Introduction
Hepatocellular carcinoma (HCC) is the fifth most
frequently occurring malignancy and the third leading cause of
cancer-associated mortality worldwide (1). Treatments, such as surgery, including
transplantation, have provided a possibility of cure; however, the
high recurrence rate has resulted in a reduced long-term survival
rate in patients with HCC (2). The
overall survival rate of patients with HCC is <10%. (3,4).
Additionally, evaluation of the individual prognostic outcome may
aid in guiding surgical and chemotherapeutic treatment. In the
present study, the prognostic significance of three HCC-associated
molecular biomarkers were evaluated.
β-catenin serves an important role in the
Wnt/β-catenin signaling pathway, and participates in the
development and progression of HCC (5). Phosphorylated hepatocyte growth factor
receptor (c-Met) triggers mitogen-activated phosphate kinase (MAPK)
signaling through the Ras-Raf-MAPK kinase signaling pathway
(6), and its overexpression is
present in the HCC phenotype with poor differentiation and
malignancy (7,8). Activation of c-Met signaling and
β-catenin mutations are frequent genetic occurrences observed in
liver cancer initiation (9–11). Previous studies have reported that
c-Met and β-catenin are coactivated in HCC, and possess a possible
correlation with hepatic carcinogenesis (12,13).
Focal adhesion kinase (FAK) is a nonreceptor
tyrosine kinase that can be phosphorylated, and activated by growth
factors and integrins (6,9). Multiple downstream signaling pathways,
including extracellular receptor kinase (Erk), protein kinase B
(PKB or Akt) and Ras-related C3 botulinum toxin substrate (Rac)
have been identified to be regulated by FAK (14). Thus, FAK activation can regulate the
adhesion, motility, proliferation and survival of various cells
types (14). In a variety of cancers,
including breast (15), intestine
(16) and brain (17) cancer, FAK has been demonstrated to be
activated and/or overexpressed, thus promoting the progression and
metastasis of the aforementioned cancer types. Additionally, in
certain HCC studies, FAK has been demonstrated to be overexpressed
in HCC specimens, suggesting that it serves a role in
hepatocarcinogenesis (18,19).
Although the association between HCC and these three
molecules has been separately investigated, a recent in vivo
study investigated the potential role of FAK in
hepatocarcinogenesis, and it association with c-Met and β-catenin
(3). In that report, the deletion of
FAK in hepatocytes did not affect their morphology, proliferation
or apoptosis rates. However, FAK deficiency significantly repressed
MET/DN90-b-catenin (CAT)-induced tumor development and prolonged
the survival of mice with MET/CAT-induced HCC. In the livers of
mice with HCC and in HCC cell lines, FAK was demonstrated to be
activated by c-Met, thus inducing Akt/Erk activation, cyclin D1
upregulation and tumor cell proliferation. CAT enhanced
c-Met-stimulated FAK activation and synergistically induced the
activation of the Akt/Erk-cyclin D1 signaling pathway in a
FAK-dependent manner. In addition, FAK was demonstrated to be
required for CAT-induced cyclin D1 expression in a
kinase-independent manner (3).
Following the review of previous studies on FAK,
c-Met and β-catenin oncoproteins, their correlation with HCC has
been identified in a variety of studies (12,13,18–22).
However, the intercorrelation among them requires further
investigation. Irrespective of the coactivation of c-Met and
β-catenin in HCC or the FAK's potential role in regulation of
tumors described previously (3,12,22), those previous studies were mainly
focused on identifying the underlying molecular mechanisms of
c-Met/β-catenin-driven hepatocarcinogenesis. By contrast, the
present study aimed to evaluate the prognostic value of these three
oncoproteins and the possible correlations with each other in
patients with HCC.
Materials and methods
Patients
A total of 86 patients with HCC who underwent
surgery were included in the present study. All information and
tumor tissue samples were collected from the database of the
Department of Pathology of the First Affiliated Hospital of Sun
Yat-Sen University (Guangzhou, China). Patients with radically
resected HCCs who received surgery between January 2004 and
December 2008 were recruited. Patients who had been treated with
transhepatic artery chemoembolization (TACE), percutaneous ethanol
injection (PEI) or radiofrequency ablation (RFA) were not included
in the present study. A total of 68 males and 18 females were
recruited, with a mean age of 50.2 years (age range, 26–77 years).
With respect to the basic liver disease preceding HCC development,
65 cases (78.3%) of liver cirrhosis were identified. Hepatitis B
surface antigen was positive in 73 patients and 3 hepatitis C
patients (88.0%), and 10 patients (12.0%) did not have any
underlying liver disease. The median follow-up period was 28.5
months (range, 3–60 months). The current study was approved by the
First Affiliated Hospital of Sun Yat-Sen University. Written
informed consent was obtained from all patients.
Tissue sections
Paraffin tissue blocks from all resected HCC
specimens were obtained for immunohistochemical analysis.
Hematoxylin and eosin-stained slides were examined to identify
normal and tumor tissue. The tissue sections were produced by
placing single, 3-mm cores of tumor or normal tissue in a recipient
block with manually created holes.
The examined clinicopathological features of the
HCCs included the gender, age, hepatitis infection, tumor size,
tumor-node-metastasis (TNM) stage, histological differentiation of
tumor cells according to the Edmondson and Steiner grading system
(23), presence of liver cirrhosis,
portal vein invasion, hepatic artery invasion, and microvascular
invasion (Table I).
 | Table I.Clinicopathological characteristics
of patients with hepatocellular carcinoma. |
Table I.
Clinicopathological characteristics
of patients with hepatocellular carcinoma.
| Clinicopathological
characteristic | No. of patients
(n=86) |
|---|
| Sex |
|
|
Male | 68 |
|
Female | 18 |
| HBV infection
status |
|
|
(+) | 73 |
|
(−) | 13 |
| AFP level,
ng/ml |
|
|
<500 | 39 |
|
≥500 | 47 |
| Liver cirrhosis
status |
|
|
(+) | 65 |
|
(−) | 21 |
| Size, cm |
|
|
<2 | 1 |
|
≤2–5 | 23 |
|
≤5–10 | 39 |
|
≤10 | 23 |
| Edmondson
grade |
|
| I | 23 |
| II | 33 |
|
III | 26 |
| IV | 4 |
| Microvascular
invasion status |
|
|
(+) | 4 |
|
(−) | 82 |
| Portal vein
invasion status |
|
|
(+) | 22 |
|
(−) | 64 |
| TNM stage |
|
| I | 21 |
| II | 31 |
|
III | 29 |
| IV | 5 |
Immunohistochemical staining
Formalin-fixed paraffin-embedded sections of tumor
tissue that were obtained from the resected liver specimens of
patients with HCC were cut into 3-µm thick sections. The specific
antibodies used, sources, dilutions and detection system
(GTVisionTM III Detection System/Mo&Rb; cat. no. GK500710;
Dako; Agilent Technologies, Inc., Santa Clara, CA, USA) are listed
in Table II. The detection system
included three major reagents: Horseradish peroxidase-labeled
secondary antibody (anti-rabbit/mouse), 3,3′-diaminobenzidine (DAB)
buffer diluent and DAB stock solution (a dye). Antigen retrieval
was performed with citrate buffer. Sections were placed in citrate
buffer (pH 6.0), heated at 95°C for 7 min, removed from the heat
for 10 min and subsequently heated at 95°C for 7 min. Sections were
left at room temperature to cool and washed three times with PBS
(10 mM, pH 7.5±0.1) for 3 min each. The sections were
deparaffinized in xylene, and the xylene was subsequently removed
using absolute ethanol. Endogenous peroxidase activity was blocked
by treatment with 0.3% hydrogen peroxide for 10 min and normal goat
serum (CWBIO, Beijing, China; catalog no. CW0130) for 1 h at room
temperature. The sections were then incubated with the primary
antibodies overnight at 4°C. Then, sections were incubated with
secondary antibodies for 1 h in room temperature.
3,3′-Diaminobenzidine tetrahydrochloride was used as a chromogen,
and Mayer's hematoxylin counterstain was applied. Omission of
primary antibody was used as a negative control. The distribution
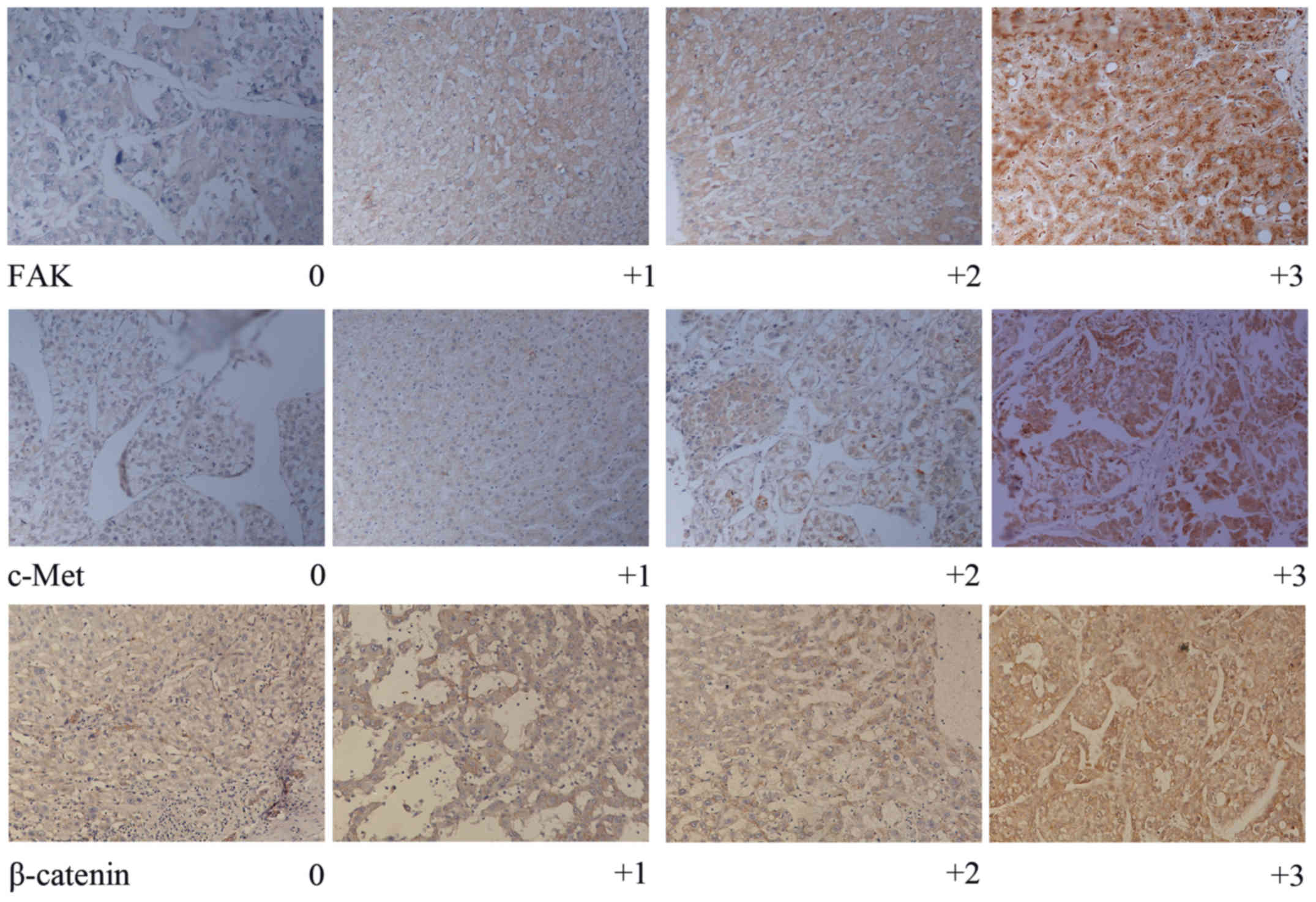
of staining for FAK, c-Met and β-catenin was semi-quantitatively
assessed by the percentage of positively stained cells. Staining
for FAK, c-Met and β-catenin was categorized into four groups as
follows: 0 (<5%), 1+ (6–25%), 2+ (26–50%) and 3+ (>50%)
(Fig. 1). Two observers independently
evaluated the staining results, and interpretation differences were
resolved by consensus. Olympus CX23 light microscope (Olympus
Corporation, Tokyo, Japan) was used (magnification, ×200).
 | Table II.Primary antibodies used for
immunohistochemical analysis. |
Table II.
Primary antibodies used for
immunohistochemical analysis.
| Antibody | Source | Catalogue no. | Dilution |
|---|
| Anti-focal adhesion
kinase | Epitomics (Abcam,
Cambridge, UK) | ab6094 | 1:300 |
| Rabbit anti-c-Met
mAb | Abcam | ab51067 | 1:250 |
| Mouse
anti-β-catenin mAb | BD Biosciences
(Franklin Lakes, NJ, USA) | 610153 | 1:400 |
Statistical analysis
The SPSS statistical software (version 21; IBM
Corp., Armonk, NY, USA) was used in the present study. The Fisher's
exact test was used for categorical variables, and the logistic
regression model was used for multivariate analysis. Cumulative
overall survival and disease-free survival curves were constructed
using the Kaplan-Meier estimator method, and survival curves were
calculated using the log-rank test. Correlation factors that were
identified as statistically significant were included in the Cox's
multiple regression model. Correlation analyses were used to
evaluate the correlation among the three molecular markers.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Immunohistochemical expression of
FAK
High FAK expression was observed in 39/86 HCC cases
and low expression was observed in 47/86 HCC cases. The correlation
between the overexpression of all three biomarkers and
clinicopathological parameters of HCC are illustrated in Table III. Univariate analysis identified a
significant difference between FAK overexpression and other
clinicopathological factors such as the Edmondson grade, TNM stage
and portal vein invasion (all P<0.001). Logistic analysis
revealed a positive correlation between FAK expression and
Edmondson grade (P=0.002; Table
IV).
 | Table III.Correlation between molecular marker
overexpression and the clinicopathological characteristics of
patients with hepatocellular carcinoma. |
Table III.
Correlation between molecular marker
overexpression and the clinicopathological characteristics of
patients with hepatocellular carcinoma.
|
| FAK | β-catenin | c-Met |
|---|
|
|
|
|
|
|---|
| Clinicopathological
characteristic | L (n=47) | H (n=39) | P-value | L (n=34) | H (n=52) | P-value | L (n=32) | H (n=54) | P-value |
|---|
| Sex |
|
| 0.249 |
|
| 0.632 |
|
| 0.702 |
|
Male | 35 | 33 |
| 26 | 42 |
| 26 | 42 |
|
|
Female | 12 | 6 |
| 8 | 10 |
| 6 | 12 |
|
| Age, years |
|
| 0.647 |
|
| 0.968 |
|
| 0.129 |
|
≤60 | 38 | 33 |
| 28 | 43 |
| 29 | 42 |
|
|
>60 | 9 | 6 |
| 6 | 9 |
| 3 | 12 |
|
| Size, cm |
|
| 0.164 |
|
| <0.001 |
|
| 0.012 |
|
<3 | 16 | 8 |
| 17 | 7 |
| 14 | 10 |
|
| ≤3 | 31 | 31 |
| 17 | 45 |
| 18 | 44 |
|
| HBV infection
status |
|
| 0.588 |
|
| <0.001 |
|
| 0.919 |
|
(−) | 8 | 5 |
| 12 | 1 |
| 5 | 8 |
|
|
(+) | 39 | 34 |
| 22 | 51 |
| 27 | 46 |
|
| AFP level
ng/ml |
|
| 0.314 |
|
| 0.130 |
|
| 0.014 |
|
<500 | 28 | 19 |
| 22 | 25 |
| 23 | 24 |
|
|
≥500 | 19 | 20 |
| 12 | 27 |
| 9 | 30 |
|
| Edmondson
grade |
|
| <0.001 |
|
| <0.001 |
|
| 0.001 |
|
I/II | 41 | 15 |
| 30 | 26 |
| 28 | 28 |
|
|
III/IV | 6 | 24 |
| 4 | 26 |
| 4 | 26 |
|
| TMN stage |
|
| <0.001 |
|
| 0.001 |
|
| 0.010 |
|
I/II | 39 | 13 |
| 28 | 24 |
| 25 | 27 |
|
|
III/IV | 8 | 26 |
| 6 | 28 |
| 7 | 27 |
|
| Portal vein
invasion status |
|
| <0.001 |
|
| 0.018 |
|
| 0.032 |
|
(−) | 42 | 22 |
| 30 | 34 |
| 28 | 36 |
|
|
(+) | 5 | 17 |
| 4 | 18 |
| 4 | 18 |
|
| Cirrhosis
status |
|
| 0.810 |
|
| 0.058 |
|
| 0.346 |
|
(−) | 11 | 10 |
| 12 | 9 |
| 6 | 15 |
|
|
(+) | 36 | 29 |
| 22 | 43 |
| 26 | 39 |
|
| Microvascular
invasion status |
|
| 0.848 |
|
| 0.098 |
|
| 0.605 |
|
(−) | 45 | 37 |
| 34 | 48 |
| 31 | 51 |
|
|
(+) | 2 | 2 |
| 0 | 4 |
| 1 | 3 |
|
 | Table IV.Logistic analysis for the correlation
between the overexpression of molecular markers and the
clinicopathological parameters of patients with hepatocellular
carcinomaa. |
Table IV.
Logistic analysis for the correlation
between the overexpression of molecular markers and the
clinicopathological parameters of patients with hepatocellular
carcinomaa.
| A, Correlation
between FAK expression and Edmonson grade |
|---|
| Variable | B | SE | Wald | df | P-value | OR |
|---|
| Edmonson grade | 1.872 | 0.600 | 9.735 | 1 | 0.002 | 6.500 |
| Constant | −1.872 | 0.758 | 6.102 | 1 | 0.014 | 0.154 |
|
| B, Correlation
between β-catenin expression and clinicopathological
characteristics |
|
|
Variable | B | SE | Wald | df | P-value | OR |
|
| Edmonson grade | 2.242 | 0.813 | 7.597 | 1 | 0.006 | 9.408 |
| Tumor size | 1.635 | 0.624 | 6.875 | 1 | 0.009 | 5.132 |
| HBV infection | 3.787 | 1.247 | 9.224 | 1 | 0.002 | 44.139 |
| Constant | −8.521 | 2.094 | 16.567 | 1 | <0.001 | <0.001 |
Immunohistochemical expression of
c-Met
High c-Met expression was observed in 54/86 HCC
cases, and low expression was observed in 32/86 HCC cases.
Univariate analysis identified significant correlations between
c-Met overexpression, and portal vein invasion (P=0.032), tumor
size (P=0.012), α-fetoprotein (AFP) (P=0.014), poor histological
differentiation (Edmondson grade) (P=0.001) and TNM stage
(P=0.010). However, multivariate analysis identified no significant
correlation between c-Met overexpression and clinicopathological
factors.
Immunohistochemical expression of
β-catenin
High β-catenin expression was observed in 52/86 HCC
cases, and low expression was observed in 34/86 HCC cases.
Univariate analysis identified significant correlations between
β-catenin overexpression, and tumor size (P<0.001), hepatitis B
virus (HBV) infection (P<0.001), poor histological
differentiation (Edmondson grade) (P<0.001) and TNM stage
(P=0.001). Multivariate analysis identified significant
correlations between β-catenin overexpression, and HBV infection
(P=0.002), Edmondson grade (P=0.006) and tumor size (P=0.009)
(Table IV).
Survival rates, biomarkers and
clinicopathological profiles Clinicopathological factors
The differences in survival rates of patients with
mild expression or overexpression of the three biomarkers were
evaluated. The Kaplan-Meier survival curves for ≤60 months of
follow-up are shown in Fig. 2. The
Kaplan-Meier survival for patients with Edmondson grade III/IV vs.
Edmondson grade I/II was significantly correlated with the survival
period (mean survival period, 18 vs. 34 months; P<0.001;
Fig. 2A). In addition, the TNM stage
(mean survival period, 19 vs. 35 months; P<0.001; Fig. 2B), AFP levels (mean survival period,
24 vs. 32 months; P=0.038; Fig. 2C),
portal vein invasion (mean survival period, 18 vs. 32 months;
P=0.001; Fig. 2D) and age (mean
survival period, 21 vs. 30 months; P=0.043; Fig. 2E) were all identified to possess
significant prognostic value. Thus, Edmondson grade, AFP levels,
age, TNM stage and portal vein invasion were negatively correlated
with the survival period.
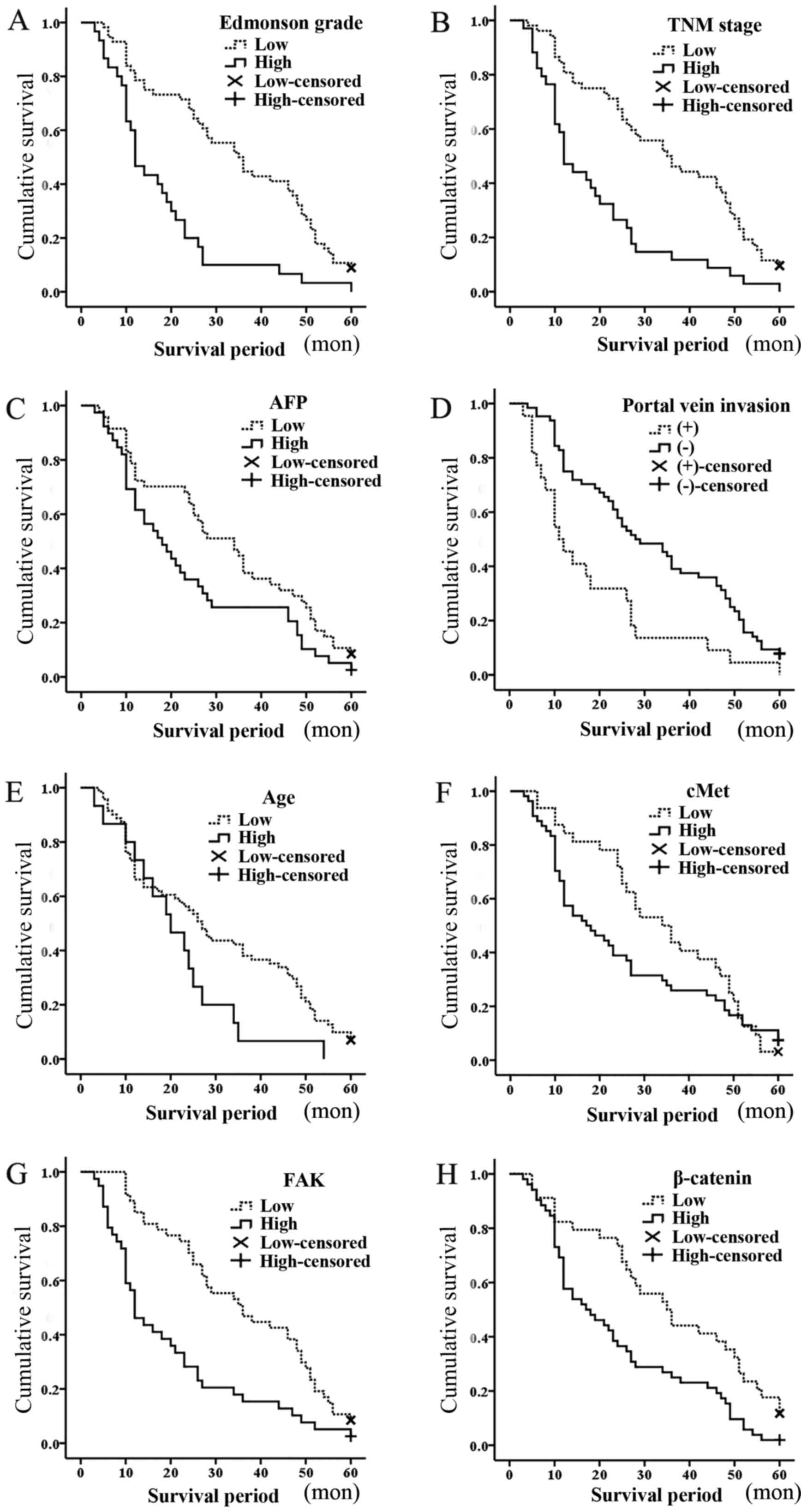 | Figure 2.Kaplan-Meier survival curves for
patients with hepatocellular carcinoma over 60 months: (A) Mean
survival period (months), 18 vs. 34 for Edmonson grade III/IV vs.
Edmondson grade I/II, respectively; P<0.001. (B) Mean survival
period (months), 19 vs. 35 for TNM stage III/IV vs. TNM stage I/II,
respectively; P<0.001. (C) Mean survival period (months), 24 vs.
32 for AFP levels high (≥500 ng/ml) vs. low (<500 ng/ml),
respectively; P=0.038. (D) Mean survival period (months), 18 vs. 32
for portal vein invasion, positive vs. negative, respectively;
P=0.001. (E) Mean survival period (months), 21 vs. 30 for >60
vs. ≤60 years, respectively; P=0.043. (F) Mean survival period
(months), 25 vs. 34 for c-Met high expression vs. low expression,
respectively; P=0.254; (G) Mean survival period (months), 20 vs. 36
for FAK high expression vs. low expression, respectively;
P<0.001. (H) Mean survival period (months), 24 vs. 36 for
β-catenin high expression vs. low expression, respectively;
P=0.003. Censored refers to the state of the patient at follow-up,
whereby 0 means censored and 1 means mortality.; high, high
expression; low, low expression; TNM, tumor-node-metastasis; AFP,
α-fetoprotein; FAK, focal adhesion kinase; c-Met, hepatocyte growth
factor receptor. |
FAK, β-catenin and c-Met
The different survival rates of the patients with
mild or over expression of biomarkers were evaluated. No
significant correlations were identified between c-Met expression
and overall survival rates (mean survival period, 25 vs. 34 months;
P=0.254; Fig. 2F). The Kaplan-Meier
survival for patients with high vs. low expression of FAK (mean
survival period, 20 vs. 36 months; P<0.001; Fig. 2G) and β-catenin (mean survival period,
24 vs. 36 months; P=0.003; Fig. 2H)
was demonstrated to be significantly different. Cox's regression
analysis demonstrated that FAK (P=0.004), β-catenin (P=0.036), age
(P=0.007) and AFP (P=0.027) were significantly associated with the
survival period, whereas, Edmondson grade (P=0.083) was markedly
associated with the survival period (Table V). High FAK expression exhibited a
~2.107-fold higher risk of mortality compared with that of low FAK
expression. High β-catenin expression exhibited a 1.690-fold higher
risk of mortality compared with that of low expression. Other
factors that influenced the survival period included the age
(2.295-fold), AFP levels (1.699-fold) and Edmondson grade
(1.632-fold). However, the results of the present study for age as
a prognostic factor have yet to be discussed, because it has been
demonstrated that the tumor grows at an increased pace in younger
people compared with older people in other types of tumor (24–26).
 | Table V.Cox's regression analysis of
parameters in patients with hepatocellular carcinoma. |
Table V.
Cox's regression analysis of
parameters in patients with hepatocellular carcinoma.
| Parameter | B | SE | Wald | df | P-value | OR |
|---|
| FAK expression | 0.745 | 0.262 | 8.082 | 1 | 0.004 | 2.107 |
| β-catenin
expression | 0.525 | 0.250 | 4.389 | 1 | 0.036 | 1.690 |
| Age | 0.831 | 0.306 | 7.372 | 1 | 0.007 | 2.295 |
| AFP level | 0.530 | 0.239 | 4.918 | 1 | 0.027 | 1.699 |
| Edmonson grade | 0.490 | 0.283 | 2.998 | 1 | 0.083 | 1.632 |
Correlation between FAK, β-catenin and
c-Met expression
Following univariate analysis (Table VI), no significant difference was
identified between FAK and β-catenin expression, while a marked
difference was demonstrated between c-Met and β-catenin expression
(P=0.068). However, FAK expression was significantly different
compared with c-Met expression (P=0.015) in 86 patients with HCC.
Following correlation analysis (Table
VII), β-catenin was identified to be markedly correlated with
c-Met expression (P=0.052; Pearson's r=0.21), but the other
comparison, including c-Met vs. FAK (P=0.855; Pearson's r=0.020)
and FAK vs. β-catenin (P=0.164; Pearson's r=0.151), lacked an
identifiable outcome.
 | Table VI.Correlation between β-catenin, FAK
and c-Met expression in patients with hepatocellular carcinoma. |
Table VI.
Correlation between β-catenin, FAK
and c-Met expression in patients with hepatocellular carcinoma.
| A, Correlation
between β-catenin and FAK expression (n=86) |
|---|
|
|---|
|
| β-catenin |
|---|
|
|
|
|---|
| Variable | L (n=34) | H (n=52) | P-value |
|---|
| FAK expression |
|
| 0.376 |
| L (n=47) | 21 | 26 |
|
| H (n=39) | 13 | 26 |
|
|
| B, Correlation
between β-catenin and c-Met expression (n=86) |
|
|
|
β-catenin |
|
|
|
|
Variable | L
(n=34) | H
(n=52) | P-value |
| c-Met
expression |
|
| 0.068 |
| L (n=32) | 17 | 15 |
|
| H (n=54) | 17 | 37 |
|
|
| C, Correlation
between c-Met and FAK expression (n=86) |
|
|
| c-Met |
|
|
|
|
Variable | L
(n=34) | H
(n=52) | P-value |
| FAK expression |
|
| 0.015 |
| L (n=47) | 23 | 24 |
|
| H (n=39) | 9 | 30 |
|
 | Table VII.Correlation between molecular markers
in patients with hepatocellular carcinoma. |
Table VII.
Correlation between molecular markers
in patients with hepatocellular carcinoma.
|
| Molecular
marker |
|---|
|
|
|
|---|
| Molecular
marker | c-Met | β-catenin | FAK |
|---|
| c-Met |
|
|
|
|
Pearson's r | 1 | 0.210 | 0.020 |
| P-value
(2-tailed) |
| 0.052 | 0.855 |
| No. of
patients | 86 | 86 | 86 |
| β-catenin |
|
|
|
|
Pearson's r | 0.210 | 1 | 0.151 |
| P-value
(2-tailed) | 0.052 |
| 0.164 |
| No. of
patients | 86 | 86 | 86 |
| FAK |
|
|
|
|
Pearson's r | 0.020 | 0.151 | 1 |
| P-value
(2-tailed) | 0.855 | 0.164 |
|
| No. of
patients | 86 | 86 | 86 |
Discussion
A variety of molecular events have been identified
to be involved in the etiopathogenesis of HCC, including
insulin-like growth factors, hepatocyte growth factor and the
Wnt/β-catenin signaling axis (27,28).
Specific signaling pathways include Wnt, insulin like growth factor
and mechanistic target of rapamycin (29–33).
Previous studies identified an association between c-Met and
β-catenin expression in hepatocarcinogenesis using in vivo
models (12,13,22). Shang
et al (3) demonstrated that
their effects may be regulated by FAK. As the correlation between
the three oncoproteins has been established in animal models, their
potential prognostic value in patients with HCC was investigated in
the present study. Firstly, the correlation between the
clinicopathological features of patients with HCC and the
expression of the three oncoproteins was assessed. Kaplan-Meier
survival and Cox's regression analyses were used to assess their
correlation with the survival period. The correlations between the
three molecular markers were also analyzed.
FAK is a cytoplasmic protein tyrosine kinase
(34). Previous studies have
identified FAK overexpression and activation in several
advanced-stage solid cancer types (12,19).
Furthermore, cancer and stromal cells have been demonstrated to be
affected by FAK in the process of carcinogenesis; for example,
integrin-FAK signaling activated a number of signaling pathways via
phosphorylation and protein-protein interactions to promote
tumorigenesis (9–11). In the present study, FAK expression
was revealed to be significantly different compared with different
Edmondson grades (P<0.001), different TNM stages (P<0.001)
and patients with or without portal vein invasion (P<0.001).
Logistic analysis demonstrated a significant correlation between
FAK and Edmondson grade (P=0.002). Following Kaplan-Meier survival
curve analysis, different survival rates were identified between
subjects with high and low FAK expression (mean survival period, 20
vs. 36 months; P<0.001). Furthermore, Cox's regression analysis
revealed a correlation between FAK expression and the survival
period; it appears that patients with high FAK expression have a
~2.107-fold higher risk of mortality compared with that of patients
with low FAK expression (P=0.004). Thus, the results of the present
study suggest that FAK is correlated with certain
clinicopathological features of patients with HCC. Notably, FAK
expression was identified to be correlated with the survival
period, indicating its potential prognostic value in patients with
HCC.
c-Met tyrosine kinase is a cell surface receptor for
hepatocyte growth factor (HGF) (35).
HGF is secreted by mesenchymal cells that are regenerated in the
liver (35). c-Met activation by HGF
can induce cell scattering, invasion, evasion from apoptosis and
angiogenesis, thus acting as promoter of cancer dissemination
(20). The HGF/c-Met signaling
pathway has been demonstrated to simultaneously activate multiple
signal transduction pathways that promote cancer cell infiltration
(36–41). In addition, genetic mutations of the
c-Met receptor and its overexpression have been reported in various
cancer types, including papillary renal, pulmonary, gastric and
hepatic cancer (36–42). According to the potential role of
c-Met in liver regeneration, its correlation with cirrhosis should
have been easily identified in the present study; however, no
correlation was identified between the two. By contrast, c-Met
expression was significantly different compared with that of
certain tumor-associated factors such as tumor size (P=0.012), AFP
(P=0.014), poor histological differentiation (Edmondson grade)
(P=0.001) and TNM stage (P=0.010). This indicates that c-Met may
possess a more specific role than previously considered. Despite
successful detection of c-Met expression differences in the
specimens, no significant correlation was identified between c-Met
expression and the survival period in the Kaplan-Meier survival
curve or Cox's regression analyses. Considering these results, it
is suggested that the prognostic significance of c-Met in HCC is
limited.
β-catenin protein can be located in the cell
membrane, cytoplasm or nucleus (43).
In inactivated cells, the majority of β-catenin is primarily
located in the membrane and integrated into the adhesion complex,
which is responsible for maintaining cell junctions. The remaining
β-catenin that is free in the cytoplasm binds to a degradation
complex and is degraded. Once the Wnt/β-catenin signaling pathway
is aberrantly activated, membranous expression of β-catenin is
reduced and cytoplasmic degradation of β-catenin is prevented. This
allows free β-catenin to accumulate in the cytoplasm and
translocate to the nucleus, where it interacts with transcription
factors of the T-cell factor/lymphoid enhancer factor family to
regulate various target genes (31).
Numerous studies have reported that β-catenin
overexpression in the cytoplasm and/or nucleus is correlated with
cancer metastasis and poor prognosis (43–51). In
the present study, higher Edmondson grade, poor TNM stage and
positive portal vein invasion cases were identified to have
increased cytoplasmic expression of β-catenin. Furthermore,
univariate analysis confirmed a significant difference and
correlation between β-catenin overexpression, and poor histological
differentiation, Edmondson grade (P<0.001) and TNM stage
(P=0.001). Following multivariate analysis, a significant
correlation was identified between β-catenin overexpression and
Edmondson grade (P=0.006). Other clinicopathological factors such
as tumor size (P<0.001) and HBV infection (P<0.001) were
identified to be significantly associated with β-catenin
expression. β-catenin demonstrated a significant correlation with
the survival period according to the Kaplan-Meier survival curve
(24 vs. 36%; P=0.003) and Cox's regression (P=0.036) analyses.
Patients with high β-catenin expression exhibited a 1.690-fold
higher risk of mortality compared with that of patients with low
β-catenin expression. The results of the present study suggest that
β-catenin may be correlated with specific clinicopathological
factors and survival period of patients with HCC. Thus, β-catenin
may potentially be used as a prognostic factor for HCC.
The clinicopathological factors investigated in the
current study are generally considered to be correlated with HCC,
including sex, HBV infection, liver cirrhosis, tumor size, portal
vein invasion, microvascular invasion, Edmondson grade and TNM
stage (23,50,51). In
the present study, the correlation between these factors and the
expression of the three oncoproteins was investigated; in addition,
their correlation with survival period was verified. Based on the
results obtained, the majority of factors were consistent with
general considerations, such as portal vein invasion, Edmondson
grade and TNM stage possessing a negative correlation with the
survival period in the Kaplan-Meier survival curve analysis, and
Edmondson grade serving as an independent risk factor in Cox's
regression analysis. However, certain factors, such as age and HBV
infection demonstrated controversial outcomes that require further
analysis. In the present study, different β-catenin expression
levels were identified in patients that had been infected by HBV
vs. non-infected patients. To the best of our knowledge, no
correlation between HBV infection and β-catenin expression has been
previously identified. The HBV infection rate among the
participants in the present study was relatively high (73/86; 85%),
and as only 13 patients were HBV-negative, there may have been
selective bias.
Although significant correlations were identified
between the three oncoproteins and certain clinicopathological
factors, no significant correlation was identified among the three
oncoproteins themselves following logistic analysis. Thus, further
studies on the three molecular markers were performed. Firstly, the
differences between each set of two markers were analyzed, and
univariate analysis demonstrated a significant difference between
FAK and β-catenin expression (P=0.015), and a marked difference
between c-Met and β-catenin expression (P=0.068). Following
correlation analysis amongst the three molecular markers, no
significant correlation was identified between any pair; however,
β-catenin was identified to be correlated with c-Met (P=0.052;
Pearson's r=0.21). The established association with c-Met and
β-catenin in hepatocarcinogenesis, identified previously (1,10), was
consistent with the results of the present study. Although
significant differences between FAK and β-catenin expression were
established, their correlation with β-catenin and c-Met cannot be
concluded.
Other established criteria, including poor
histological differentiation (Edmondson grade and TNM stage), were
in agreement with the general consensus (23,52–54). Apart
from different statistical results of c-Met, FAK and β-catenin, FAK
and β-catenin performed well according to a variety of statistical
indicators in the present study. As prognostic predictors, FAK and
β-catenin were effective in the present study; however,
coeffectiveness of c-Met and β-Catenin or their regulation by FAK
was not observed in the present study (3,12,22).
Certain clinicopathological factors such as tumor
size, cirrhosis and microvascular invasion were not identified to
be correlated with a high expression of the FAK, β-catenin and
c-Met. TNM stage, which has been previously demonstrated to be
correlated with the survival period, was excluded from the Cox's
regression analysis (23,50,51).
Although the association between c-Met and β-catenin expression in
HCC was not identified to be significant in the present study,
their association should be studied further.
In conclusion, c-Met demonstrated conflicting
results regarding its correlation with the clinicopathology and
survival period of patients with HCC. As a result, its prognostic
value cannot be confirmed by the results of the present study.
c-Met displayed variable expression in 86 patients, and was
expressed in 54/86 (62.8%) HCC cases. Although no correlation was
identified between the three oncoproteins, FAK and β-catenin
demonstrated promising results regarding their correlations with
clinicopathological characteristics and survival period of patients
with HCC. The results of the current study suggest that FAK and
β-catenin may be used as prognosis markers of HCC. Considering that
single agent or combined chemotherapy were used as treatment for
all patients following surgery, and the results of the present
study on the prognostic value of FAK and β-catenin, more aggressive
treatments such as TACE, PEI or RFA may be required post-surgery
for patients with high FAK and β-catenin expression.
Acknowledgements
This work was supported by grants from the National
Natural Science Foundation of China (grant nos: 81472253, 81472258,
81572424, 81101862, 81172079, 81072047 and 81201930); the Project
of Guangzhou Municipal Science & Technology Planning (grant
nos: 201607010309 and 201607010164) and the Science and Technology
Planning Project of Guangdong Province (grant nos: 2016A020215047,
2017A020215016, 2014A020212083).
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kim KH and Choi YK: Long-term survival
after resection of hepatocellular carcinoma. Korean J Hepatobiliary
Pancreat Surg. 16:98–104. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shang N, Arteaga M, Zaidi A, Stauffer J,
Cotler SJ, Zeleznik-Le NJ, Zhang J and Qiu W: FAK is required for
c-Met/β-catenin-driven hepatocarcinogenesis. Hepatology.
61:214–226. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bruix J and Sherman M: Practice Guidelines
Committee, American Association for the Study of Liver Diseases:
Management of hepatocellular carcinoma. Hepatology. 42:1208–1236.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Clevers H: Wnt/beta-catenin signaling in
development and disease. Cell. 127:469–480. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao X and Guan JL: Focal adhesion kinase
and its signaling pathways in cell migration and angiogenesis. Adv
Drug Deliv Rev. 63:610–615. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Llovet JM, Ricci S, Mazzaferro V, Hilgard
P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A,
et al: Sorafenib in advanced hepatocellular carcinoma. N Engl J
Med. 359:378–390. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mitra SK, Hanson DA and Schlaepfer DD:
Focal adhesion kinase: In command and control of cell motility. Nat
Rev Mol Cell Biol. 6:56–68. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schaller MD: Cellular functions of FAK
kinases: Insight into molecular mechanisms and novel functions. J
Cell Sci. 123:1007–1013. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhao J and Guan JL: Signal transduction by
focal adhesion kinase in cancer. Cancer Metastasis Rev. 28:35–49.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Parsons JT: Focal adhesion kinase: The
first ten years. J Cell Sci. 116:1409–1416. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Patil MA, Lee SA, Macias E, Lam ET, Xu C,
Jones KD, Ho C, Rodriguez-Puebla M and Chen X: Role of cyclin D1 as
a mediator of c-Met and beta-catenin induced hepatocarcinogenesis.
Cancer Res. 69:253–261. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tward AD, Jones KD, Yant S, Cheung ST, Fan
ST, Chen X, Kay MA, Wang R and Bishop JM: Distinct pathways of
genomic progression to benign and malignant tumors of the liver.
Proc Natl Acad Sci USA. 104:pp. 14771–14776. 2007; View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schlaepfer DD, Hauck CR and Sieg DJ:
Signaling through focal adhesion kinase. Prog Biophys Mol Biol.
71:435–478. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pylayeva Y, Gillen KM, Gerald W, Beggs HE,
Reichardt LF and Giancotti FG: Ras- and PI3K-dependent breast
tumorigenesis in mice and humans requires focal adhesion kinase
signaling. J Clin Invest. 119:252–266. 2009.PubMed/NCBI
|
|
16
|
Ashton GH, Morton JP, Myant K, Phesse TJ,
Ridgway RA, Marsh V, Wilkins JA, Athineos D, Muncan V, Kemp R, et
al: Focal adhesion kinase is required for intestinal regeneration
and tumorigenesis downstream of Wnt/c-Myc signaling. Dev Cell.
19:259–269. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee J, Borboa AK, Chun HB, Baird A and
Eliceiri BP: Conditional deletion of the focal adhesion kinase FAK
alters remodeling of the blood-brain barrier in glioma. Cancer Res.
70:10131–10140. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Itoh S, Maeda T, Shimada M, Aishima S,
Shirabe K, Tanaka S and Maehara Y: Role of expression of focal
adhesion kinase in progression of hepatocellular carcinoma. Clin
Cancer Res. 10:2812–2817. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fujii T, Koshikawa K, Nomoto S, Okochi O,
Kaneko T, Inoue S, Yatabe Y, Takeda S and Nakao A: Focal adhesion
kinase is overexpressed in hepatocellular carcinoma and can be
served as an independent prognostic factor. J Hepatol. 41:104–111.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kaposi-Novak P, Lee JS, Gòmez-Quiroz L,
Coulouarn C, Factor VM and Thorgeirsson SS: Met-regulated
expression signature defines a subset of human hepatocellular
carcinomas with poor prognosis and aggressive phenotype. J Clin
Invest. 116:1582–1595. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cieply B, Zeng G, Proverbs-Singh T, Geller
DA and Monga SP: Unique phenotype of hepatocellular cancers with
exon-3 mutations in beta-catenin gene. Hepatology. 49:821–831.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Stauffer JK, Scarzello AJ, Andersen JB, De
Kluyver RL, Back TC, Weiss JM, Thorgeirsson SS and Wiltrout RH:
Coactivation of AKT and β-catenin in mice rapidly induces formation
of lipogenic liver tumors. Cancer Res. 71:2718–2727. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Edmondson HA and Steiner PE: Primary
carcinoma of the liver: A study of 100 cases among 48,900
necropsies. Cancer. 7:462–503. 1954. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Calabrese CT, Adam YG and Volk H:
Geriatric colon cancer. Am J Surg. 125:181–184. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ershler WB, Socinski MA and Greene CJ:
Bronchogenic cancer, metastases and aging. J Am Geriatr Soc.
31:673–676. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ershler WB: Why tumors grow more slowly in
old people. J Natl Cancer Inst. 77:837–839. 1986.PubMed/NCBI
|
|
27
|
Breuhahn K, Longerich T and Schirmacher P:
Dysregulation of growth factor signaling in human hepatocellular
carcinoma. Oncogene. 25:3787–3800. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Farazi PA and DePinho RA: Hepatocellular
carcinoma pathogenesis: From genes to environment. Nat Rev Cancer.
6:674–687. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tabor E: Tumor suppressor genes, growth
factor genes, and oncogenes in hepatitis B virus-associated
hepatocellular carcinoma. J Med Virol. 42:357–365. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rogler CE and Chisari FV: Cellular and
molecular mechanisms of hepatocarcinogenesis. Semin Liver Dis.
12:265–278. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sung CO, Yoo BC, Koh KC, Cho JW and Park
CK: Prognostic significance of p53 overexpression after hepatic
resection of hepatocellular carcinoma. Korean J Gastroenterol.
45:425–430. 2005.PubMed/NCBI
|
|
32
|
Pang RW and Poon RT: From molecular
biology to targeted therapies for hepatocellular carcinoma: The
future is now. Oncology. 72 Suppl 1:S30–S44. 2007. View Article : Google Scholar
|
|
33
|
Llovet JM and Bruix J: Molecular targeted
therapies in hepatocellular carcinoma. Hepatology. 48:1312–1327.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
André E and Becker-André M: Expression of
an N-terminally truncated form of human focal adhesion kinase in
brain. Biochem Biophys Res Commun. 190:140–147. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fausto N, Campbell JS and Riehle KJ: Liver
regeneration. Hepatology. 43(2 Suppl 1): S45–S53. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Schmidt L, Duh FM, Chen F, Kishida T,
Glenn G, Choyke P, Scherer SW, Zhuang Z, Lubensky I, Dean M, et al:
Germline and somatic mutations in the tyrosine kinase domain of the
MET proto-oncogene in papillary renal carcinomas. Nat Genet.
16:68–73. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Schmidt L, Junker K, Weirich G, Glenn G,
Choyke P, Lubensky I, Zhuang Z, Jeffers M, Vande Woude G, Neumann
H, et al: Two North American families with hereditary papillary
renal carcinoma and identical novel mutations in the MET
proto-oncogene. Cancer Res. 58:1719–1722. 1998.PubMed/NCBI
|
|
38
|
Park WS, Dong SM, Kim SY, Na EY, Shin MS,
Pi JH, Kim BJ, Bae JH, Hong YK, Lee KS, et al: Somatic mutations in
the kinase domain of the Met/hepatocyte growth factor receptor gene
in childhood hepatocellular carcinomas. Cancer Res. 59:307–310.
1999.PubMed/NCBI
|
|
39
|
Ma PC, Kijima T, Maulik G, et al: c-MET
mutational analysis in small cell lung cancer: Novel juxtamembrane
domain mutations regulating cytoskeletal functions. Cancer Res.
63:6272–6281. 2003.PubMed/NCBI
|
|
40
|
Ma PC, Jagdeesh S, Jagadeeswaran R, Fox
EA, Christensen J, Maulik G, Naoki K, Schaefer E, Lader A, Richards
W, et al: c-MET expression/activation, functions and mutations in
non-small cell lung cancer. Proc Am Assoc Cancer Res. 44:pp.
18752004;
|
|
41
|
Lee JH, Han SU, Cho H, Jennings B, Gerrard
B, Dean M, Schmidt L, Zbar B and Vande Woude GF: A novel germ line
juxtamembrane Met mutation in human gastric cancer. Oncogene.
19:4947–4953. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Christensen JG, Burrows J and Salgia R:
c-Met as a target for human cancer and characterization of
inhibitors for therapeutic intervention. Cancer Lett. 225:1–26.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chen Z, He X, Jia M, Liu Y, Qu D, Wu D, Wu
P, Ni C, Zhang Z, Ye J, et al: β-catenin overexpression in the
nucleus predicts progress disease and unfavourable survival in
colorectal cancer: A meta-analysis. PLoS One. 8:e638542013.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Gough NR: Focus issue: Wnt and
beta-catenin signaling in development and disease. Sci Signal.
5:eg22012.PubMed/NCBI
|
|
45
|
Inagawa S, Itabashi M, Adachi S, Kawamoto
T, Hori M, Shimazaki J, Yoshimi F and Fukao K: Expression and
prognostic roles of beta-catenin in hepatocellular carcinoma:
Correlation with tumor progression and postoperative survival. Clin
Cancer Res. 8:450–456. 2002.PubMed/NCBI
|
|
46
|
Liu L, Zhu XD, Wang WQ, Shen Y, Qin Y, Ren
ZG, Sun HC and Tang ZY: Activation of beta-catenin by hypoxia in
hepatocellular carcinoma contributes to enhanced metastatic
potential and poor prognosis. Clin Cancer Res. 16:2740–2750. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lee JM, Yang J, Newell P, Singh S, Parwani
A, Friedman SL, Nejak-Bowen KN and Monga SP: β-Catenin signaling in
hepatocellular cancer: Implications in inflammation, fibrosis, and
proliferation. Cancer Lett. 343:90–97. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Jin J, Jung HY, Wang Y, Xie J, Yeom YI,
Jang JJ and Lee KB: Nuclear expression of phosphorylated TRAF2- and
NCK-interacting kinase in hepatocellular carcinoma is associated
with poor prognosis. Path Res Pract. 210:621–627. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zulehner G, Mikula M, Schneller D, van
Zijl F, Huber H, Sieghart W, Grasl-Kraupp B, Waldhör T,
Peck-Radosavljevic M, Beug H and Mikulits W: Nuclear beta-catenin
induces an early liver progenitor phenotype in hepatocellular
carcinoma and promotes tumor recurrence. Am J Pathol. 176:472–481.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Liver cancer study group of Japan, . The
general rules for the clinical and pathological study of primary
liver cancer. 3rd. Kanehara Shuppan; Tokyo: 1992
|
|
51
|
Sumie S, Kuromatsu R, Okuda K, Ando E,
Takata A, Fukushima N, Watanabe Y, Kojiro M and Sata M:
Microvascular invasion in patients with hepatocellular carcinoma
and its predictable clinicopathological factors. Ann Surg Oncol.
15:1375–1382. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Minagawa M, Ikai I, Matsuyama Y, Yamaoka Y
and Makuuchi M: Staging of hepatocellular carcinoma: Assessment of
the Japanese TNM and AJCC/UICC TNM systems in a cohort of 13,772
patients in Japan. Ann Surg. 245:909–922. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Henderson J, Sherman M, Tavill A,
Abecassis M, Chejfec G and Gramlich T: AHPBA/AJCC consensus
conference on staging of hepatocellular carcinoma: Consensus
statement. HPB(Oxford). 5:243–250. 2003.PubMed/NCBI
|
|
54
|
Leung TW, Tang AM, Zee B, Lau WY, Lai PB,
Leung KL, Lau JT, Yu SC and Johnson PJ: Construction of the Chinese
University Prognostic Index for hepatocellular carcinoma and
comparison with the TNM staging system, the Okuda staging system,
and the Cancer of the Liver Italian Program staging system: A study
based on 926 patients. Cancer. 94:1760–1769. 2002. View Article : Google Scholar : PubMed/NCBI
|