Introduction
Breast cancer is a severe disease that presents a
great threat to female health worldwide (1). Breast cancer-associated morbidity
increases each year and ~1.2 million individuals are diagnosed with
breast cancer annually (2). Although
surgery, radiotherapy, chemotherapy and endocrine therapy are
widely applied in clinics, long-term survival rates have not
significantly improved (3). Each year
~500,000 individuals succumb to the disease; the leading cause of
breast cancer-associated mortality is tumor metastasis, which
remains a challenge for the prophylaxis and treatment of breast
cancer (4).
Autophagy is an important process that aids with the
turnover of intracellular proteins. During autophagy, proteins or
organelles are encased by double-membrane structures, which
eventually fuse with the lysosome in order for protein degradation
to take place (2). Major functions of
autophagy include cell clearance, and the degradation of impaired
organelles and excessive biomacromolecules (5). The products of degradation can be used
to provide energy and reconstruct cellular structures in order to
maintain metabolic balance and homeostasis. A previous study has
reported important functions for autophagy in the metastasis of
breast cancer (6). Certain molecules
and anti-cancer drugs have been reported to influence the
proliferation and metastasis of breast cancer by regulating
autophagy (6). For instance, B-cell
lymphoma-2 (Bcl-2) can promote the proliferation of MCF-7 cells, an
effect that is associated with the inhibition of autophagy
(7). In addition, a previous study
demonstrated that inhibition of the phosphoinositide 3-kinase
(PI3K)/RAC-α serine-threonine-protein kinase (Akt)/mammalian target
of rapamycin (mTOR) signaling pathway inhibits MDA-MB-231 cell
proliferation, and that the PI3K/Akt/mTOR cascade is a key
signaling pathway that regulates autophagy (8).
Licochalcone A is a chalconoid (a type of natural
phenol) that can be isolated from Glycyrrhiza glabra
(licorice) and Glycyrrhiza inflata (Chinese licorice)
(9), although the quantity of
Licochalcone A that can be extracted from licorice is the highest.
Previous research has demonstrated that Licochalcone A possesses
antimalarial and antitumor effects, in addition to antioxidant,
anti-inflammatory, anti-bacterial, anti-leishmaniasis and
estrogenic effects (10,11). Additionally, Licochalcone A has wide
applications in the food and medical industry (10,11).
Materials and methods
Reagents and chemicals
Dulbecco's modified Eagle's medium (DMEM) and fetal
bovine serum (FBS) were obtained from Gibco (Thermo Fisher
Scientific, Inc., Waltham, MA, USA). MTT was obtained from
Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). The Annexin
V-fluorescein isothiocyanate (FITC) and propidium iodide (PI) kit
was purchased from BD Biosciences (San Jose, CA, USA). The chemical
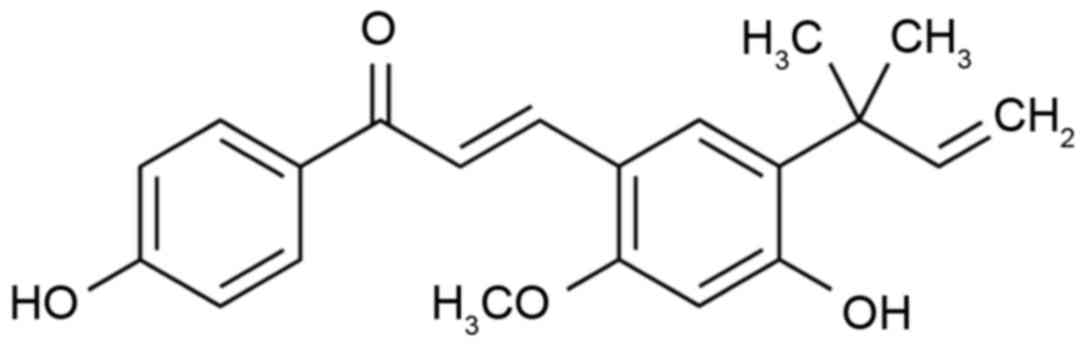
structure of Licochalcone A (purchased from Sigma-Aldrich; Merck
KGaA) is illustrated in Fig. 1.
Cell culture
The human MCF-7 cell line was obtained from the Cell
Bank of Type Culture Collection of Chinese Academy of Sciences
(Shanghai, China) and cultured in DMEM supplemented with 10% FBS at
37°C with 5% CO2.
Cell viability assay
The effect of Licochalcone A on cell viability was
determined using an MTT assay and untreated cells were used as a
comparison. A total of 8,000–10,000 MCF-7 cells/well were seeded
into 96-well plates and cultured with 20 µl MTT for 4 h at 37°C.
Following the removal of culture medium, 150 µl dimethyl sulfoxide
was added to each well to dissolve the formazan crystals. The
results were assessed by measuring the absorbance at 495 nm.
Annexin V-FITC/PI staining
The effect of Licochalcone A on the apoptosis rate
of MCF-7 cells was determined using an Annexin V-FITC/PI kit (BD
Biosciences). A total of 1–2×106 MCF-7 cells/well were
seeded in 6-well plates and cultured with 100 µl Annexin V-FITC at
4°C in the dark for 30 min. Then, 10 µl PI was added to each well
and incubated in the dark for 5 min at 37°C.
Acridine orange (AO) staining of
autophagic cells
A total of 1–2×106 MCF-7 cells/well were
seeded in 6-well plates and washed twice with ice-cold PBS. Then,
MCF-7 cells were incubated with 1 µg/ml AO (Sigma-Aldrich; Merck
KGaA) for 30 min at 37°C. MCF-7 cells were observed with
fluorescence microscopy using 490-nm band-pass blue excitation
filters and a 515-nm long-pass barrier filter.
Western blot analysis
A total of 1–2×106 MCF-7 cells/well were
seeded in 6-well plates and washed twice with ice-cold PBS. Then,
MCF-7 cells were harvested at 2,000 × g for 10 min at 4°C and
gently lysed for 1 h in ice-cold cell lysis buffer (Beijing Dingguo
Biotechnology, Co., Ltd., Beijing, China). Supernatants were
collected following centrifugation at 12,000 × g for 10 min at 4°C.
Protein concentrations were measured using a BCA assay. The samples
(50 µg protein) were loaded onto a 10–12% SDS-PAGE gel and then
transferred to a polyvinylidene difluoride (PVDF) membrane. The
PVDF membrane was blocked with PBS containing 5% non-fat milk and
0.1% Tween-20 for 1 h at 37°C. Then, the PVDF membrane was
incubated with anti-PI3K (cat. no. 4249; dilution, 1:2,000),
anti-Akt (cat. no. 4691; dilution, 1:2,000), anti-phosphorylated
(p)-Akt (cat. no. 4060; dilution, 1:2,000), anti-p-mTOR (cat. no.
5536; dilution, 1:2,000), anti-mTOR (cat. no. 2983; dilution,
1:2,000), anti-GFP-microtubule-associated proteins 1A/1B light
chain 3 (LC3-II), anti-LC3-II, anti-Bcl-2 (cat. no. 3498; dilution,
1:2,000) and anti-β-actin (cat. no. 4970; dilution, 1:5,000)
antibodies (all from Cell Signaling Technology, Inc., Danvers, MA,
USA) at a dilution of 1:1,000 overnight at 4°C. Following washing
with TBST for 20 min, the membrane was incubated with a horseradish
peroxidase-conjugated anti-mouse antibody (cat. no. 14708;
dilution, 1:10,000; Cell Signaling Technology, Inc.) at room
temperature for 2 h. Protein blank was visualized using BeyoECL
Plus (Beyotime Institute of Biotechnology, Haimen, China).
Capase-3 activity assay
A total of 1–2×106 MCF-7 cells/well were
seeded in 6-well plates and washed twice with ice-cold PBS. Then,
cells were incubated with caspase-3 activity kits (C1115; Beyotime
Institute of Biotechnology) according to the manufacturer's
protocol for 2 h at room temperature. The caspase-3 activity was
detected at 405 nm using a Sunrise absorbance reader (Tecan Group,
Ltd., Männedorf, Switzerland).
Statistical analysis
Data was calculated by mean ± standard deviation.
All statistical analyses were performed with SPSS software (version
17.0; SPSS, Inc., Chicago, IL, USA) using an unpaired Student's
t-test. Experiments were repeated three times. P<0.05 was
considered to indicate a statistically significant difference.
Results
Licochalcone A decreases the viability
of MCF-7 cells
Prior to investigating the anticancer effects of
Licochalcone A on a culture of MCF-7 cells, the effect of
Licochalcone A on cell viability was measured using an MTT assay.
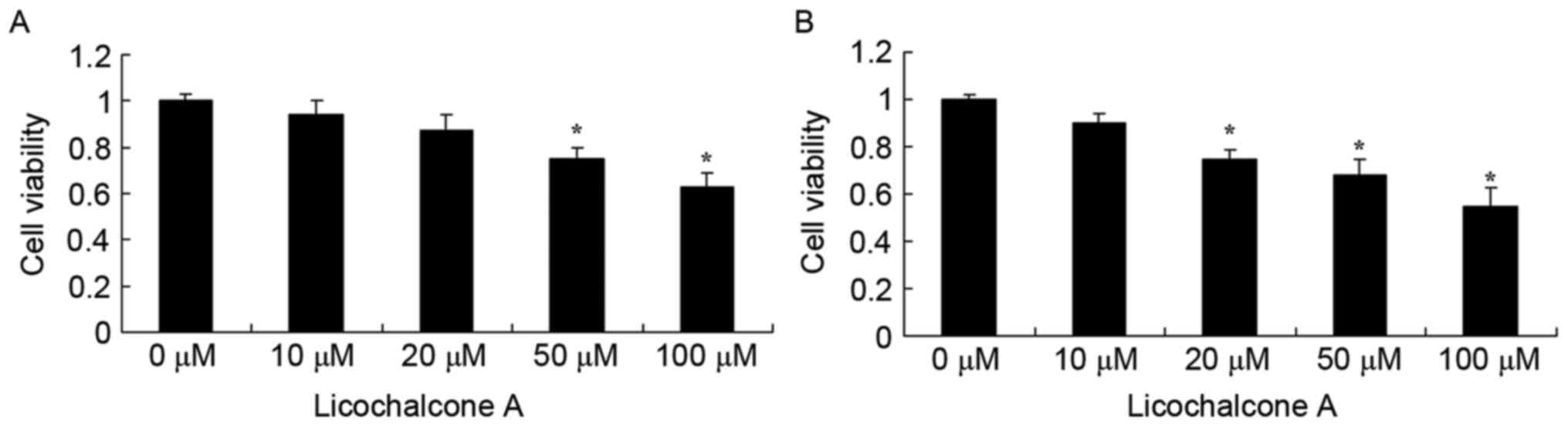
Licochalcone A treatment decreased MCF-7 cell viability in a dose-
and time-dependent manner at 24 and 48 h (Fig. 2A and B, respectively). At 24 h, 50 or
100 µM Licochalcone A significantly decreased cell viability
compared with the 0 µM control group (P<0.05; Fig. 2A). At 48 h, 20, 50 or 100 µM
Licochalcone A significantly decreased the cell viability of MCF-7
cells compared with the 0 µM control group (P<0.01; Fig. 2B). Subsequently, 10, 20 and 50 µM
Licochalcone A treatment for 48 h was selected to evaluate the
underlying molecular mechanisms of Licochalcone A on breast cancer
cells.
Licochalcone A inhibits Akt and mTOR
signaling in MCF-7 cells
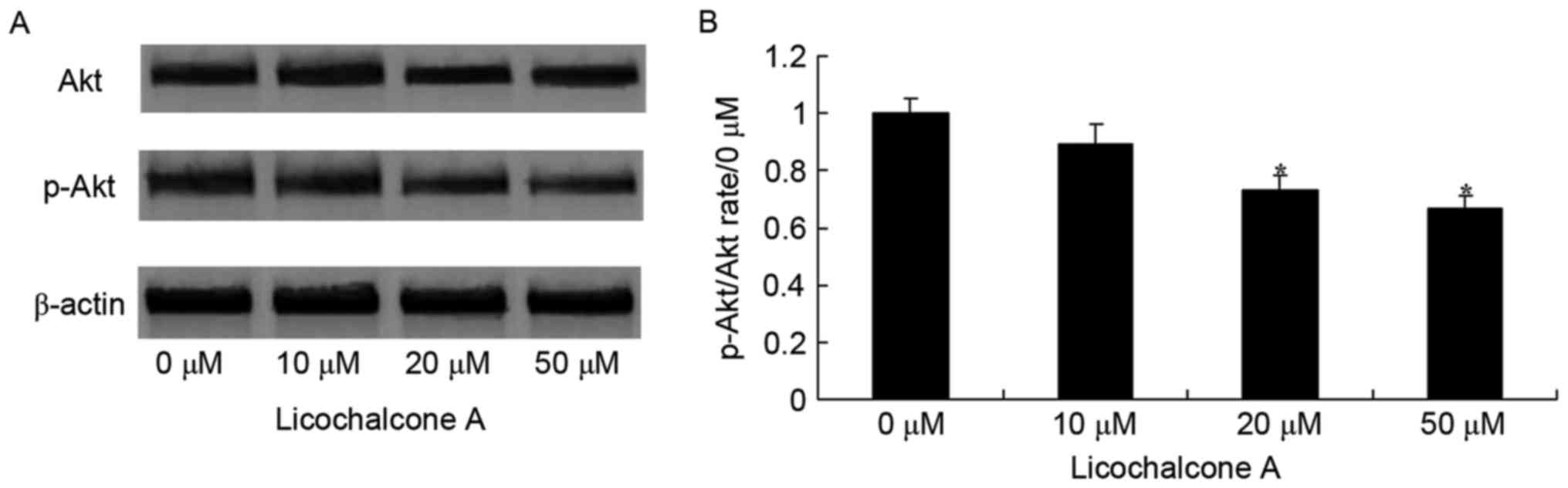
To determine whether the anticancer effect of
Licochalcone A on MCF-7 cells was mediated by the Akt/mTOR
signaling pathway, p-Akt and Akt expression was detected in MCF-7
cells incubated with various concentrations of Licochalcone A. The
expression of p-Akt/Akt and p-mTOR/mTOR was significantly reduced
in MCF-7 cells following treatment with 20 and 50 µM Licochalcone
A, compared with the 0 µM control group (P<0.01; Figs. 3 and 4).
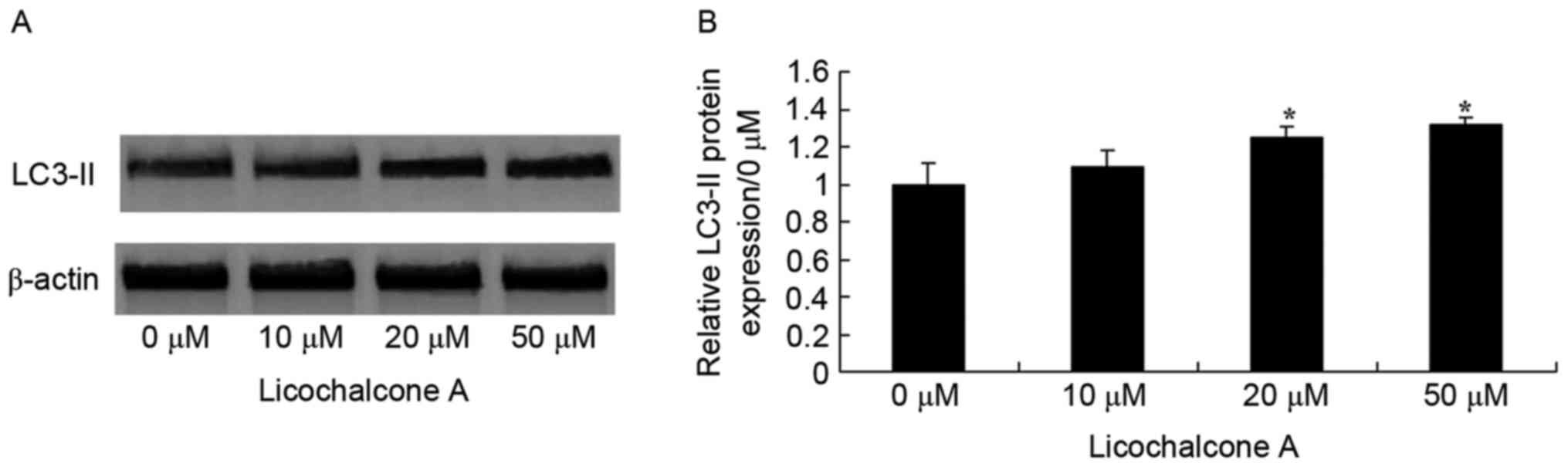
Licochalcone A promotes autophagy of
MCF-7 cells
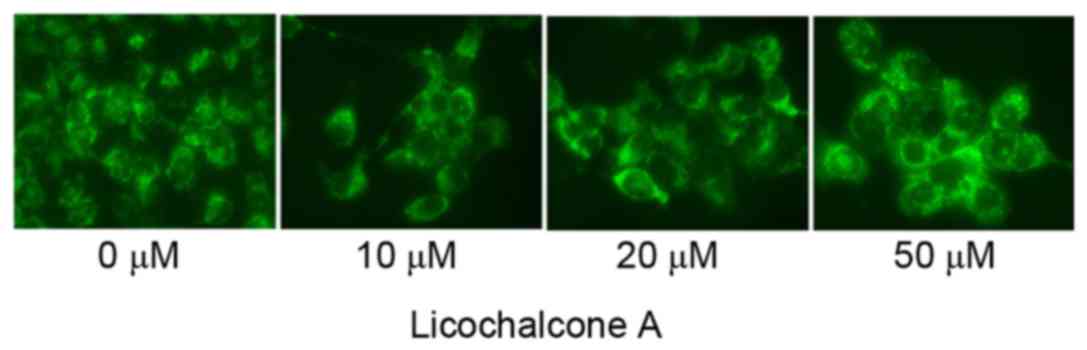
To determine the association between the anticancer
effects of Licochalcone A and autophagy following treatment of
MCF-7 cells with Licochalcone A, AO staining was performed.
Fluorescence microscopy indicated that 20 or 50 µM Licochalcone A
induces autophagy of MCF-7 cells (Fig.
5). In addition, western blot analysis demonstrated that the
expression of LC3-II, a protein recruited to autophagosomal
membranes, was significantly increased in MCF-7 cells treated with
20 or 50 µM Licochalcone A, as compared with in the 0 µM control
group (P<0.01; Fig. 6).
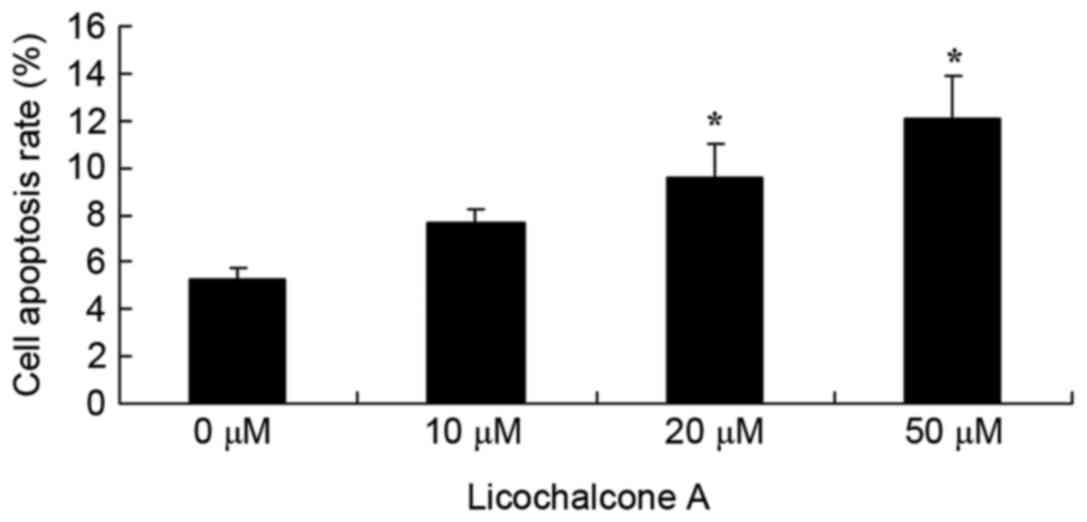
Licochalcone A promotes apoptosis of
MCF-7 cells
To elucidate the effects of Licochalcone A on the
apoptosis rate of MCF-7 cells, an Annexin V-FITC/PI staining assay
was performed on MCF-7 cells treated with various concentrations of
Licochalcone A. Concentrations of 20 or 50 µM Licochalcone A
significantly increased the apoptosis rate of MCF-7 cells compared
with the 0 µM control group (P<0.01; Fig. 7).
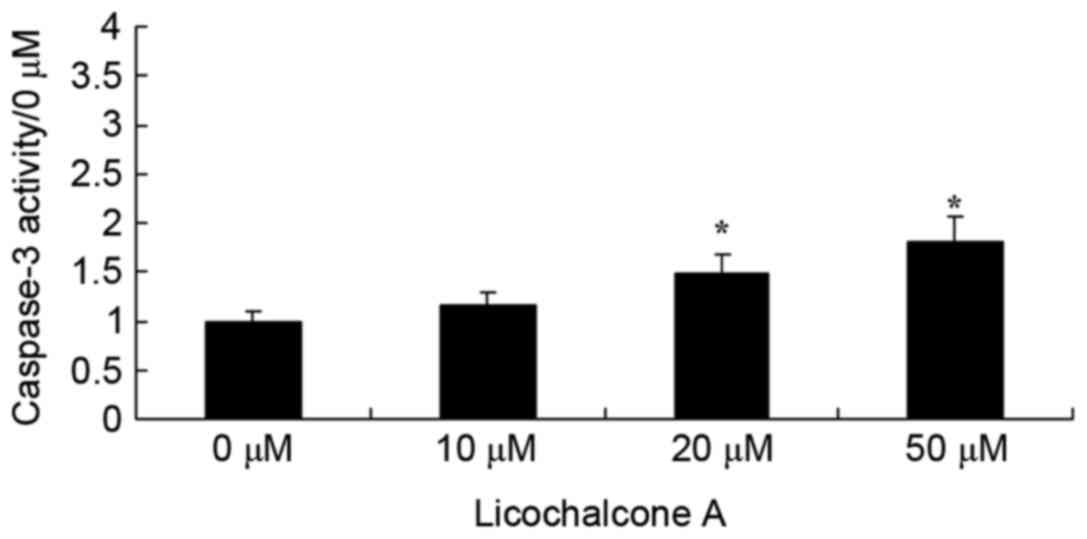
Licochalcone A increases caspase-3
activity in MCF-7 cells
To determine the effects of Licochalcone A on the
caspase-3 activity of MCF-7 cells, caspase-3 activity was detected
using a caspase-3 activity kit. Following the administration of
Licochalcone A at 20 or 50 µM in MCF-7 cells, caspase-3 activity
was significantly enhanced compared with the 0 µM control group
(P<0.01; Fig. 8).
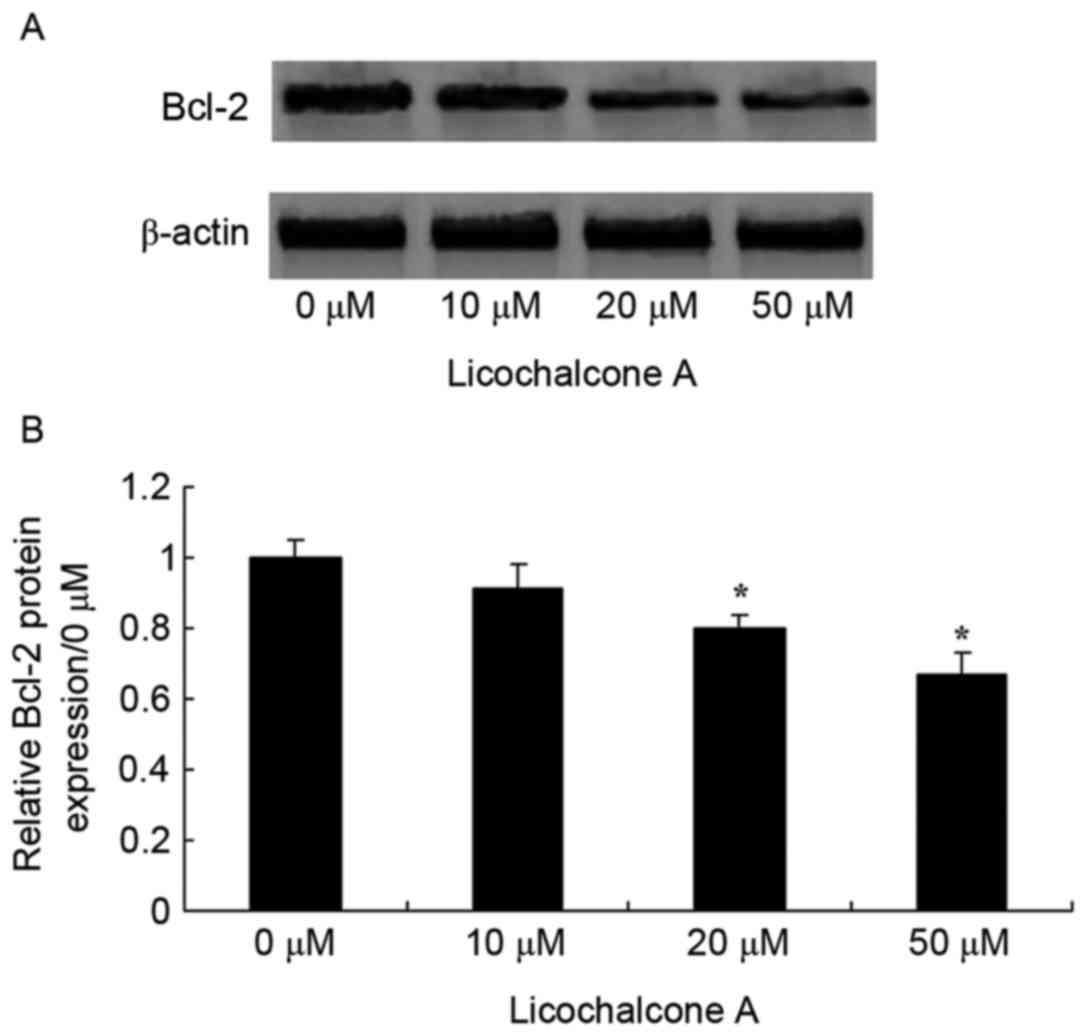
Licochalcone A increases apoptosis
regulator Bcl-2 signaling pathway activity in MCF-7 cells
To confirm the anticancer effects of Licochalcone A
on the Bcl-2 signaling pathway activity of MCF-7 cells, Bcl-2
expression was examined in MCF-7 cells treated with various
concentrations of Licochalcone A. Bcl-2 expression decreased in a
dose-dependent manner and was significantly decreased following
treatment with 20 and 50 µM Licochalcone A, as compared with the 0
µM control group (P<0.01; Fig. 9)
These results suggest that Licochalcone A induces the apoptosis of
human breast cancer cells.
Discussion
Breast cancer is a common type of malignant cancer
with an incidence that increases each year (12). Although the incidence of breast cancer
is low in China, compared with in western countries, the incidence
in China is rising annually (13).
Autophagy is a process that leads to the degradation of proteins
and organelles in eukaryotic cells. A previous study suggested that
autophagy is associated with a number of diseases, including
cancer, neurodegeneration and cardiac hypertrophy (6). The data from the present study
demonstrated that Licochalcone A decreases cell viability, induces
apoptosis and increases the caspase-3 activity in MCF-7 cells.
Previous studies have demonstrated that Licochalcone A suppresses
growth of human esophageal carcinoma (14), human lung cancer (15) and human oral cancer cells (16).
Autophagy promotes the survival of cells and
maintains homeostasis by degrading damaged organelles and proteins
(17). The PI3K/Akt/mTOR signaling
pathway promotes cellular growth, migration, protein synthesis,
survival and metabolism in response to growth factors and nutrient
availability (8). PI3K activates Akt,
which leads to the phosphorylation of mTOR via a number of
regulators (17). In the present
study, Licochalcone A suppressed the PI3K/Akt/mTOR signaling
pathway in MCF-7 cells. Tsai et al (18) demonstrated that Licochalcone A induces
autophagy through the inhibition of the PI3K/Akt/mTOR signaling
pathway in human cervical cancer cells (18). In addition, Hao et al (19) suggested that Licochalcone A induces
the apoptosis of BGC-823 human gastric cancer cells via the
PI3K/AKT signaling pathway.
Apoptosis and autophagy are forms of programmed cell
death. The morphological manifestations of apoptosis include cell
contraction, nuclear fragmentation, chromatin condensation and DNA
fragmentation (20). mTOR complex 1
is a negative regulator of autophagy (21). LC3 and autophagy-related protein
(Atg)8 serve essential functions in metastasis and the maturation
of autophagosomes (21). Prior to
induction of autophagy, LC3-I and phosphatidylethanolamine are
combined under the actions of Atg3 and Atg7 (22), forming LC3-II. LC3-II is absorbed by
the autophagosomes, which then degrade. At present, to the best of
our knowledge, no cancer therapies currently exist that act on
apoptosis and autophagy (23). Data
from the present study demonstrated that Licochalcone A increases
the expression of LC3-II, inhibits Bcl-2 expression and increases
caspase-3 activity in MCF-7 cells. Tsai et al (18) demonstrated that Licochalcone A induces
autophagy through LC3-II and the inactivation of the PI3K/Akt/mTOR
signaling pathway in human cervical cancer cells. The results from
the present study revealed that Licochalcone A could induce
apoptosis in MCF-7 cells via the caspase-dependent Bcl-2 apoptosis
signaling pathway.
In conclusion, in the present study, Licochalcone A
was observed to suppress the viability of MCF-7 cells, induce
autophagy and apoptosis, and significantly increase the levels of
LC3-II protein expression in human breast cancer cells through the
suppression of PI3K/Akt/mTOR signaling pathway activation.
References
|
1
|
Bouvet V, Jans HS, Wuest M, Soueidan OM,
Grant T, Mercer J, McEwan AJ, West FG, Cheeseman CI and Wuest F:
Erratum: Automated synthesis and dosimetry of
6-deoxy-6-[F]fluoro-D-fructose (6-[F]FDF): A radiotracer for
imaging of GLUT5 in breast cancer. Am J Nucl Med Mol Imaging.
5:952014.PubMed/NCBI
|
|
2
|
Zarzynska JM: The importance of autophagy
regulation in breast cancer development and treatment. Biomed Res
Int. 2014:7103452014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pu Z, Zhang X, Chen Q, Yuan X and Xie H:
Establishment of an expression platform of OATP1B1 388GG and 521CC
genetic polymorphism and the therapeutic effect of tamoxifen in
MCF-7 cells. Oncol Rep. 33:2420–2428. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang LW, Qu AP, Yuan JP, Chen C, Sun SR,
Hu MB, Liu J and Li Y: Computer-based image studies on tumor nests
mathematical features of breast cancer and their clinical
prognostic value. PLoS One. 8:e823142013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chukkapalli S, Amessou M, Dilly AK, Dekhil
H, Zhao J, Liu Q, Bejna A, Thomas RD, Bandyopadhyay S, Bismar TA,
et al: Role of the EphB2 receptor in autophagy, apoptosis and
invasion in human breast cancer cells. Exp Cell Res. 320:233–246.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fang WB, Yao M, Jokar I, Alhakamy N,
Berkland C, Chen J, Brantley-Sieders D and Cheng N: The CCL2
chemokine is a negative regulator of autophagy and necrosis in
luminal B breast cancer cells. Breast Cancer Res Treat.
150:309–320. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lamparska-Przybysz M, Gajkowska B and
Motyl T: BID-deficient breast cancer MCF-7 cells as a model for the
study of autophagy in cancer therapy. Autophagy. 2:47–48. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang H, Guo M, Chen JH, Wang Z, Du XF,
Liu PX and Li WH: Osteopontin knockdown inhibits αv,β3
integrin-induced cell migration and invasion and promotes apoptosis
of breast cancer cells by inducing autophagy and inactivating the
PI3K/Akt/mTOR pathway. Cell Physiol Biochem. 33:991–1002. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim KH, Yoon G, Cho JJ, Cho JH, Cho YS,
Chae JI and Shim JH: Licochalcone A induces apoptosis in malignant
pleural mesothelioma through downregulation of Sp1 and subsequent
activation of mitochondria-related apoptotic pathway. Int J Oncol.
46:1385–1392. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen M, Theander TG, Christensen SB, Hviid
L, Zhai L and Kharazmi A: Licochalcone A, a new antimalarial agent,
inhibits in vitro growth of the human malaria parasite Plasmodium
falciparum and protects mice from P. yoelii infection. Antimicrob
Agents Chemother. 38:1470–1475. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shibata S, Inoue H, Iwata S, Ma RD, Yu LJ,
Ueyama H, Takayasu J, Hasegawa T, Tokuda H, Nishino A, et al:
Inhibitory effects of licochalcone A isolated from Glycyrrhiza
inflata root on inflammatory ear edema and tumour promotion in
mice. Planta Med. 57:221–224. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rovito D, Giordano C, Plastina P, Barone
I, De Amicis F, Mauro L, Rizza P, Lanzino M, Catalano S, Bonofiglio
D and Andò S: Omega-3 DHA- and EPA-dopamine conjugates induce
PPARgamma-dependent breast cancer cell death through autophagy and
apoptosis. Biochim Biophys Acta. 1850:2185–2195. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jain K, Paranandi KS, Sridharan S and Basu
A: Autophagy in breast cancer and its implications for therapy. Am
J Cancer Res. 3:251–265. 2013.PubMed/NCBI
|
|
14
|
Yang P, Tuo L, Wu Q and Cao X:
Licochalcone-A sensitizes human esophageal carcinoma cells to
TRAIL-mediated apoptosis by proteasomal degradation of XIAP.
Hepatogastroenterology. 61:1229–1234. 2014.PubMed/NCBI
|
|
15
|
Huang HC, Tsai LL, Tsai JP, Hsieh SC, Yang
SF, Hsueh JT and Hsieh YH: Licochalcone A inhibits the migration
and invasion of human lung cancer cells via inactivation of the Akt
signaling pathway with downregulation of MMP-1/−3 expression.
Tumour Biol. 35:12139–12149. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zeng G, Shen H, Yang Y, Cai X and Xun W:
Licochalcone A as a potent antitumor agent suppresses growth of
human oral cancer SCC-25 cells in vitro via caspase-3 dependent
pathways. Tumour Biol. 35:6549–6555. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chang L, Graham PH, Hao J, Ni J, Bucci J,
Cozzi PJ, Kearsley JH and Li Y: PI3K/Akt/mTOR pathway inhibitors
enhance radiosensitivity in radioresistant prostate cancer cells
through inducing apoptosis, reducing autophagy, suppressing NHEJ
and HR repair pathways. Cell Death Dis. 5:e14372014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tsai JP, Lee CH, Ying TH, Lin CL, Lin CL,
Hsueh JT and Hsieh YH: Licochalcone A induces autophagy through
PI3K/Akt/mTOR inactivation and autophagy suppression enhances
Licochalcone A-induced apoptosis of human cervical cancer cells.
Oncotarget. 6:28851–28866. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hao W, Yuan X, Yu L, Gao C, Sun X, Wang D
and Zheng Q: Licochalcone A-induced human gastric cancer BGC-823
cells apoptosis by regulating ROS-mediated MAPKs and PI3K/AKT
signaling pathways. Sci Rep. 5:103362015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu C, Xu P, Chen D, Fan X, Xu Y, Li M,
Yang X and Wang C: Roles of autophagy-related genes Beclin-1 and
LC3 in the development and progression of prostate cancer and
benign prostatic hyperplasia. Biomed Rep. 1:855–860.
2013.PubMed/NCBI
|
|
21
|
Pan ST, Qin Y, Zhou ZW, He ZX, Zhang X,
Yang T, Yang YX, Wang D, Qiu JX and Zhou SF: Plumbagin induces G2/M
arrest, apoptosis, and autophagy via p38 MAPK- and
PI3K/Akt/mTOR-mediated pathways in human tongue squamous cell
carcinoma cells. Drug Des Devel Ther. 9:1601–1626. 2015.PubMed/NCBI
|
|
22
|
Zou M, Lu N, Hu C, Liu W, Sun Y, Wang X,
You Q, Gu C, Xi T and Guo Q: Beclin 1-mediated autophagy in
hepatocellular carcinoma cells: implication in anticancer
efficiency of oroxylin A via inhibition of mTOR signaling. Cell
Signal. 24:1722–1732. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li JP, Yang YX, Liu QL, Zhou ZW, Pan ST,
He ZX, Zhang X, Yang T, Pan SY, Duan W, et al: The pan-inhibitor of
Aurora kinases danusertib induces apoptosis and autophagy and
suppresses epithelial-to-mesenchymal transition in human breast
cancer cells. Drug Des Devel Ther. 9:1027–1062. 2015.PubMed/NCBI
|