Introduction
Acute pancreatitis (AP) is a severe disease that
affects the abdomen and its incidence is increasing from 13 to
45/100,000 (1,2). In the majority of cases, AP is a mild
and self-limiting disease; however, ~30% of patients will develop
severe acute pancreatitis (SAP), which is characterized by severe
attacks, including pancreatic necrosis, intestinal barrier
dysfunction and bacterial translocation, leading to multiple organ
dysfunction (mortality rate, 15–30%) (3–5).
Currently, the mechanisms involved in the pathogenesis of AP and
associated pancreatic injury have not been fully elucidated. Among
the various hypotheses used to explain the development of AP,
microcirculatory disturbance and inflammatory mediation have
attracted the most attention (6).
Pancreatic microcirculatory disorders may be important pathogenic
factors in determining acute pancreatitis (7) and it has been suggested that a number of
factors are involved in the development of pancreatic
microcirculatory disturbance (8). A
number of pro-inflammatory cytokines may be released from damaged
pancreatic tissue (9). These
cytokines may cause multiple organ injury by instigating and
aggravating microcirculatory disturbances (6). Current pharmaceutical therapies used to
treat AP focuses on reducing pancreatic secretion and secondary
injury (including fasting, protease inhibitors, antibiotics and
fluid resuscitation). Due to unpredictable side effects and poor
patient compliance, these therapies have a limited impact on the
incidence and severity of AP (10).
Therefore, a more in-depth understanding of the underlying
molecular mechanisms, and the development of novel treatment
strategies are required for AP.
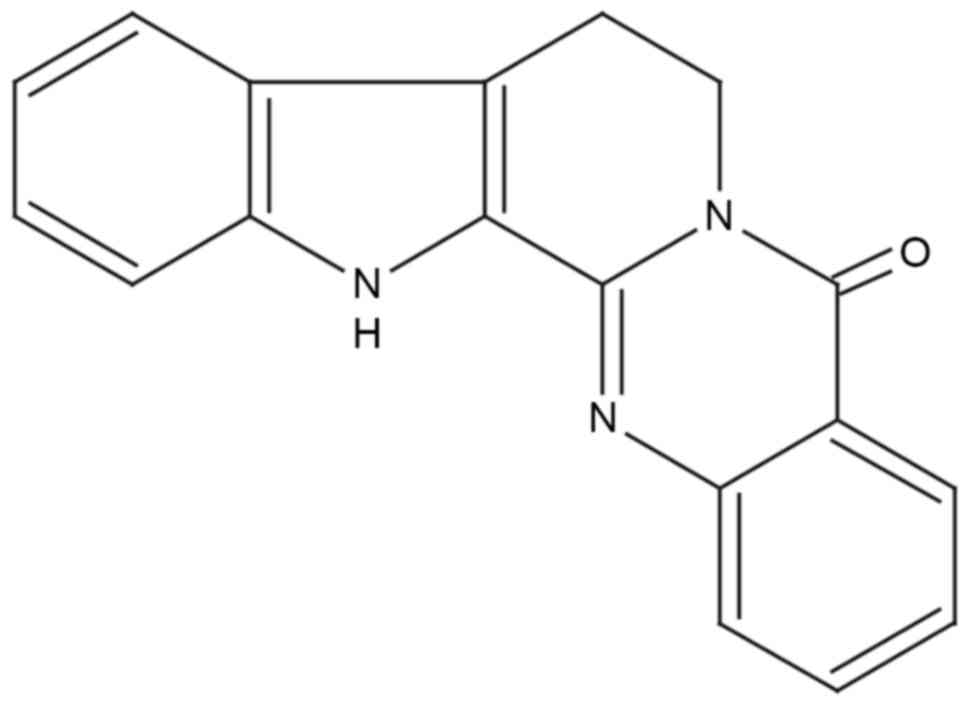
Rutaecarpine (Fig. 1)
is a quinazolinocarboline alkaloid isolated from Wu-Chu-Yu, the
dried fruit of Evodia rutaecarpa Bentham (Rutaceae), a
Chinese herbal drug (11).
Rutaecarpine possesses a number of biological properties, including
anti-hypertension, anti-thrombotic, anticancer and
anti-inflammatory activities, particularly on relaxing vascular
smooth muscle (12,13). Previous studies have revealed that the
multiple pharmacological effects elicited by rutaecarpine are
driven by the increase in endogenous calcitonin gene-related
peptide (CGRP) release following the activation of vanilloid
receptor subtype 1 (VR1) (11,14). VR1,
also known as the capsaicin receptor, is primarily expressed in
sensory nerves. Primary sensory nerves sensitive to capsaicin are
extensively distributed among different tissues and organs, and
serve an important function in regulating peripheral vascular
resistance (15). The activation of
VR1 leads to the release of multiple neurotransmitters, including
substance P (SP) and CGRP (16).
Sensory nerves are important in limiting the development of AP and
the stimulation of sensory nerves. Furthermore, the administration
of CGRP may protect against pancreatic injury (17–19).
Additionally, CGRP is a competitive VR antagonist, and therefore
may be able to abolish the effects of VR (20).
It has been previously demonstrated that
rutaecarpine has a therapeutic effect on SAP (21). However, to the best of our knowledge,
the mechanism(s) responsible for the action of rutaecarpine in AP
has not yet been reported. Previous studies performed in rats have
indicated that the effects of rutaecarpine on gastroprotection and
vasodilation may be due to the increase in endogenous CGRP release
following VR1 activation (11,22). CGRP
immunoreactivity has been detected in nerve fibers innervating the
pancreas (23). Therefore, the
present study investigated the protective effects of rutaecarpine
on AP in rats and examined whether the functional mechanisms of
rutaecarpine are associated with an increase in endogenous CGRP
release following the activation of VR1.
Materials and methods
Animals
A total of 100 male Sprague-Dawley (SD) rats
(Laboratory Animal Center, Xiangya Hospital, Central South
University, China), weighing 250±50 g, were used in the present
study. All animals were housed in a controlled temperature
environment (25°C; 50% humidity) with a 12-hour day/night rhythm
with free access to standard laboratory chow and water. All animals
received humane care in compliance with the National Institutes of
Health standards (Guide for the Care and Use of Laboratory Animals,
revised 1996) (24). The present
study was approved by the Ethics Committee of the Xiangya School of
Medicine, Central South University (Changsha, China).
Reagents
Rutaecarpine (purity, >98%) was purchased from
Shanghai Yuanye Biotechnology Co., Ltd. (Shanghai, China).
Capsazepine (a competitive VR antagonist) (purity, >98%) and 45%
sodium taurocholate were purchased from Sigma-Aldrich (Merck KGaA,
Darmstadt, Germany). Dimethyl sulfoxide and ethanol were mixed at a
ratio of 1:4 and used to dissolve rutaecarpine. A vehicle
containing 8% ethanol, 2% dimethyl sulfoxide and 90% saline was
used to dissolve capsazepine.
Modeling and grouping of animals
SD rats were randomly divided into 10 different
groups (all n=10). The groups included were as follows: i)
Sham-operated group (Sham), AP was not induced during surgery; ii)
AP group (AP), 5% sodium taurocholate solution (1.0 ml/kg) was
injected into rats to induce AP during surgery; iii) Sham-operated
+ rutaecarpine (100 µg/kg) group (Sham+Rut), rats were injected
with 100 µg/kg rutaecarpine into the sublingual vein 20 min prior
to surgery, during which AP was not induced; iv) AP + rutaecarpine
(30 µg/kg) group (AP+Rut L), rats received an injection of 30 µg/kg
rutaecarpine into the sublingual vein 20 min prior to surgery, in
which AP was induced; v) AP + rutaecarpine (100 µg/kg) group
(AP+Rut M), rats were injected with 100 µg/kg rutaecarpine into the
sublingual vein 20 min prior to surgery, in which AP was induced;
vi) AP + rutaecarpine (300 µg/kg) group (AP+Rut H), rats were
injected with 300 µg/kg rutaecarpine into the sublingual vein 20
min prior to surgery, in which AP was induced; vii) sham-operated +
capsazepine group (Sham+Cap), animals were injected with 3 mg/kg
capsazepine into the sublingual vein 30 min prior to surgery, in
which AP was not induced; viii) AP + capsazepine group (AP+Cap),
animals were injected with 3 mg/kg capsazepine into the sublingual
vein 30 min prior to surgery; ix) AP + capsazepine + rutaecarpine
(100 µg/kg) group (AP+Cap+Rut), mice were injected with 3 mg/kg
capsazepine into the sublingual vein 30 min prior to surgery and
were subsequently injected with 100 µg/kg rutaecarpine into the
sublingual vein 20 min prior to surgery, in which AP was induced;
and x) vehicle control group (AP+Sol), mice were injected with the
vehicle [a mixture of dimethyl sulfoxide and ethanol (1:4) in a
volume of 0.125 ml/kg] into the sublingual vein 20 min prior to
surgery, in which AP was induced.
SD rats were fasted for 12 h prior to surgery.
Subsequently, rats were administered 3% pentobarbital sodium (40
mg/kg; Sigma-Aldrich; Merck KGaA) intraperitoneally to induce
anesthesia. Following a conventional disinfection (first with 2.5%
iodine inunction, followed by drying with 70% alcohol twice) and
towel spreading, an incision (~1.5 cm) along the white line of
abdomen was created. Freshly prepared 5% sodium taurocholate
solution was used to induce AP by retrograde infusion through the
cholangiopancreatic duct following laparotomy (25). Instead of 5% sodium taurocholate
solution, an equivalent volume of normal saline solution was
administered to the Sham group. The incision was closed with a
continuous silk suture. All rats were sacrificed 24 h after surgery
and arterial blood and pancreatic tissue were collected. The serum
was collected following centrifugation (4°C, 1,500 × g for 5 min)
and stored at −20°C. Pancreatic tissue was fixed in 4%
phosphate-buffered formaldehyde at 4°C prior to histopathological
examination.
Detection of ascite volume
The abdomen was opened following euthanasia of the
rats (all rats were sacrificed 24 h after surgery), ascites were
removed from the abdominal cavity using a syringe and a measuring
cylinder was used to measure the volume of the ascites.
Histopathology
Formaldehyde-fixed pancreatic tissues obtained from
the rats were embedded in paraffin, sectioned at 4 µm thick, and
were dewaxed in xylene, rehydrated through decreasing
concentrations of ethanol and then washed in PBS. Sections were
stained with hematoxylin (4 min), washed with H2O, and
then 0.5% eosin (1 min) at room temperature. Two independent
pathologists, who were blinded to this experiment, evaluated the
sections under a light microscope. As outlined by the
histopathological features scoring criteria (26), the severity of pancreatitis was scored
according to edema, vacuolization, necrosis and inflammation.
Measurement of serum amylase
activity
Serum amylase activity was measured using a Hitachi
7170A full-automatic biochemical analyzer (Hitachi, Ltd., Tokyo,
Japan) according to the manufacturer's protocol (α-Amy-DR; Autec
Diagnostics, Bötzingen, Germany).
Cytokines assay
ELISA was performed to measure the serum
concentrations of interleukin (IL)-6 (Rat IL-6 ELISA kit; cat. no.
JER-04), IL-10 (Rat IL-10 ELISA kit; cat. no. JER-05) and tumor
necrosis factor (TNF)-α (Rat TNF-α ELISA kit; cat. no. JER-06),
according to the manufacturer's protocol (all products were
purchased from Joyee Biotechnics Co., Ltd., Shanghai, China).
Absorbance values were used to determine cytokine concentrations
using a standard curve. All samples were tested three times.
Measurement of plasma CGRP
concentration
Plasma was collected when rats were sacrificed 24 h
after surgery by centrifugation at 1,000 × g for 30 min at 4°C.
Subsequently, CGRP concentration in plasma was measured using a
radioimmunoassay kit, according to the manufacturer's
protocols.
Statistical analysis
All results are presented as the mean ± standard
deviation and were analyzed using SPSS software (version 17.0;
SPSS, Inc., Chicago, IL, USA). Results were compared between all
groups using one-way analysis of variance and least significant
difference t tests were used to compare two different groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Pathological changes of the
pancreas
To investigate the protective role of rutaecarpine
in a rat model of AP, histopathological changes of the pancreas in
rats from each group were assessed by evaluating H&E-stained
tissue. Representative histological sections are presented in
Fig. 2. There were no evident
histopathological changes identified in the pancreas of mice from
the Sham, Sham+Rut and Sham+Cap groups (Fig. 2A, C and G); however, the interlobular
septum was slightly broadened in Sham+Rut group. There were varied
degrees of pathological changes in the pancreatic acinar, including
acute inflammation, necrosis, dilated intercellular spaces and
interlobular septum in the other groups (Fig. 2B, D-F and H-J). Conspicuous
hemorrhagic necrosis, pancreatic edema, interstitial leukocyte and
erythrocyte infiltration, and acinar cell vacuolization were
observed in the AP and AP+Sol groups (Fig. 2B and J). Compared with the AP group,
the extent and severity of pancreatic injuries were markedly
alleviated in the AP+Rut L, AP+Rut M and AP+Rut H groups (Fig. 2D-F and K). Rutaecarpine (30, 100 and
300 µg/kg) significantly decreased the severity of pathological
changes in a dose-dependent manner (Fig.
2D-F and K). As presented in Fig.
2K, pancreatic histological scores were highest in the AP+Cap
and AP+Cap+Rut groups; they were even significantly higher than in
the AP group (P<0.05). Coagulative necrosis was observed in
these two groups, which also exhibited widened interlobular septums
and the disappearance of acinic structures (Fig. 2H and I).
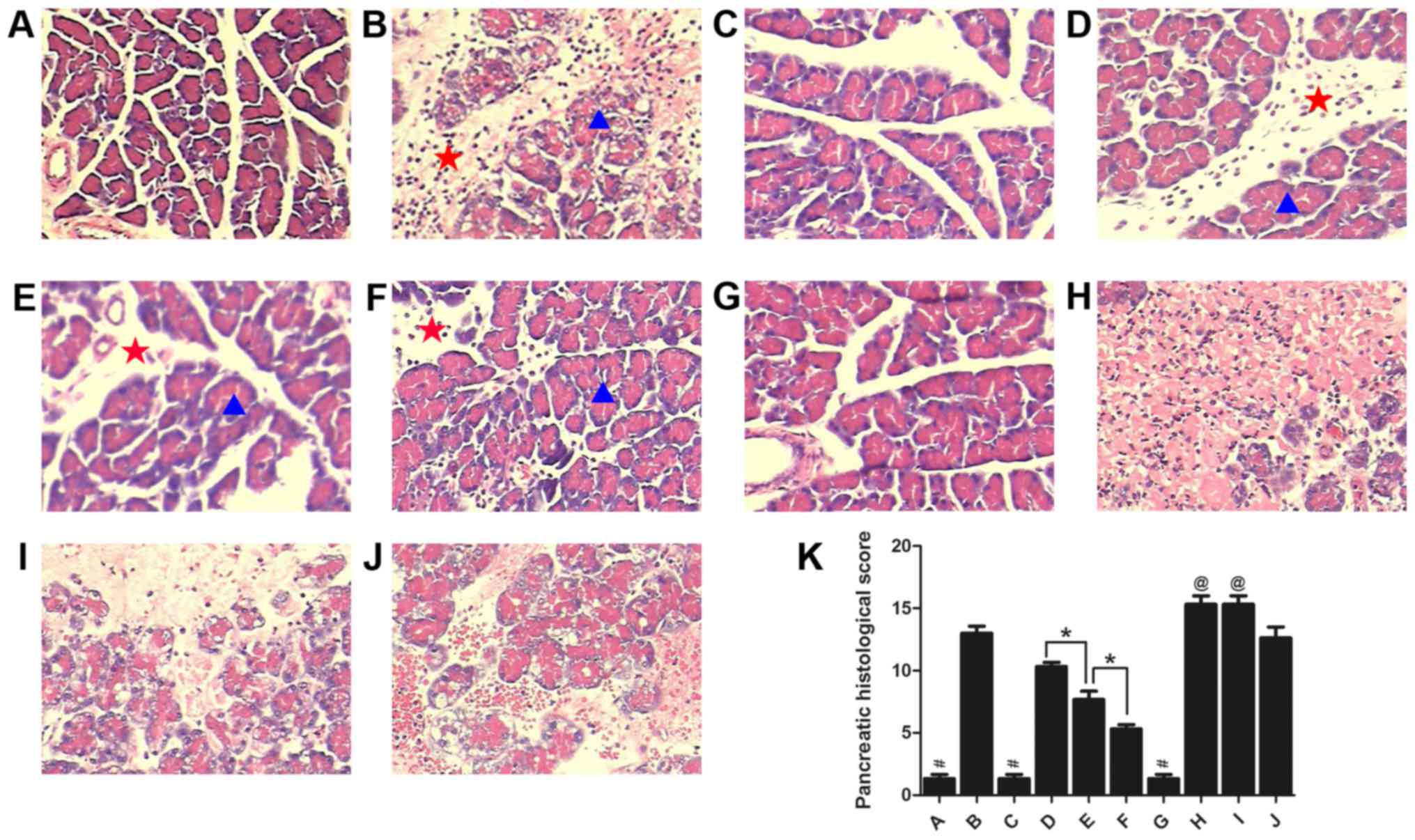 | Figure 2.Morphological changes in the pancreas
and the pancreatic histological scores in each group (n=10).
Stained sections from the (A) Sham, (B) AP, (C) Sham+Rut (100
µg/kg), (D) AP+Rut L (30 µg/kg), (E) AP+Rut M (100 µg/kg), (F)
AP+Rut H (300 µg/kg), (G) Sham+Cap, (H) AP+Cap, (I) AP+Cap+Rut (100
µg/kg) and (J) AP+Sol groups. Magnification, ×200. (K)
Quantification of histological scores of the different groups.
Results are presented as the mean ± standard deviation. Pancreatic
acinar are indicated with a blue triangle and the interlobular
septum is indicated with a red star. Conspicuous hemorrhagic
necrosis, pancreatic edema, interstitial leukocyte and erythrocyte
infiltration and acinar cell vacuolization were demonstrated in the
AP only group. However, pancreatic injuries were markedly
alleviated in the AP+Rut groups. Magnification, ×200. *P<0.05;
#P<0.05 vs. (B), (D-F) and (H-J);
@P<0.05 vs. (A-G) and (J); AP, acute pancreatitis;
Rut, rutaecarpine; Cap, capsazepine. |
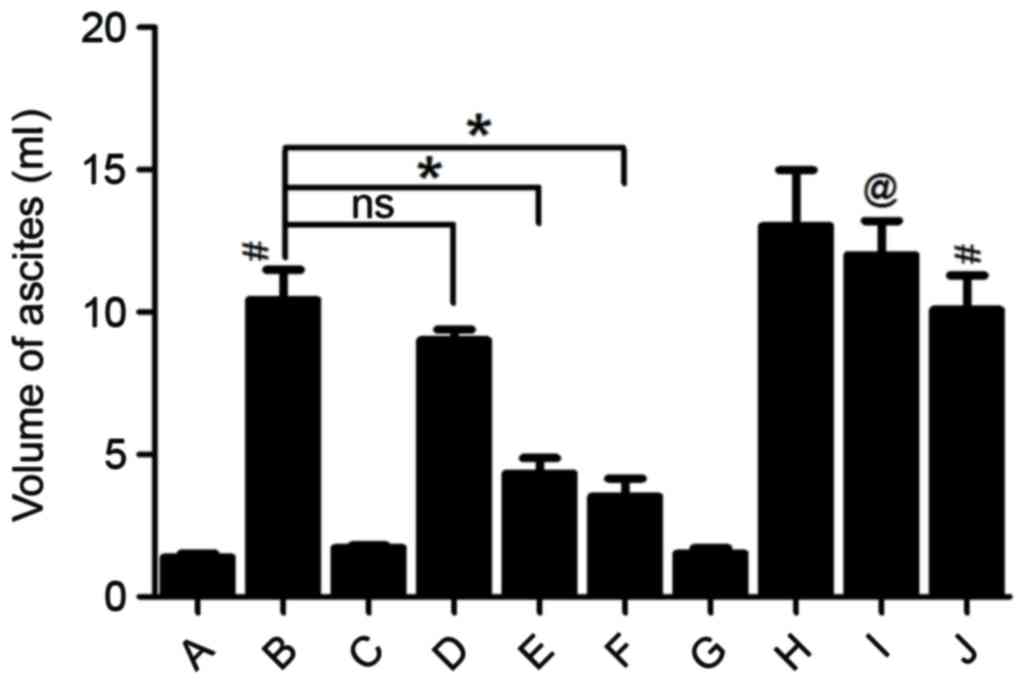
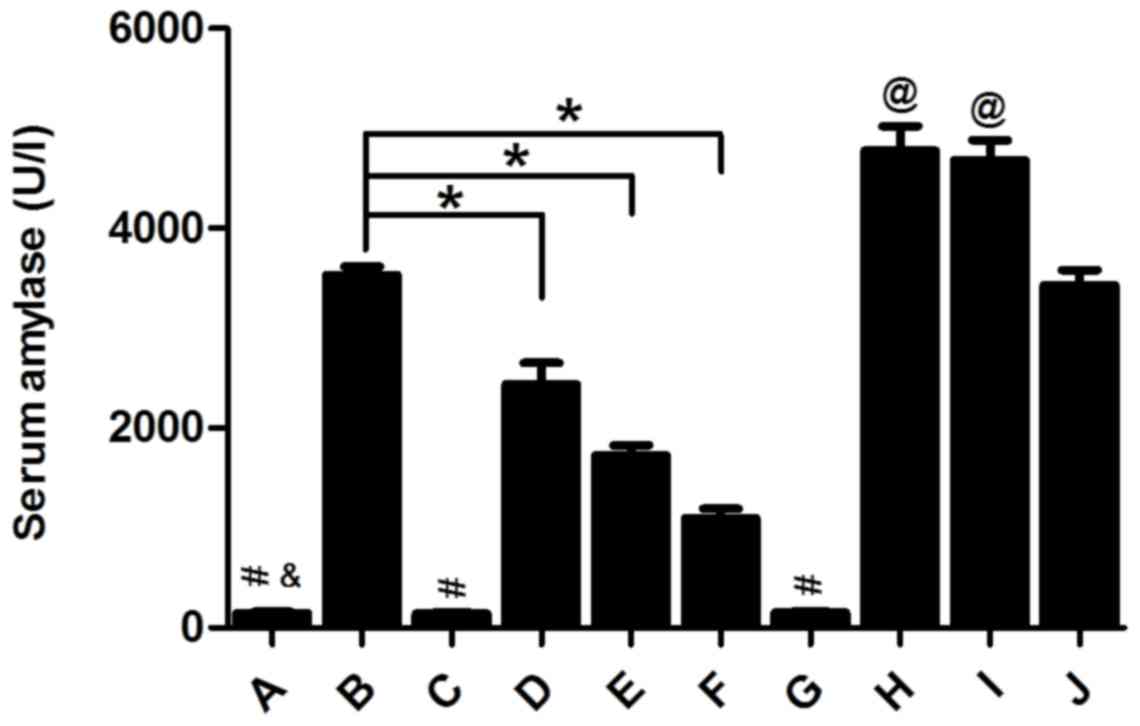
Changes in the volume of ascites and
serum amylase activity
In the Sham, Sham+Rut and Sham+Cap groups, ascite
volume in the abdominal cavity of rats was low and barely
detectable (Fig. 3) and serum amylase
activity remained low in these 3 groups (Fig. 4). Ascite volume significantly
increased in the AP and AP+Sol groups (P<0.05; Fig. 3). Furthermore, compared with the Sham
groups, the AP groups exhibited markedly increased levels of serum
amylase (P<0.05; Fig. 4). However,
pre-treatment with 30, 100 and 300 µg/kg rutaecarpine significantly
reduced the volume of ascites and serum amylase activity in a
dose-dependent manner (P<0.05). Capsazepine, a competitive VR1
antagonist reversed these effects (Figs.
3 and 4).
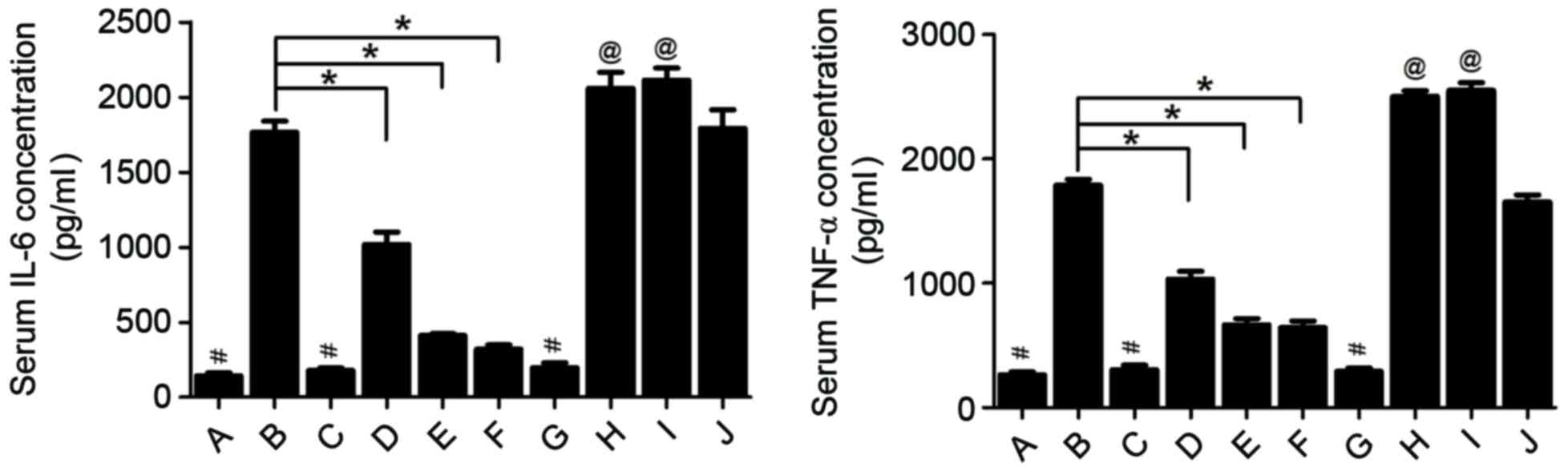
Changes in inflammatory cytokines
Cytokines serve an important function in the
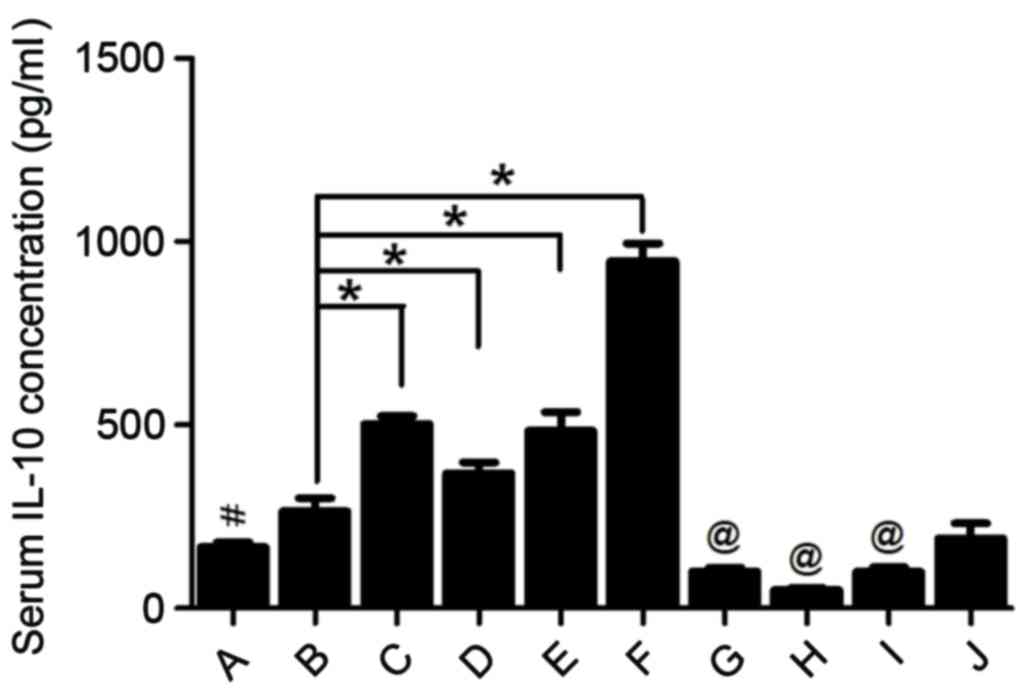
systemic response in AP; therefore, changes in the levels of IL-10,
IL-6 and TNF-α were assessed in the serum of the rats to identify
the mechanism by which rutaecarpine protects against rutaecarpine
in AP. Groups in which AP was induced by injection of 5% sodium
taurocholate exhibited a marked increase of IL-6 and TNF-α serum
concentrations (Fig. 5). IL-6 and
TNF-α are two pro-inflammatory cytokines expressed in response to
local damage to the pancreas. Pre-treatment with 30, 100 and 300
µg/kg rutaecarpine significantly reduced IL-6 and TNF-α levels
compared with the AP group in a dose-dependent manner (P<0.05;
Fig. 5). Furthermore, pre-treatment
with rutaecarpine significantly increased serum concentrations of
IL-10 (P<0.05; Fig. 6), an
anti-inflammatory cytokine that may attenuate pancreatic damage
(27). However, rats injected with
capsazepine prior to surgery exhibited significantly higher IL-6
and TNF-α concentrations (P<0.05) and significantly lower serum
IL-10 concentrations (P<0.05) compared with those treated with
rutaecarpine. This indicates that rutaecarpine suppresses the
inflammatory response in AP, an effect that is reversed by
capsazepine.
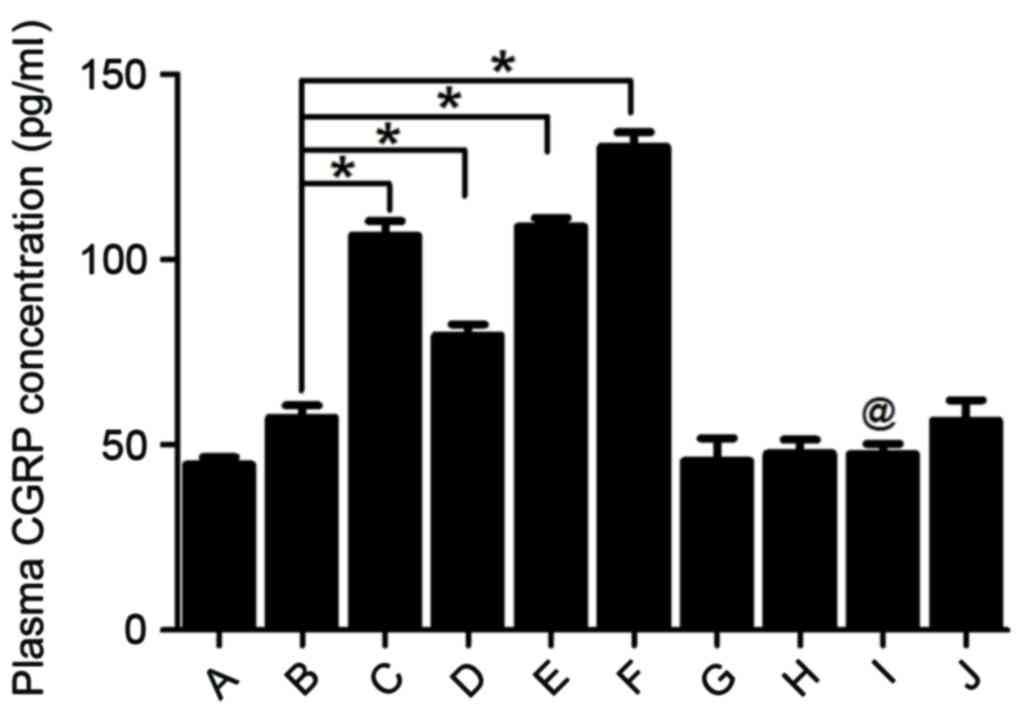
CGRP concentration in plasma
Pre-treatment with 30, 100 or 300 µg/kg rutaecarpine
significantly upregulated CGRP concentrations in a dose-dependent
manner (P<0.05; Fig. 7).
Contrastingly, treatment with capsazepine led to a significant
decrease in plasma CGRP levels even when rutaecarpine was
administered (P<0.05; Fig. 7).
Taken together, these results indicate that capsazepine may
attenuate the effect of rutaecarpine on CGRP concentration.
Discussion
The morbidity of patients with acute pancreatitis
has increased in recent years. However, the pharmacological
therapies currently used to treat AP are limited. Therefore, a more
in-depth understanding of the pathogenic mechanisms of AP and the
identification of novel treatment strategies to treat it, are
urgently required. Microcirculatory disorders serve an important
function in the pathogenesis of AP, as they cause hypoxic damage in
focal tissue and eventually induce edema formation and necrosis
(28). A variety of pro-inflammatory
cytokines are released by injured pancreas tissue (9). These inflammatory mediators are involved
in the entire AP process, triggering and aggravating the
microcirculatory disorders, which leads to injuries in multiple
organs (28).
Rutaecarpine is a vasodilator that modulates
peripheral vascular resistance and may be associated with the
upregulation of endogenous CGRP release via activation of VR1
(15,29). VR1 is almost exclusively distributed
in the primary sensory neurons (30).
Various vasodilator neuropeptides, including CGRP, are released by
sensory afferent fibers. CGRP regulates regional organ blood flow
and vascular tone, and is a potent vasodilator (31). The mammalian pancreas is richly
innervated by a number of different nerve fibers and CGRP
immunoreactivity has been observed in these nerve fibers (23). Previous studies have demonstrated that
sensory nerves limit the development of AP and that stimulation of
sensory nerves or administration of CGRP may protect against
pancreatic injury (17–19). It has been demonstrated that
rutaecarpine has a therapeutic effect on SAP (21). However, to the best of our knowledge,
the mechanism of rutaecarpine action in AP has not yet been
described. The results of the present study indicate that
pre-treatment with rutaecarpine alleviates pancreatic inflammation
and necrosis in a rat model of pancreatitis, reducing the volume of
ascites and serum amylase activity, whilst significantly increasing
CGRP plasma concentration. The effect of rutaecarpine treatment
increased as the drug dose increased; however, its protective
effects were attenuated by capsazepine. These results indicated
that the protective effect of rutaecarpine against AP is mediated
by upregulating endogenous CGRP release via the activation of
VR1.
Inflammatory mediators are key players in the
systemic response to AP (9) and
induce and aggravate microcirculatory disturbance throughout the
body (28). Local damage of the
pancreas is accompanied by the presence of leukocytes that release
various pro-inflammatory cytokines, including TNF-α, IL-6 and
IL-1β. The balance between pro- and anti-inflammatory mediators
modulates the inflammatory response to AP (32). The presence of IL-10, which is a major
anti-inflammatory mediator in AP, diminishes pancreatic damage
(27). Therefore, further research
into treatments that can modulate various inflammatory mediators
may provide a novel method of treating AP. Previous studies
determined the anti-inflammatory role of CGRP: CGRP reduces the
expression of IL-6, TNF-α and IL-8 by inhibiting stimulation of
nuclear factor-κB (33)
butupregulates the anti-inflammatory mediator IL-10 (34). The present study confirmed that
rutaecarpine increases serum concentrations of IL-10 but reduces
IL-6 and TNF-α levels by stimulating the release of CGRP. This
effect was dose-dependent and was abolished by capsazepine.
Notably, although the rats in the AP group had not been treated
with rutaecarpine prior to surgery, serum IL-10 concentrations
increased slightly. This may be due to the protective effect
against acute inflammation exhibited by living organisms.
In conclusion, the results of the present study
indicate that rutaecarpine protects against injuries caused by AP
in rats and that these effects are mediated by the release of CGRP
via activation of VR1. Rutaecarpine induces an anti-inflammatory
response in the treatment of AP. The results of the present study
provide novel insights into the pharmacological therapy of AP.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81670589), the
Project Sponsored by the Scientific Research Foundation for the
Returned Overseas Chinese Scholars, State Education Ministry [grant
no. (2015) 311] and the Project from Science and Technology
Department, Hunan, China (grant no. 2015SF2020-3).
Glossary
Abbreviations
Abbreviations:
|
AP
|
acute pancreatitis
|
|
Rut
|
rutaecarpine
|
|
CGRP
|
calcitonin gene-related peptide
|
|
Cap
|
capsazepine
|
References
|
1
|
Maksimow M, Kyhälä L, Nieminen A, Kylänpää
L, Aalto K, Elima K, Mentula P, Lehti M, Puolakkainen P, Yegutkin
GG, et al: Early prediction of persistent organ failure by soluble
CD73 in patients with acute pancreatitis*. Crit Care Med.
42:2556–2564. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yadav D and Lowenfels AB: The epidemiology
of pancreatitis and pancreatic cancer. Gastrenterology.
144:1252–1261. 2013. View Article : Google Scholar
|
|
3
|
Yang ZW, Meng XX and Xu P: Central role of
neutrophil in the pathogenesis of severe acute pancreatitis. J Cell
Mol Med. 19:2513–2520. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dellinger EP, Forsmark CE, Layer P, Lévy
P, Maraví-Poma E, Petrov MS, Shimosegawa T, Siriwardena AK, Uomo G,
Whitcomb DC, et al: Determinant-based classification of acute
pancreatitis severity: An international multidisciplinary
consultation. Ann Surg. 256:875–880. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Forsmark CE and Toskes PP: Acute
pancreatitis. Medical management. Crit Care Clin. 11:295–309.
1995.PubMed/NCBI
|
|
6
|
Zhang XP, Li ZJ and Zhang J: Inflammatory
mediators and microcirculatory disturbance in acute pancreatitis.
Hepatobiliary Pancreat Dis Int. 8:351–357. 2009.PubMed/NCBI
|
|
7
|
Klar E, Schratt W, Foitzik T, Buhr H,
Herfarth C and Messmer K: Impact of microcirculatory flow pattern
changes on the development of acute edematous and necrotizing
pancreatitis in rabbit pancreas. Dig Dis Sci. 39:2639–2644. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhou ZG and Chen YD: Influencing factors
of pancreatic microcirculatory impairment in acute panceatitis.
World J Gastroenterol. 8:406–412. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gómez-Cambronero LG, Sabater L, Pereda J,
Cassinello N, Camps B, Viña J and Sastre J: Role of cytokines and
oxidative stress in the pathophysiology of acute pancreatitis:
Therapeutical implications. Curr Drug Targets Inflamm Allergy.
1:393–403. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xiang H, Zhang Q, Qi B, Tao X, Xia S, Song
H, Qu J and Shang D: Chinese herbal medicines attenuate acute
pancreatitis: Pharmacological activities and mechanisms. Front
Pharmacol. 8:2162017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang L, Hu CP, Deng PY, Shen SS, Zhu HQ,
Ding JS, Tan GS and Li YJ: The protective effects of rutaecarpine
on gastric mucosa injury in rats. Planta Med. 71:416–419. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee SH, Son JK, Jeong BS, Jeong TC, Chang
HW, Lee ES and Jahng Y: Progress in the studies on rutaecarpine.
Molecules. 13:272–300. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yu J, Tan GS, Deng PY, Xu KP, Hu CP and Li
YJ: Involvement of CGRP in the inhibitory effect of rutaecarpine on
vasoconstriction induced by anaphylaxis in guinea pig. Regul Pept.
125:93–97. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li JZ, Peng J, Xiao L, Zhang YS, Liao MC,
Li XH, Hu CP, Deng HW and Li YJ: Reversal of isoprenaline-induced
cardiac remodeling by rutaecarpine via stimulation of calcitonin
gene-related peptide production. Can J Physiol Pharmacol.
88:949–959. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Deng PY, Ye F, Cai WJ, Tan GS, Hu CP, Deng
HW and Li YJ: Stimulation of calcitonin gene-related peptide
synthesis and release: Mechanisms for a novel antihypertensive
drug, rutaecarpine. J Hypertens. 22:1819–1829. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Peng J and Li YJ: The vanilloid receptor
TRPV1: Role in cardiovascular and gastrointestinal protection. Eur
J Pharmacol. 627:1–7. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Warzecha Z, Dembiński A, Jaworek J,
Ceranowicz P, Szlachcic A, Walocha J and Konturek SJ: Role of
sensory nerves in pancreatic secretion and caerulein-induced
pancreatitis. J PhysiolPharmacol. 48:43–58. 1997.
|
|
18
|
Warzecha Z, Dembiński A, Ceranowicz P,
Konturek PC, Stachura J, Konturek SJ and Niemiec J: Protective
effect of calcitonin gene-related peptide against caerulein-induced
pancreatitis in rats. J Physiol Pharmacol. 48:775–787.
1997.PubMed/NCBI
|
|
19
|
Dembiński A, Warzecha Z, Ceranowicz P,
Jaworek J, Sendur R, Knafel A, Dembiński M, Bilski J, Pawlik WW,
Tomaszewska R, et al: Stimulation of sensory nerves and CGRP
attenuate pancreatic damage in ischemia/reperfusion induced
pancreatitis. Med Sci Monit. 9:BR418–BR425. 2003.PubMed/NCBI
|
|
20
|
van den Worm E, de Vires A, Nijkamp FP and
Engels F: Capsazepine, a vanilloid receptor antagonist, inhibits
allergen-induced tracheal contraction. Eur J Pharmacol. 518:77–78.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huan G, Jie P and Jixiang M: Experimental
study of therapeutic use of rutecarpin in severe acute
pancreatitis. Chin J Gen Surg. 23:2014.
|
|
22
|
Hu CP, Xiao L, Deng HW and Li YJ: The
cardioprotection of rutaecarpine is mediated by endogenous
calcitonin related-gene peptide through activation of vanilloid
receptors in guinea-pig hearts. Planta Med. 68:705–709. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sternini C, De Giorgio R and Furness JB:
Calcitonin gene-related peptide neurons innervating the canine
digestive system. Regul Pept. 42:15–26. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
National Research Council (US) Institute
for Laboratory Animal Research, . Guide for the Care and Use of
Laboratory Animals. National Academies Press (US); Washington, DC:
1996, http://oacu.od.nih.gov/regs/guide/guide.pdf
|
|
25
|
Aho HJ and Nevalainen TJ: Experimental
pancreatitis in the rat. Ultrastructure of sodium
taurocholate-induced pancreatic lesions. Scand J Gastroenterol.
15:417–424. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yilmaz M, Topsakal S, Herek O, Ozmen O,
Sahinduran S, Buyukoglu T and Yonetci N: Effects of etanercept on
sodium taurocholate-induced acute pancreatitis in rats. Transl Res.
154:241–249. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Keceli M, Kucuk C, Sozuer E, Kerek M, Ince
O and Arar M: The effect of interleukin-10 on acute pancreatitis
induced by cerulein in a rat experimental model. J Invest Surg.
18:7–12. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Menger MD, Plusczyk T and Vollmar B:
Microcirculatory derangements in acute pancreatitis. J
Hepatobiliary Pancreat Surg. 8:187–194. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hu CP, Xiao L, Deng HW and Li YJ: The
depressor and vasodilator effects of rutaecarpine are mediated by
calcitonin gene-related peptide. Planta Med. 69:125–129. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Caterina MJ and Julius D: The vanilloid
receptor: A molecular gateway to the pain pathway. Annu Rev
Neurosci. 24:487–517. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wimalawansa SJ: Calcitonin gene-related
peptide and its receptors: Molecular genetics, physiology,
pathophysiology, and therapeutic potentials. Endocr Rev.
17:533–585. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pérez S, Pereda J, Sabater L and Sastre J:
Redox signaling in acute pancreatitis. Redox Biol. 5:1–14. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Monneret G, Pachot A, Laroche B, Picollet
J and Bienvenu J: Procalcitonin and calcitonin gene-related peptide
decrease LPS-induced tnf production by human circulating blood
cells. Cytokine. 12:762–764. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Granger J and Remick D: Acute
pancreatitis: Models, markers, and mediators. Shock. 24 Suppl
1:S45–S51. 2005. View Article : Google Scholar
|