, Kanemitsu K, Sumi
Y, Yamamoto M, Kanaji S, Imanishi T, Nakamura T, Suzuki S, Tanaka K
and Kakeji Y: Prognostic significance of pathological response to
preoperative chemoradiotherapy in patients with locally advanced
rectal cancer. Int J Clin Oncol. 21:344–349. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hamburger AW and Salmon SE: Primary
bioassay of human tumor stem cells. Science. 197:461–463. 1977.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Reya T, Morrison SJ, Clarke MF and
Weissman IL: Stem cells, cancer, and cancer stem cells. Nature.
414:105–111. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bao S, Wu Q, McLendon RE, Hao Y, Shi Q,
Hjelmeland AB, Dewhirst MW, Bigner DD and Rich JN: Glioma stem
cells promote radioresistance by preferential activation of the DNA
damage response. Nature. 444:756–760. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Espersen ML, Olsen J, Linnemann D, Høgdall
E and Troelsen JT: Clinical implications of intestinal stem cell
markers in colorectal cancer. Clin Colorectal Cancer. 14:63–71.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ricci-Vitiani L, Lombardi DG, Pilozzi E,
Biffoni M, Todaro M, Peschle C and De Maria R: Identification and
expansion of human colon-cancer-initiating cells. Nature.
445:111–115. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Carmon KS, Gong X, Lin Q, Thomas A and Liu
Q: R-spondins function as ligands of the orphan receptors LGR4 and
LGR5 to regulate Wnt/beta-catenin signaling. Proc Natl Acad Sci
USA. 108:pp. 11452–11457. 2011, View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Barker N, van Es JH, Kuipers J, Kujala P,
van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H,
Peters PJ and Clevers H: Identification of stem cell in small
intestine and colon by marker gene Lgr5. Nature. 449:1003–1017.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xi HQ, Cai AZ, Wu XS, Cui JX, Shen WS,
Bian SB, Wang N, Li JY, Lu CR, Song Z, et al: Leucine-rich
repeat-containing G-protein-coupled receptor 5 is associated with
invasion, metastasis, and cloud be a potential therapeutic target
in human gastric cancer. Br J Cancer. 110:2011–2020. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
McClanahan T, Koseoglu S, Smith K, Grein
J, Gustafson E, Black S, Kirschmeier P and Samatar AA:
Identification of overexpression of orphan G protein-coupled
receptor GPR49 in human colon and ovarian primary tumors. Cancer
Biol Ther. 5:419–426. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Michelotti G, Jiang X, Sosa JA, Diehl AM
and Henderson BB: LGR5 is associated with tumor aggressiveness in
papillary thyroid cancer. Oncotarget. 6:34549–34560.
2015.PubMed/NCBI
|
|
13
|
Yang L, Tang H, Kong Y and Xie X, Chen J,
Song C, Liu X, Ye F, Li N, Wang N and Xie X: LGR5 promotes breast
cancer progression and maintains stem-like cells through activation
of Wnt/β-catenin signaling. Stem Cells. 33:2913–2924. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu XS, Xi HQ and Chen L: Lgr5 is a
potential marker of colorectal carcinoma stem cells that correlates
with patient survival. World J Surg Oncol. 10:2442012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen Q, Zhang X, Li WM, Ji YQ, Cao HZ and
Zheng P: Prognostic value of LGR5 in colorectal cancer: A
meta-analysis. PLoS One. 9:e1070132014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Han Y, Xue X, Jiang M, Guo X, Li P, Liu F,
Yuan B, Shen Y, Guo X, Zhi Q and Zhao H: LGR5, a relevant marker of
cancer stem cells, indicates a poor prognosis in colorectal cancer
patients: A meta-analysis. Clin Res Hepatol Gastroenterol.
39:267–273. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Saigusa S, Inoue Y, Tanaka K, Toiyama Y,
Matsushita K, Kawamura M, Okugawa Y, Hiro J, Uchida K, Mohri Y and
Kusunoki M: Clinical significance of LGR5 and CD44 expression in
locally advanced rectal cancer after preoperative
chemoradiotherapy. Int J Oncol. 41:1643–1652. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ma S: Biology and clinical implications of
CD133(+) liver cancer stem cells. Exp Cell Res. 319:126–132. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Long H, Xie R, Xiang T, Zhao Z, Lin S,
Liang Z, Chen Z and Zhu B: Autocrine CCL5 signaling promotes
invasion and migration of CD133+ ovarian cancer stem-like cells via
NF-κB-mediated MMP-9 upregulation. Stem Cells. 30:2309–2319. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shien K, Toyooka S, Ichimura K, Soh J,
Furukawa M, Maki Y, Muraoka T, Tanaka N, Ueno T, Asano H, et al:
Prognostic impact of cancer stem cell-related markers in non-small
cell lung cancer patients treated with induction chemoradiotherapy.
Lung Cancer. 77:162–167. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhou F, Mu YD, Liang J, Liu ZX, Chen HS
and Zhang JF: Expression and prognostic value of tumor stem cell
markers ALDH1 and CD133 in colorectal carcinoma. Oncol Lett.
7:507–512. 2014.PubMed/NCBI
|
|
22
|
Chen S, Song X, Chen Z, Li X, Li M, Liu H
and Li J: CD133 expression and the prognosis of colorectal cancer:
A systematic review and meta-analysis. PLoS One. 8:e563802013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang K, Xu J, Zhang J and Huang J:
Prognostic role of CD133 expression in colorectal cancer: A
meta-analysis. BMC Cancer. 12:5732012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hongo K, Kazama S, Sunami E, Tsuno NH,
Takahashi K, Nagawa H and Kitayama J: Immunohistochemical detection
of CD133 is associated with tumor regression grade after
chemoradiotherapy in rectal cancer. Med Oncol. 29:2849–2857. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kawamoto A, Tanaka K, Saigusa S, Toiyama
Y, Morimoto Y, Fujikawa H, Iwata T, Matsushita K, Yokoe T, Yasuda
H, et al: Clinical significance of radiation-induced CD133
expression in residual rectal cancer cells after chemoradiotherapy.
Exp Ther Med. 3:403–409. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Saigusa S, Tanaka K, Toiyama Y, Yokoe T,
Okugawa Y, Koike Y, Fujikawa H, Inoue Y, Miki C and Kusunoki M:
Clinical significance of CD133 and hypoxia inducible factor-1α gene
expression in rectal cancer after preoperative chemoradiotherapy.
Clin Oncol (R Coll Radiol). 23:323–332. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Maeda S, Shinchi H, Kurahara H, Mataki Y,
Maemura K, Sato M, Natsugoe S, Aikou T and Takao S: CD133
expression is correlated with lymph node metastasis and vascular
endothelial growth factor-C expression in pancreatic cancer. Br J
Cancer. 98:1389–1397. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Saigusa S, Inoue Y, Tanaka K, Toiyama Y,
Kawamura M, Okugawa Y, Okigami M, Hiro J, Uchida K, Mohri Y and
Kusunoki M: Significant correlation between LKB1 and LGR5 gene
expression and the association with poor recurrence-free survival
in rectal cancer after preoperative chemoradiotherapy. J Cancer Res
Clin Oncol. 139:131–138. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hirsch D, Barker N, McNeil N, Hu Y, Camps
J, McKinnon K, Clevers H, Ried T and Gaiser T: LGR5 positivity
defines stem-like cells in colorectal cancer. Carcinogenesis.
35:849–858. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chao C, Carmical JR, Ives KL, Wood TG,
Aronson JF, Gomez GA, Djukom CD and Hellmich MR: CD133+ colon
cancer cells are more interactive with the tumor microenvironment
than CD133-cells. Lab Invest. 92:420–436. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu YM, Li XF, Liu H and Wu XL:
Ultrasound-targeted microbubble destruction-mediated downregulation
of CD133 inhibits epithelial-mesenchymal transition, stemness and
migratory ability of liver cancer stem cells. Oncol Rep.
34:2977–2986. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu YS, Hsu HC, Tseng KC, Chen HC and Chen
SJ: Lgr5 promotes cancer stemness and confers chemoresistance
through ABCB1 in colorectal cancer. Biomed Pharmacother.
67:791–799. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Saigusa S, Tanaka K, Toiyama Y, Yokoe T,
Okugawa Y, Kawamoto A, Yasuda H, Morimoto Y, Fujikawa H, Inoue Y,
et al: Immunohistochemical features of CD133 expression:
Association with resistance to chemoradiotherapy in rectal cancer.
Oncol Rep. 24:345–350. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lim SH, Jang J, Park JO, Kim KM, Kim ST,
Park YS, Lee J and Kim HC: CD133-positive tumor cell content is a
predictor of early recurrence in colorectal cancer. J Gastrointest
Oncol. 5:447–456. 2014.PubMed/NCBI
|
|
35
|
Planutis AK, Holcombe RF, Planoutene MV
and Planoutis KS: SW480 colorectal cancer cells that naturally
express Lgr5 are more sensitive to the most common chemotherapeutic
agents than Lgr5-negative SW480 cells. Anticancer Drugs.
26:942–947. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kobayashi S, Yamada-Okabe H, Suzuki M,
Natori O, Kato A, Matsubara K, Chen Jau Y, Yamazaki M, Funahashi S,
Yoshida K, et al: LGR5-positive colon cancer stem cells
interconvert with drug-resistant LGR5-negative cells and are
capable of tumor reconstitution. Stem Cells. 30:2631–2644. 2012.
View Article : Google Scholar : PubMed/NCBI
|