Introduction
Cerebral tumors are the second most common tumor in
pediatric patients, with morbidity rate of 3.3/10,0000 individuals
in 2012 in China (1). Due to its poor
prognosis, cerebral tumors are the leading cause of mortality in
pediatric oncology (1).
Medulloblastoma is a type of neuroepithelial tumor of the
epencephalon, and is a common pediatric central nervous system
tumor, with a morbidity rate of 25% and high paroxysmal age of 8
years (2). According to the
reclassification of nervous system neoplasms by the World Health
Organization in 2002, the types of medulloblastoma are classic
medulloblastoma, pro-fibroplasia, maxicell/anaplasia and melanin.
For classic medulloblastoma, tumor cells grow vigorously with
little cytoplasm shown on histopathological examination (3). Statistics have shown that the survival
time of 70% of patients reaches 5 years, while a certain high
risk-patients have metastasis (4).
Only 25% of these patients with metastasis have a survival time of
5 years (5).
Termed Forkhead box or winged helix domain, Forkhead
box protein Ml (FOXM1) is an evolutionarily-conserved
transcriptional regulatory factor family characterized by DNA
binding domain (6). FOXM1 is a key
regulator of the cell cycle. FOXM1 is expressed during the G1 and S
phases in the cell cycle, and can regulate the transcription
activity of numerous genes, including encoding cell division cycle
(Cdc) 25A, Cdc25B, cyclin B, cyclin A, cyclin Dl, p21cip1, p27kipl,
Aurora B kinase and Polo-like kinase 1. The loss of FOXM1
expression may lead to formation defects in the mitotic spindle and
the delay of cell division, which may result in the failure of
mitosis (7). FOXM1 is also associated
with cell proliferation and apoptosis. A previous study has found
that the signaling pathway of FOXM1 has an essential role in
maintaining self-stability during cell developmental process
(8). New evidence has indicated that
FOXM1 has abnormal high expression in malignant cancers, such as
lung cancer, spongioblastoma, prostatic cancer, hepatocellular
carcinoma, primary breast carcinoma and pancreatic carcinoma
(9). When altered using RNA
interference, the expression of FOXM1 in the mammary gland and
pancreatic cancer cells triggered the proliferation, metastasis,
invasion, migration and inhibition of carcinoma cells (10).
As an important intranuclear transcription factor,
the stress protein activator protein-1 (AP-1) directly participates
in normal growth and carcinous conversion process while its
functions in cells directly depend on cell types, the constituents
of AP-1 and the relative proportion of different parts (11). The activity of AP-1 is closely
associated with tumors. It can activate tumor-associated genes and
promotes the occurrence and malignant evolution of tumors. AP-1
participates in cell proliferation, differentiation and conversion
and has a fundamental role in tumor formation, metastasis and
invasion (12).
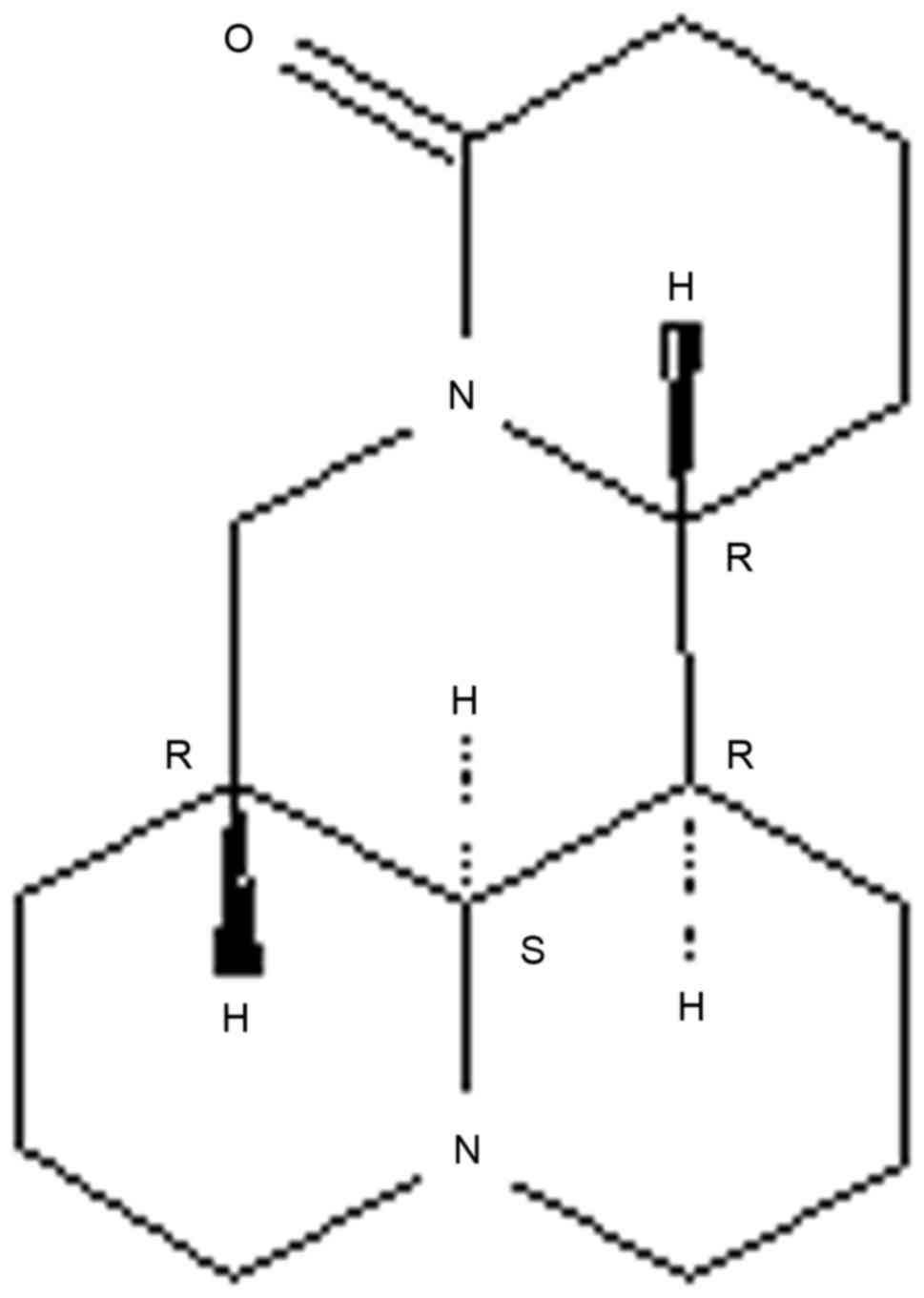
Sophoridine (Fig. 1)
is an alkaloid that occurs in high levels in Sophora
alopecuroides (13). Previous
studies have identified that sophoridine reduced inflammatory
responses caused by LPS (14,15). It has pharmacological functions such
as antitumor and anti-arrhythmia and affects the immune and central
nervous systems (12). When used at
an antitumor dosage, sophoridine can lower the heart rates of
tested animals without affecting other indicators of the
electrocardiogram or breathing (13).
Therefore, the present study assessed the effect of Sophoridine on
the suppression of cell growth in human medulloblastoma, which may
provide an important biological basis for the pathogenesis of this
disease.
Materials and methods
Cell culture
The human medulloblastoma D283-Med cell line was
obtained directly from the Central Laboratory of Tianjin Medical
University Cancer Institute and Hospital (Tianjin, China) and
maintained in DMEM-Nutrient Mixture F-12 (DMEM/F12; Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) with human
recombinant epidermal growth factor (20 ng/ml), B-27 supplement
(2%; Invitrogen; Thermo Fisher Scientific, Inc.), supplemented with
10% fetal bovine serum (Invitrogen; Thermo Fisher Scientific, Inc.)
and 1% 100 µg/ml penicillin/streptomycin solution at 37°C in a 5%
CO2 atmosphere.
Cell proliferation and cytotoxicity
analysis
D283-Med cells (5×104/ml; 200 µl) were
added to a 96-well plate and divided into groups treated with 0,
0.5, 1 and 2 mg/ml sophoridine (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) for 24, 48 and 72 h. Subsequently, 50 µl MTT (5
mg/ml; Invitrogen; Thermo Fisher Scientific, Inc.) was added to the
D283-Med cells and incubated for 4 h at 37°C in a 5% CO2
atmosphere. MTT was removed and 150 µl dimethyl sulfoxide was added
and the plate was agitated for 20 min. Lactate dehydrogenase (LDH;
60 µl; Thermo Fisher Scientific, Inc.) was added, and the plate was
incubated for 30 min at 37°C in darkness. Cell proliferation and
cytotoxicity were analyzed using a spectrophotometer (Tecan Sunrise
Rainbow; Tecan Japan Co., Ltd., Tokyo, Japan) at 490 nm.
Cell apoptosis analysis
D283-Med cells (1×108/ml; 2 ml) were
added to a 6-well plate and divided into groups treated with 0,
0.5, 1 and 2 mg/ml sophoridine for 48 h. The D283-Med cells were
incubated with 5 µl annexin V-fluorescein isothiocyanate (Caltag
Laboratories, Burlingame, CA, USA) and 5 µl of propidium iodide
(Caltag Laboratories) for 30 min at room temperature. Cells
apoptosis was analyzed using the Guava easyCyte 5HT Flow Cytometer
(Merck KGaA, Darmstadt, Germany).
Caspase-3/8 activity
D283-Med cells (1×108/ml; 2 ml) were
added to a 6-well plate and divided into groups treated with 0,
0.5, 1 and 2 mg/ml sophoridine for 48 h. D283-Med cells were lysed
on ice with 100 ml pre-cooled cell lysis buffer (Beyotime Institute
of Biotechnology, Haimen, China) for 30 min. The protein
concentration was determined by the bicinchoninic acid method
(NE-PER; Thermo Fisher Scientific, Inc.). The substrates 10 µl of
Ac-DEVD-pNA (caspase-3; 2 mM; Beyotime Institute of Biotechnology)
and 10 µl of Ac-IETD-pNA (caspase-8; 2 mM; Beyotime Institute of
Biotechnology) were added to each well for 1 h at 37°C. The
activity of caspase-3/8 was analyzed using a spectrophotometer
(Tecan Sunrise Rainbow; Tecan Japan Co., Ltd.) at 450 nm.
Western blot analysis
D283-Med cells (1×108/ml; 2 ml) were
added to a 6-well plate and divided into groups treated with 0,
0.5, 1 and 2 mg/ml sophoridine for 48 h. D283-Med cells were lysed
on ice with 100 ml pre-cooled cell lysis buffer for 30 min. The
protein concentration was determined by the bicinchoninic acid
method (NE-PER; Thermo Fisher Scientific, Inc.). Protein samples
were separated by SDS-PAGE on a 8–12% gel and then transferred onto
a polyvinylidene difluoride membrane (GE Healthcare, Little
Chalfont, UK). After blocking with 5% non-fat milk for 1 h,
membranes were incubated with primary anti-FoxM1 (cat. no. sc-502;
1:500; Santa Cruz Biotechnology, Inc., Dallas, TX, USA), anti-TrkB
(cat. no. sc-118; 1:500; Santa Cruz Biotechnology, Inc.), anti-BDNF
(cat. no. sc-20981; 1:500; Santa Cruz Biotechnology, Inc.),
anti-NF-κB (cat. no. sc-7178; 1:500; Santa Cruz Biotechnology,
Inc.), anti-AP-1 (cat. no. ab21981; 1:1,000; Abcam, Cambridge, MA,
USA) and anti-β-actin (cat. no. sc-7210; 1:500; Santa Cruz
Biotechnology, Inc.) antibodies at 4°C overnight. After washing in
Tris-buffered saline with Tween-20 (20 mM Tris-HCl, 150 mM NaCl and
0.05% Tween-20), the membranes were incubated with goat anti-rabbit
IgG-horseradish peroxidase secondary antibodies (cat. no. sc-2004;
1:5,000; Santa Cruz Biotechnology, Inc.) at 37°C for 2 h.
Subsequent to this incubation, the membranes were visualized using
a BeyoECL Plus (Beyotime Institute of Biotechnology).
Statistical analysis
The experimental data were presented as the mean ±
standard deviation and analyzed with SPSS 13.0 software (SPSS,
Inc., Chicago, IL, USA). Statistical significance was determined by
one-way analysis of variance followed by Tukey post hoc analysis.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Sophoridine suppresses cell growth of
human medulloblastoma
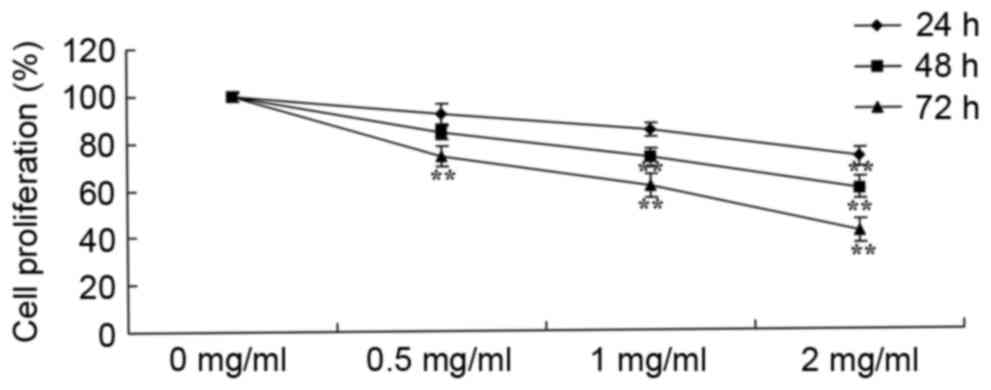
To determine the effect of sophoridine on the growth
of D283-Med cells, cell proliferation was measured using an MTT
assay. As shown in Fig. 2, the
proliferation of D283-Med cells was suppressed by treatment with
sophoridine in a dose- and time-dependent manner. In particular, 2
mg/ml sophoridine significantly suppressed cell proliferation
subsequent to treatment for 24, 48 and 72 h, 1 mg/ml sophoridine
significantly suppressed cell proliferation subsequent to treatment
for 72 h, and 0.5 mg/ml sophoridine significantly suppressed cell
proliferation subsequent to treatment for 72 h in D283-Med
cell.
Sophoridine is cytotoxic in human
medulloblastoma
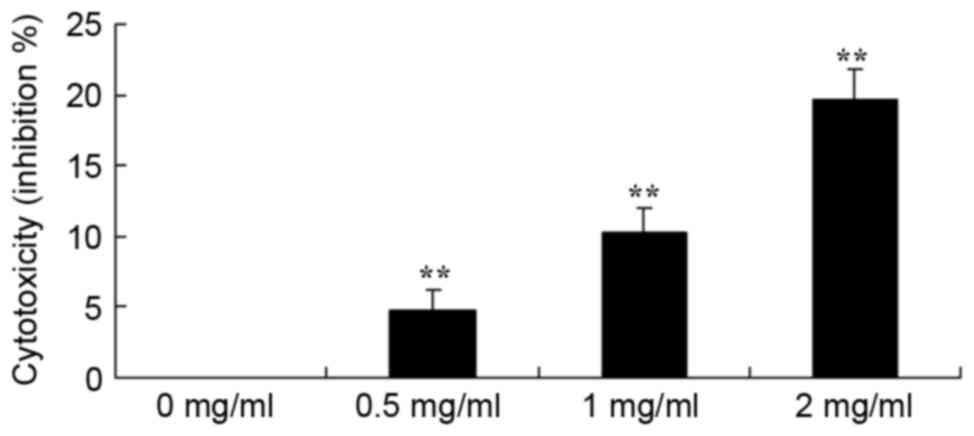
To study the effect of sophoridine on cytotoxicity
of human medulloblastoma, a LDH assay was used to analyze the
cytotoxicity of sophoridine on D283-Med cells. The results showed
that treatment with 1 or 2 mg/ml sophoridine significantly induced
cytotoxicity of D283-Med cells (Fig.
3).
Sophoridine induces apoptosis in human
medulloblastoma
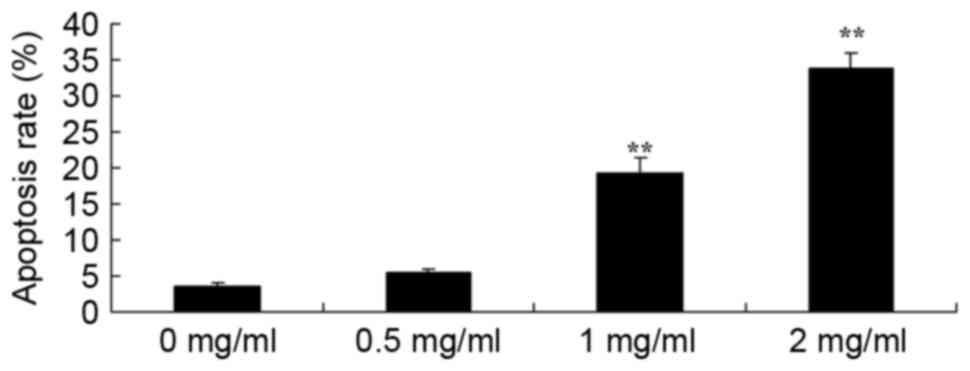
To further study the effect of sophoridine on the
apoptosis of human medulloblastoma cells, apoptosis rates were
detected using flow cytometry. Fig. 4
showed that treatment with 1 or 2 mg/ml sophoridine significantly
induced apoptosis in D283-Med cells, compared with control cells (0
mg/ml sophoridine).
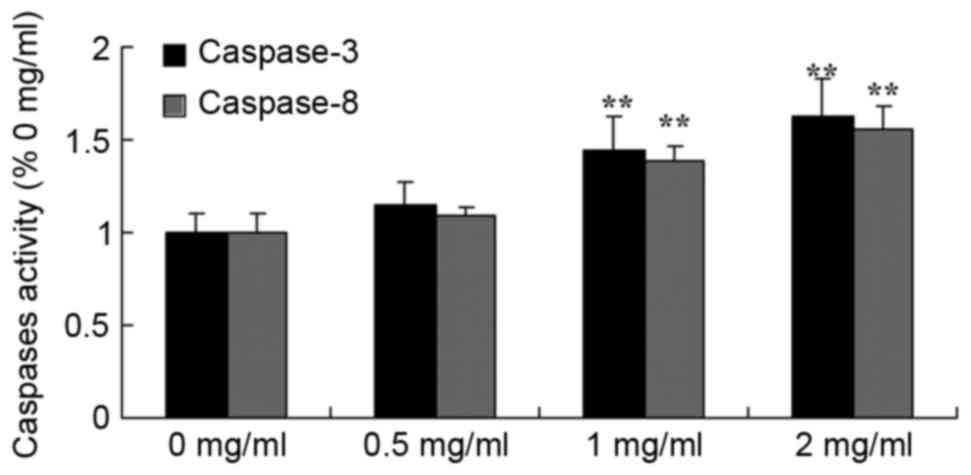
Sophoridine induces caspase-3/8
activity in human medulloblastoma
A Business kit was used to evaluate the effect of
sophoridine on caspase-3/8 activity of human medulloblastoma in
vitro subsequent to treatment for 48 h. The representative
results are shown in Fig. 5.
Sophoridine (1 or 2 mg/ml) significantly induced caspase-3/8
activity in D283-Med cells, compared with control cells (0 mg/ml
sophoridine).
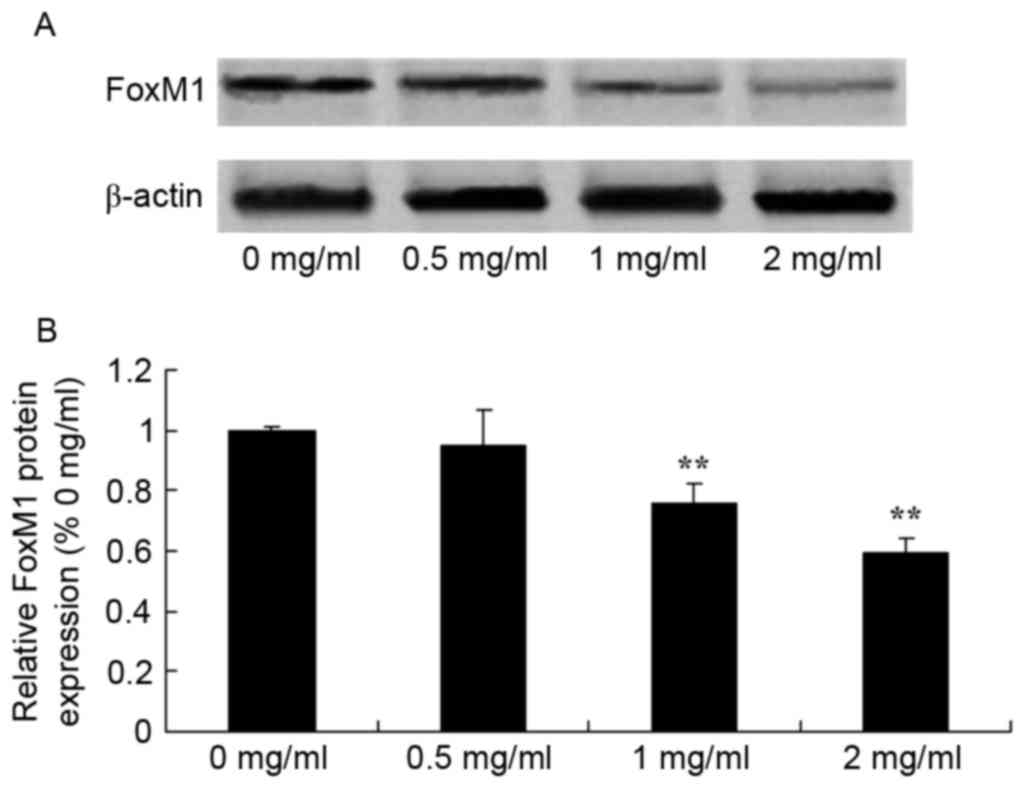
Sophoridine suppresses FoxM1 protein
expression in human medulloblastoma
To evaluate the effect of sophoridine on FoxM1
signal in human medulloblastoma, FoxM1 protein expression was
recorded using western blot analysis. Sophoridine concentrations of
1 and 2 mg/ml concentrations were found to significantly suppress
FoxM1 protein expression in D283-Med cells, compared with control
cells (0 mg/ml sophoridine; Fig.
6).
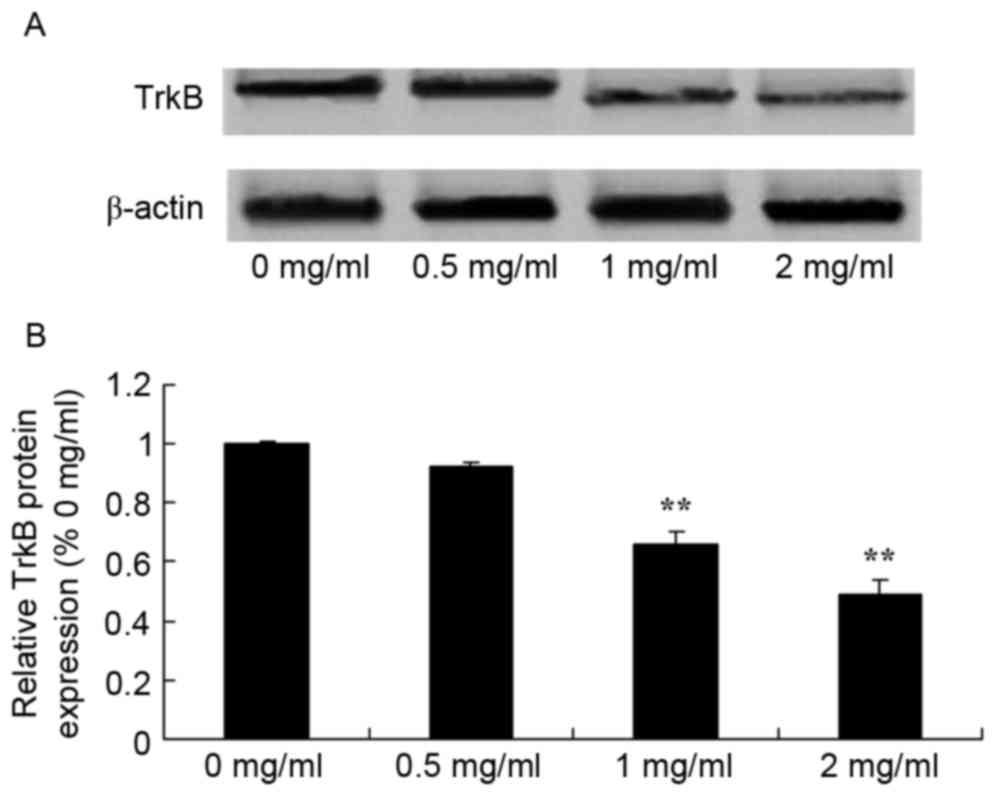
Sophoridine suppresses TrkB protein
expression in human medulloblastoma
To further evaluate the effect of sophoridine on
TrkB signaling in human medulloblastoma, western blot analysis was
used to detect TrkB protein expression in D283-Med cells. TrkB
protein expression of D283-Med cells was significantly inhibited by
1 and 2 mg/ml of sophoridine, compared with control cells (0 mg/ml
sophoridine; Fig. 7).
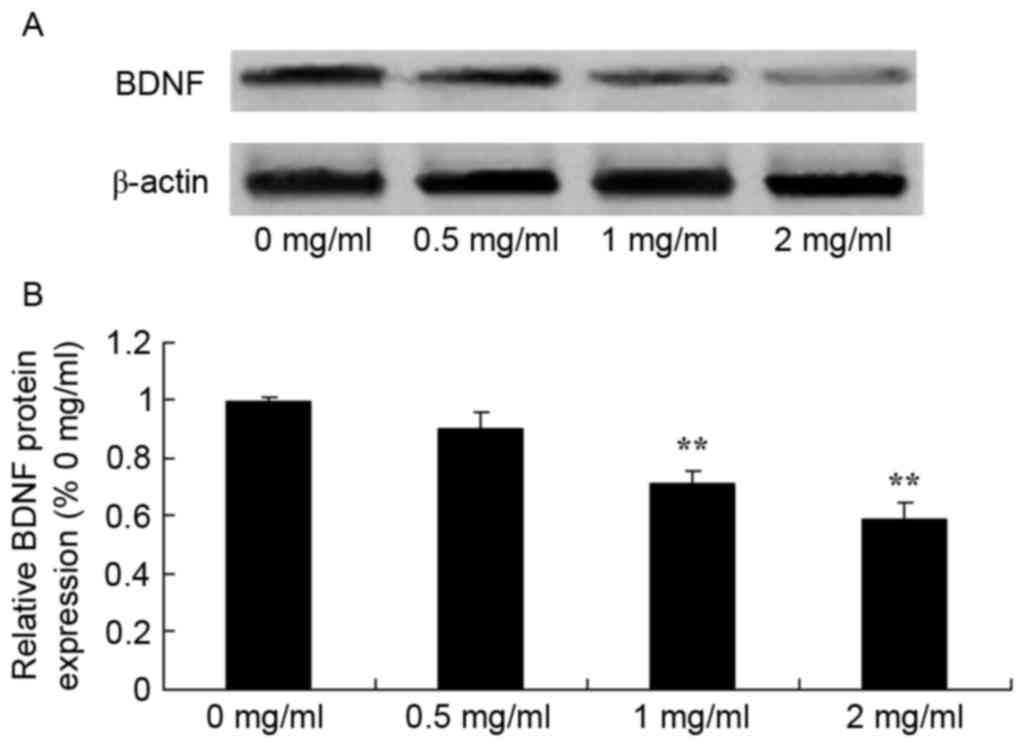
Sophoridine suppresses BDNF protein
expression in human medulloblastoma
To study the effect of sophoridine on BDNF signaling
in human medulloblastoma, BDNF protein expression in D283-Med cells
was measured using western blot analysis. The results showed that
treatment with 1 and 2 mg/ml sophoridine significantly reduced BDNF
protein expression in D283-Med cells, compared with control cells
(0 mg/ml sophoridine; Fig. 8).
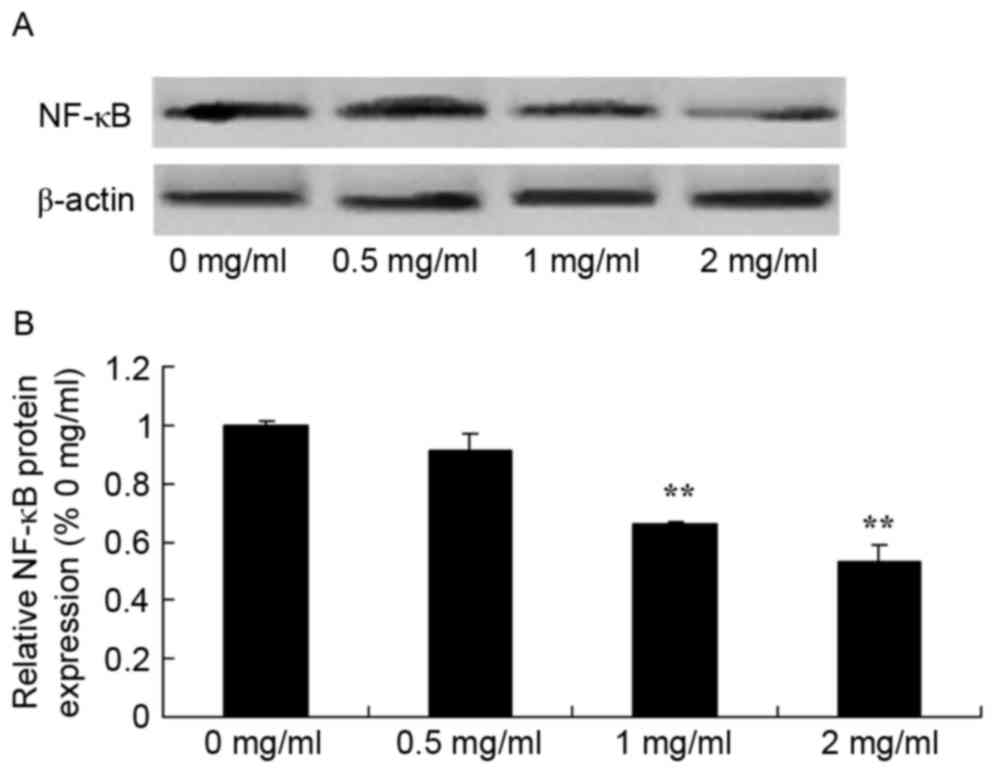
Sophoridine suppresses NF-κB protein
expression in human medulloblastoma
To further study the mechanism of sophoridine on
cell growth of human medulloblastoma, changes in NF-κB protein
expression were examined using western blot analysis. The present
study also found that NF-κB protein expression in D283-Med cells
was significantly suppressed by treatment with 1 and 2 mg/ml
sophoridine, compared with control cells (0 mg/ml sophoridine;
Fig. 9).
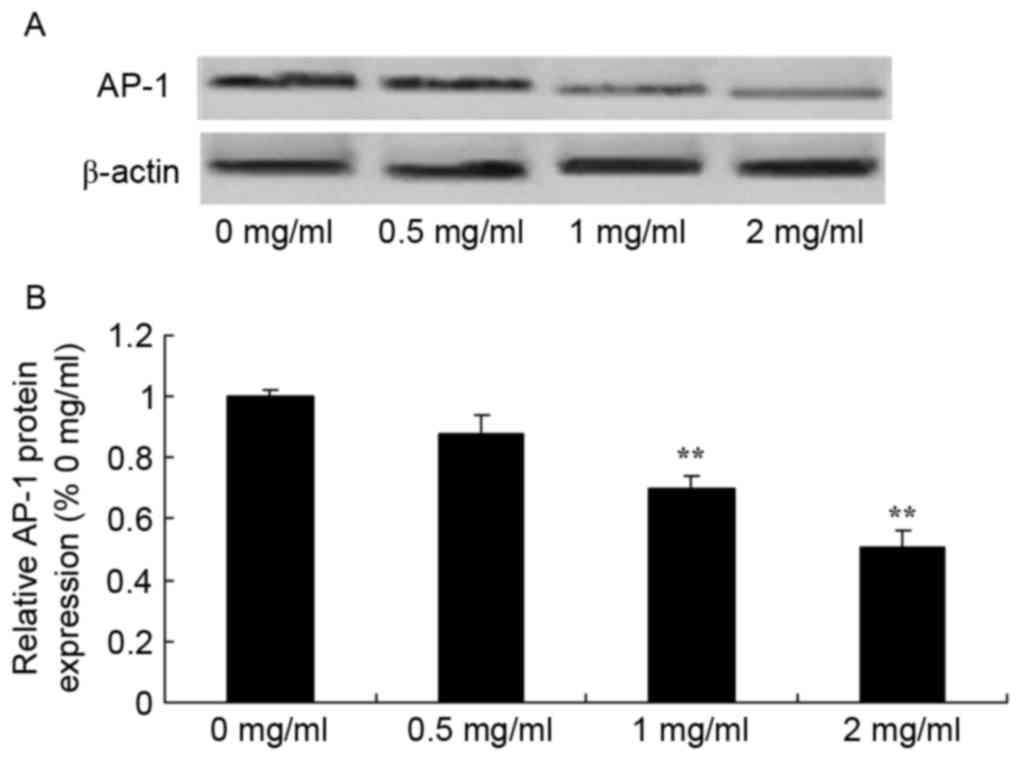
Sophoridine suppresses AP-1 protein
expression in human medulloblastoma
Since one of the main mechanisms of sophoridine is
its effect on AP-1 signaling in human medulloblastoma, the present
study evaluated whether sophoridine affects AP-1 protein expression
in D283-Med cells. As shown in Fig.
10, 1 and 2 mg/ml sophoridine significantly inhibited AP-1
protein expression in human D283-Med cells, compared with control
cells (0 mg/ml sophoridine; Fig.
10).
Discussion
Medulloblastoma is an epithelial tumor of cerebellar
neurocytes and is the most common central nervous system tumor in
children (16). The morbidity of
medulloblastoma accounts for 25% of pediatric brain tumors
(17). The most common age is 8
years, and it mostly appears in patients >20 years old.
According to statistics, the 5-year survival rate of patients is
70%, but certain high-risk patients (<3 years old) often present
with metastasis, and the 5-year survival rate is 25% in 2012 in
China (18). The present study showed
that sophoridine suppresses cell growth, induces cytotoxicity and
apoptosis and increased caspase-3/8 activity of human
medulloblastoma. Wang et al (19) reported that sophoridine induces
mitochondrial apoptosis through suppression of β-catenin/survivin
signaling in cisplatin-resistant non-small cell lung cancer
cells.
FOXM1 is a key regulator of the cell cycle and
expressed during the G1, S and mitotic phases (9). A previous study suggested that FOXM1
could significantly downregulate the expression of P2cipi and
P27kipi (7). During the transitional
phase between G2 and M, two downstream target genes of FOXM1 were
found to promote NF-κB to participate in the progression of
neurodegenerative diseases, such as Alzheimer's disease and
Parkinson's disease (20). In the
present study, it was found that sophoridine significantly
suppressed FOXM1 protein expression in D283-Med cells. Wang et
al (13) reported that
sophoridine suppresses cell proliferation of human glioma U87MG
cell line by upregulating the expression of caspase-3/8, NF-κB and
AP-1.
The nuclear transcription factor NF-κB is contains
κBα. In normal cells, NF-κB is located in the cytoplasm (21). Stimulated by extracellular functions,
κBα can be degraded, which results in the activation of p65. The
activation of NF-κB induces the expression of certain genes,
including inflammatory factors, proliferation or pro-apoptosis
factors (22). In the present study,
it was also found that sophoridine significantly decreases the
protein expression of NF-κB in D283-Med cells. Li et al
(23) reported that sophoridine
inhibits cell proliferation through regulation of the NF-κB
signaling pathway in castration-resistant prostate cancer
cells.
As target proteins of the TrkB/BDNF signaling
pathway, NF-κB and FKHR are associated with FOXM1 (24). The functions of these proteins are
associated with the survival, proliferation, invasion and
metastasis (25). The interaction of
FOXM1 with the TrkB/BDNF signaling pathway affects the biological
behaviors of multiple myeloma cells. Previous studies have
indicated that there is abnormal expression of TrkB/BDNF and FOXM1
in medulloblastoma, and the expression levels of these proteins are
positively associated (26). The
interaction between FOXM1 and TrkB/BDNF affects the apoptosis, cell
cycle progression and proliferation of multiple myeloma cells
(27). The present results showed
that sophoridine suppressed TrkB and BDNF protein expression in
D283-Med cells. Kan et al (28) reported that sophoridine protects
neuro-axon from inflammation-induced injury through BDNF.
The AP-1 compound exerts its function by combining
with genetic promoters (29). AP-1 is
essential to the biological function of oncogenes, particularly
participates in metastasis mediated by oncogenes. By importing
TAM67 to inhibit the activity of AP-1, the invasiveness of squamous
cell carcinoma in rats and humans can be markedly reduced (30). The results of the present study
suggested that sophoridine suppressed the protein expression of
NF-κB and AP-1 in D283-Med cell. Wang et al (13) reported that sophoridine suppresses
cell proliferation of human glioma U87MG cell line through
upregulating the expression of caspase-3/8, NF-κB and AP-1.
To conclude, the present findings demonstrated that
sophoridine suppresses cell growth, induces cytotoxicity and
apoptosis, and increases caspase-3/8 activity in human
medulloblastoma. In addition, it was concluded that the FOXM1,
NF-κB and AP-1 pathway has an important function in intracellular
signaling in response to the anticancer effect of sophoridine in
human medulloblastoma cells.
References
|
1
|
Robinson GW, Orr BA, Wu G, Gururangan S,
Lin T, Qaddoumi I, Packer RJ, Goldman S, Prados MD, Desjardins A,
et al: Vismodegib exerts targeted efficacy against recurrent sonic
hedgehog-subgroup medulloblastoma: Results from phase ii pediatric
brain tumor consortium studies PBTC-025B and PBTC-032. J Clin
Oncol. 33:2646–2654. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Deutsch M, Thomas PR, Krischer J, Boyett
JM, Albright L, Aronin P, Langston J, Allen JC, Packer RJ, Linggood
R, et al: Results of a prospective randomized trial comparing
standard dose neuraxis irradiation (3,600 cGy/20) with reduced
neuraxis irradiation (2,340 cGy/13) in patients with low-stage
medulloblastoma. A combined children's cancer group-pediatric
oncology group study. Pediatr Neurosurg. 24:167–177. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Allen J, Donahue B, Mehta M, Miller DC,
Rorke LB, Jakacki R, Robertson P, Sposto R, Holmes E, Vezina G, et
al: A phase II study of preradiotherapy chemotherapy followed by
hyperfractionated radiotherapy for newly diagnosed high-risk
medulloblastoma/primitive neuroectodermal tumor: A report from the
children's oncology group (CCG 9931). Int J Radiat Oncol Biol Phys.
74:1006–1011. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cefalo G, Massimino M, Ruggiero A, Barone
G, Ridola V, Spreafico F, Potepan P, Abate ME, Mascarin M, Garrè
ML, et al: Temozolomide is an active agent in children with
recurrent medulloblastoma/primitive neuroectodermal tumor: An
Italian multi-institutional phase II trial. Neuro Oncol.
16:748–753. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dhall G, Grodman H, Ji L, Gardner S,
Dunkel IJ, McCowage GB, Diez B, Allen JC, Gopalan A, Cornelius AS,
et al: Outcome of children less than three years old at diagnosis
with non-metastatic medulloblastoma treated with chemotherapy on
the ‘Head Start’ I and II protocols. Pediatr Blood Cancer.
50:1169–1175. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ito T, Kohashi K, Yamada Y, Maekawa A,
Kuda M, Furue M and Oda Y: Prognostic significance of Forkhead box
M1 (FOXM1) expression and antitumor effect of FOXM1 inhibition in
melanoma. Histopathology. 69:63–71. 2015. View Article : Google Scholar
|
|
7
|
Dai J, Yang L, Wang J, Xiao Y and Ruan Q:
Prognostic value of FOXM1 in patients with malignant solid tumor: A
meta-analysis and system review. Dis Markers. 2015:3524782015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhao F and Lam EW: Role of the forkhead
transcription factor FOXO-FOXM1 axis in cancer and drug resistance.
Front Med. 6:376–380. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Quan M, Wang P, Cui J, Gao Y and Xie K:
The roles of FOXM1 in pancreatic stem cells and carcinogenesis. Mol
Cancer. 12:1592013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shi M, Cui J and Xie K: Signaling of
miRNAs-FOXM1 in cancer and potential targeted therapy. Curr Drug
Targets. 14:1192–1202. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chambers M, Kirkpatrick G, Evans M, Gorski
G, Foster S and Borghaei RC: IL-4 inhibition of IL-1 induced Matrix
metalloproteinase-3 (MMP-3) expression in human fibroblasts
involves decreased AP-1 activation via negative crosstalk involving
of Jun N-terminal kinase (JNK). Exp Cell Res. 319:1398–1408. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Uluckan O, Guinea-Viniegra J, Jimenez M
and Wagner EF: Signalling in inflammatory skin disease by AP-1
(Fos/Jun). Clin Exp Rheumatol. 33 4 Suppl 92:S44–S49.
2015.PubMed/NCBI
|
|
13
|
Wang WX, Sun ZH, Chen HM, Xu BN and Wang
FY: Role and mechanism of Sophoridine on proliferation inhibition
in human glioma U87MG cell line. Int J Clin Exp Med. 8:464–471.
2015.PubMed/NCBI
|
|
14
|
Zhang B, Liu ZY, Li YY, Luo Y, Liu ML,
Dong HY, Wang YX, Liu Y, Zhao PT, Jin FG and Li ZC:
Antiinflammatory effects of matrine in LPS-induced acute lung
injury in mice. Eur J Pharm Sci. 44:573–579. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liou CJ, Lai YR, Chen YL, Chang YH, Li ZY
and Huang WC: Matrine attenuates COX-2 and ICAM-1 Expressions in
human lung epithelial cells and prevents acute lung injury in
LPS-induced mice. Mediators Inflamm. 2016:36304852016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gessi M, von Bueren AO, Rutkowski S and
Pietsch T: p53 expression predicts dismal outcome for
medulloblastoma patients with metastatic disease. J Neurooncol.
106:135–141. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Camara-Costa H, Resch A, Kieffer V,
Lalande C, Poggi G, Kennedy C, Bull K, Calaminus G, Grill J, Doz F,
et al: Neuropsychological outcome of children treated for standard
risk Medulloblastoma in the PNET4 european randomized controlled
trial of hyperfractionated versus standard radiation therapy and
maintenance chemotherapy. Int J Radiat Oncol Biol Phys. 92:978–985.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Palmer SL, Leigh L, Ellison SC,
Onar-Thomas A, Wu S, Qaddoumi I, Armstrong GT, Wright K, Wetmore C,
Broniscer A and Gajjar A: Feasibility and efficacy of a
computer-based intervention aimed at preventing reading decoding
deficits among children undergoing active treatment for
medulloblastoma: Results of a randomized trial. J Pediatr Psychol.
39:450–458. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang HQ, Jin JJ and Wang J: Matrine
induces mitochondrial apoptosis in cisplatin-resistant non-small
cell lung cancer cells via suppression of β-catenin/survivin
signaling. Oncol Rep. 33:2561–2566. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Arora R, Yates C, Gary BD, McClellan S,
Tan M, Xi Y, Reed E, Piazza GA, Owen LB and Dean-Colomb W:
Panepoxydone targets NF-kB and FOXM1 to inhibit proliferation,
induce apoptosis and reverse epithelial to mesenchymal transition
in breast cancer. PLoS One. 9:e983702014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Arslan S, Korkmaz Ö, Özbilüm N and Berkan
Ö: Association between NF-κBI and NF-κB BIA polymorphisms and
coronary artery disease. Biomed Rep. 3:736–740. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Qian Y, Guan T, Huang M, Cao L, Li Y,
Cheng H, Jin H and Yu D: Neuroprotection by the soy isoflavone,
genistein, via inhibition of mitochondria-dependent apoptosis
pathways and reactive oxygen induced-NF-κB activation in a cerebral
ischemia mouse model. Neurochem Int. 60:759–767. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li Q, Lai Y, Wang C, Xu G, He Z, Shang X,
Sun Y, Zhang F, Liu L and Huang H: Matrine inhibits the
proliferation, invasion and migration of castration-resistant
prostate cancer cells through regulation of the NF-κB signaling
pathway. Oncol Rep. 35:375–381. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kasemeier-Kulesa JC, Morrison JA, Lefcort
F and Kulesa PM: TrkB/BDNF signalling patterns the sympathetic
nervous system. Nat Commun. 6:82812015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ghiglieri V, Sgobio C, Patassini S,
Bagetta V, Fejtova A, Giampà C, Marinucci S, Heyden A, Gundelfinger
ED, Fusco FR, et al: TrkB/BDNF-dependent striatal plasticity and
behavior in a genetic model of epilepsy: Modulation by valproic
acid. Neuropsychopharmacology. 35:1531–1540. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Turner BA, Sparrow J, Cai B, Monroe J,
Mikawa T and Hempstead BL: TrkB/BDNF signaling regulates
photoreceptor progenitor cell fate decisions. Dev Biol.
299:455–465. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Odate S, Nakamura K, Onishi H, Kojima M,
Uchiyama A, Nakano K, Kato M, Tanaka M and Katano M: TrkB/BDNF
signaling pathway is a potential therapeutic target for pulmonary
large cell neuroendocrine carcinoma. Lung Cancer. 79:205–214. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kan QC, Lv P, Zhang XJ, Xu YM, Zhang GX
and Zhu L: Matrine protects neuro-axon from CNS
inflammation-induced injury. Exp Mol Pathol. 98:124–130. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xu L, Ning H, Gu L, Wang Q, Lu W, Peng H,
Cui W, Ying B, Ross CR, Wilson GM, et al: Tristetraprolin induces
cell cycle arrest in breast tumor cells through targeting
AP-1/c-Jun and NF-κB pathway. Oncotarget. 6:41679–41691. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kuang H, Hua X, Zhou J and Yang R:
Resolvin D1 and E1 alleviate the progress of hepatitis toward liver
cancer in long-term concanavalin A-induced mice through inhibition
of NF-κB activity. Oncol Rep. 35:307–317. 2016. View Article : Google Scholar : PubMed/NCBI
|