Introduction
Esophageal cancer is the eighth most common type of
cancer worldwide and the sixth leading cause of cancer-related
mortality (1). Squamous-cell
carcinoma is the predominant histological type, however in the USA,
and Western European countries the incidence of esophageal
adenocarcinoma (EAC) is steadily rising and exceeds that of
esophageal squamous-cell carcinoma (ESCC) (2–6).
State-of-the-art treatment algorithms for esophageal cancer consist
of multidisciplinary approaches, including surgical resection,
combinatory chemo- and radiotherapy, as well as endoscopic
procedures (7,8). However, despite aggressive and
multimodal treatment concepts the prognosis of patients with
esophageal cancer remains disappointing, with overall 5-year
survival rates of approximately 20% (9). Poor outcome in esophageal cancer
patients is particularly related to late diagnosis at advanced
stages of the disease and high rates of cancer recurrence even
after an adequate initial therapy with curative intend (9–11).
Unfortunately, the efficacy of current chemo- and radiotherapy
regimens has been largely exhausted and further intensification is
predominantly associated with an increase in undesirable systemic
toxicity. To overcome the difficulty of adverse effects, novel
therapeutic concepts focus on the development of targeted
anticancer therapies that specifically inhibit aberrant molecular
pathways triggered by genomic and proteomic alterations in cancer
cells.
In this context, during the last decades the
Inhibitor of apoptosis protein (IAP) family attracted considerable
attention. Considering their overexpression as well as their
association with tumor progression, treatment resistance and poor
prognosis in various human cancers, IAPs represent promising
targets for cancer therapy. Initially, these proteins were found to
function as endogenous inhibitors of caspases, however today it has
become increasingly clear that IAPs affect additional cellular
functions such as proliferation, migration, invasion and metastasis
(12–14). The two most extensively studied
members of the IAP family are survivin/BIRC5 and X-linked inhibitor
of apoptosis protein (XIAP)/BIRC4 (12,14–19).
Interestingly, survivin and XIAP have been demonstrated to be
important partners accomplishing their antiapoptotic and
pro-metastatic functions by direct interaction (13,20).
Aim of this study was to analyze the expression of
survivin and XIAP in a large number of tissue specimens from
esophageal cancer patients, including primary tumors and tumor
adjacent non-malignant mucosa. Expression levels of both IAPs were
correlated with clinicopathological variables and overall survival
according to the REporting recommendations for tumor MARKer
prognostic studies (REMARK) (21). In
addition, we analyzed the antitumor activity of small molecule
survivin inhibitor YM155 and XIAP inhibitors Birinapant and
GDC-0152 in esophageal cancer cell lines originating from both,
ESCC as well as EAC.
Materials and methods
Patient selection and
clinicopathological data
Previously constructed tissue microarrays (TMA)
containing tissue samples retrieved from human EAC and ESCC were
used to assess survivin and XIAP expression (22). All formalin-fixed and
paraffin-embedded (FFPE) tissue specimens originated from the
Institutes of Pathology of the University Hospitals in Duesseldorf
and Cologne. The patients who had undergone radical en bloc
esophagectomy and lymphadenectomy with curative intent irrespective
of tumor stage and microscopic resection margin at the University
Hospital of Duesseldorf and Cologne between 1986 and 2005 were
included in this study. Exclusion criteria were preoperative
neoadjuvant therapy, macroscopic incomplete resection (R2),
esophageal tumors other than squamous cell carcinoma or
adenocarcinoma and samples with insufficient tumor material. In
addition, 73 tissue samples of tumor adjacent, non-malignant
esophageal mucosa were analyzed for survivin and XIAP expression.
Information on TNM staging (depth of invasion, lymph node and
distant metastasis) as well as grading were retrospectively
obtained from the original pathological reports. Data regarding
overall survival as well as age at the time of surgery and gender
were reviewed. The study was carried out in accordance to Good
Clinical Practice, the Declaration of Helsinki and an Institutional
Review Board (IRB)-approval of the Medical Faculty, Heinrich Heine
University Duesseldorf (IRB-no. 3821) was retrieved.
Immunohistochemistry
Two µm thick sections were cut from each TMA block
and mounted on superfrost microscope slides. Immunohistochemical
staining was performed as recently described (23,24). Two
independent investigators (LD and LMJ) blinded to
clinicopathological information evaluated the expression of
survivin and XIAP using the immunoreactivity score (IRS) according
to Remmele (25). This score is
calculated by multiplying the intensity of staining (0, no
staining; 1, weak staining; 2, strong staining; 3, very strong
staining) with the percentage of positive cells (0, no positive
cells; 1, <10% positive cells; 2, 11–50% positive cells; 3,
51–80% positive cells; 4, 81–100% positive cells). In case of
differing results the samples in question were re-examined by both
observers simultaneously and a consensus decision was made. For
survivin, nuclear and cytoplasmic protein expression were
separately determined. A tissue slide of pretested human colon and
renal cell carcinoma, known to express survivin or XIAP
intensively, served as a positive control. Sections incubated with
isotype control antibodies were used as negative controls.
Cell lines
ESCC cell lines KYSE30, KYSE270, KYSE410 and
KYSE520, established by Shimada et al (26), were obtained from the German
collective of microorganisms and cell cultures (DSMZ, Braunschweig,
Germany). EAC cell lines OE19 and OE33 were acquired from the
European collection of cell cultures (ECACC, Salisbury, UK). All
cell lines were maintained in RPMI medium supplemented with 10%
heat inactivated FCS, penicillin and streptomycin at 37°C in an
atmosphere with 5% CO2. DNA fingerprinting, conducted as
previously described, confirmed that no cross contamination had
occurred (27).
Functional in vitro assays
Cell viability and proliferation were assessed in
96-well culture plates with 2×103 cells per well. After
24 h cells were incubated with YM155, Birinapant, GDC-0152 or
dimethyl sulfoxid (DMSO) vehicle control for 48 h. The CellTiter
96® AQueous Non-Radioactive Cell Proliferation Assay
(Promega Corporation,, Madison, WI, USA) was used to measure cell
viability. Changes in cell proliferation were quantified based on
BrdU-incorporation using a Cell Proliferation ELISA BrdU assay
(Roche Applied Science, Mannheim, Germany). Both assays were
conducted according to the manufacturer's protocols. Absorbance was
measured using the Infinite® 200 microplate reader
(Tecan Group Ltd., Crailsheim, Germany). The absorbance values of
treated cells are presented as a percentage of the absorbance of
DMSO treated control cells.
Western blot analysis
1×105 cells were seeded in 25
cm2 cell culture flasks, grown overnight and treated
with YM155 or DMSO vehicle control for 24 h. Subsequently, cells
were lysed in RIPA buffer (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) and supplemented with protease inhibitor mix (cOmplete;
Roche Diagnostics, Indianapolis, IN, USA). Lysates (20 µg) were
separated on SDS-PAGE gels and transferred to nitrocellulose
membranes. Membranes were blocked with TBS-T buffer containing 5%
nonfat dry milk and incubated with primary antibodies overnight at
4°C. Blots were washed and incubated with secondary antibodies.
Immune-Star™ Western C™ Kit (Bio-Rad Laboratories, Inc., Hercules,
CA, USA) and the Versa Doc Imaging System (Bio-Rad Laboratories
GmbH, Munich, Germany) were used for signal detection. The
experiments were repeated three times and one representative
western blot (WB) was chosen for presentation.
Reagents
Sepantronium Bromide (YM155), Birinapant and
GDC-0152 were purchased from Selleckchem (Houston, TX, USA).
Antibodies used for immunohistochemistry (IHC) or WB analysis were
raised against survivin (NB500-201; 1:750 dilution for IHC and
1:1,000 dilution for WB; Novus, Littleton, CO, USA), XIAP (clone
48, 1:35 dilution for IHC or Clone 28, 1:1,000 dilution for WB;
both BD Biosciences, San Jose, CA, USA), PARP (9542; 1:1,000
dilution; Cell Signaling, Denver, MA, USA), α-tubulin (Clone DM1A;
1:5,000 dilution; Sigma-Aldrich; Merck KGaA) and GAPDH (Clone 6C5;
1:5,000 dilution; Abcam, Cambridge, UK). Isotype control was
performed using mouse IgG1k (MOPC-21; 1:70 dilution; Abcam) and
rabbit immunoglobulin fraction (Code X0903; 1:15,000 dilution;
Dako, Glostrup, Denmark).
Statistical analysis
Differences of IAP expression levels in esophageal
cancer specimens and adjacent non-neoplastic mucosa were analyzed
using the Mann-Whitney U test. For numerical data, a correlation
between clinicopathological variables and expression levels of
survivin or XIAP was examined using the Mann-Whitney U test. The
chi-square test was implemented for categorical data. Spearman's
correlation coefficient was used to test a relationship between
survivin and XIAP expression levels. For some analyses
immunoreactivity scores were categorized into high (IRS>2) or
low (IRS≤2) expression of survivin and XIAP, respectively. The
cut-off value for this categorization was set according to the
median IRS for survivin and XIAP expression in all investigated EC
tissue samples. Outcome measures included overall survival, defined
as the period from the date of surgery until the date of last
follow up or until death of any cause. Patients with incomplete
tumor resection or who died within 30 days after operation were
excluded from the survival analysis. Kaplan-Meier curves were
generated and assessed using the log-rank (Mantel Cox) test and
hazard ratios (HRs) with 95% confidence intervals (CIs) were
estimated. For multivariate survival analysis all variables were
included into a logistic regression analysis. Analyses were
performed using GraphPad Prism for Windows (version 5; GraphPad
Software, Inc., La Jolla, CA, USA) and SPSS statistics for Windows
(version 17.0; SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Patients and outcome
Using our selection criteria, a total number of 90
EAC and 120 ESCC patients who underwent radical en bloc
esophagectomy between 1986 and 2005 could be enrolled into our
study. Unfortunately, 10 EAC patients and 6 ESCC patients had to be
excluded from our analysis because of insufficient evaluable tumor
material after immunohistochemical staining procedure.
Clinicopathological characteristics of these remaining 80 EAC and
114 ESCC patients are summarized in Table
I. The median age of EAC patients at the time of surgery was 66
years (range, 36–82) and 58 years in the group of ESCC patients
(range, 37–83). A total of 67 EAC and 108 ESCC patients met all
predefined inclusion criteria for our survival analysis. EAC and
ESCC patients had a mean follow-up time of 38.0 month (range,
1–120) and 22.8 month (range, 1–120 month), respectively. A total
of 47 EAC patients and 89 ESCC patients died during the follow up
period. Mean overall survival of EAC patients was 49.3 month
(range, 1–120 month; 95% CI: 38.4–60.2 month) and 28.3 month for
ESCC patients (range, 1–120 month; 95% CI: 21.8–34.8).
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
|
| EAC | ESCC |
|---|
|
|
|
|
|---|
| Variable | No. of patients
(%) | No. of patients
(%) |
|---|
| Total | 80 | 114 |
| Age |
|
|
| Median
(range); years | 66 (36–82) | 59 (37–83) |
| Sex |
|
|
|
Male | 62 (77.5) | 84 (73.7) |
|
Female | 18 (22.5) | 30 (26.3) |
| Tumor stage |
|
|
|
T1/2 | 44 (55.0) | 39 (34.2) |
|
T3/4 | 33 (41.3) | 75 (65.8) |
|
Missing | 3 (3.8) | 0 (0) |
| Lymph node
metastasis |
|
|
| N0 | 26 (32.5) | 39 (34.2) |
|
N1+ | 51 (63.8) | 75 (65.8) |
|
Missing | 3 (3.8) | 0 (0) |
| Distant
metastasis |
|
|
| M0 | 71 (88.8) | 112 (98.2) |
| M1 | 6 (7.5) | 2 (1.8) |
|
Missing | 3 (3.8) | 0 (0) |
| Grading |
|
|
|
G1/2 | 21 (26.3) | 66 (57.9) |
|
G3/4 | 53 (66.3) | 48 (42.1) |
|
Missing | 6 (7.5) | 0 (0) |
| Resection
status |
|
|
| R0 | 75 (93.8) | 114 (100) |
| R+ | 2 (2.5) | 0 (0) |
|
Missing | 3 (3.8) | 0 (0) |
Survivin and XIAP expression in
esophageal cancer
As expected, immunohistochemical staining of TMAs
showed a cytoplasmic and nuclear expression for survivin, whereas
XIAP was exclusively localized in the cytoplasm (Fig. 1A). Both IAPs were aberrantly expressed
in esophageal cancer tissue, when compared to adjacent
non-neoplastic tumor mucosa, with significantly increased
expression levels in both ESCC and EAC (Fig. 1B). Interestingly, XIAP and nuclear
survivin expression levels were significantly higher in EAC when
compared to ESCC (Fig. 1B). Of note,
cytoplasmic survivin expression correlated positively with XIAP
expression in EAC (rs=0.442; P<0.001) (Fig. 1D). In contrast, we did not detect a
correlation between survivin and XIAP expression in ESCC
samples.
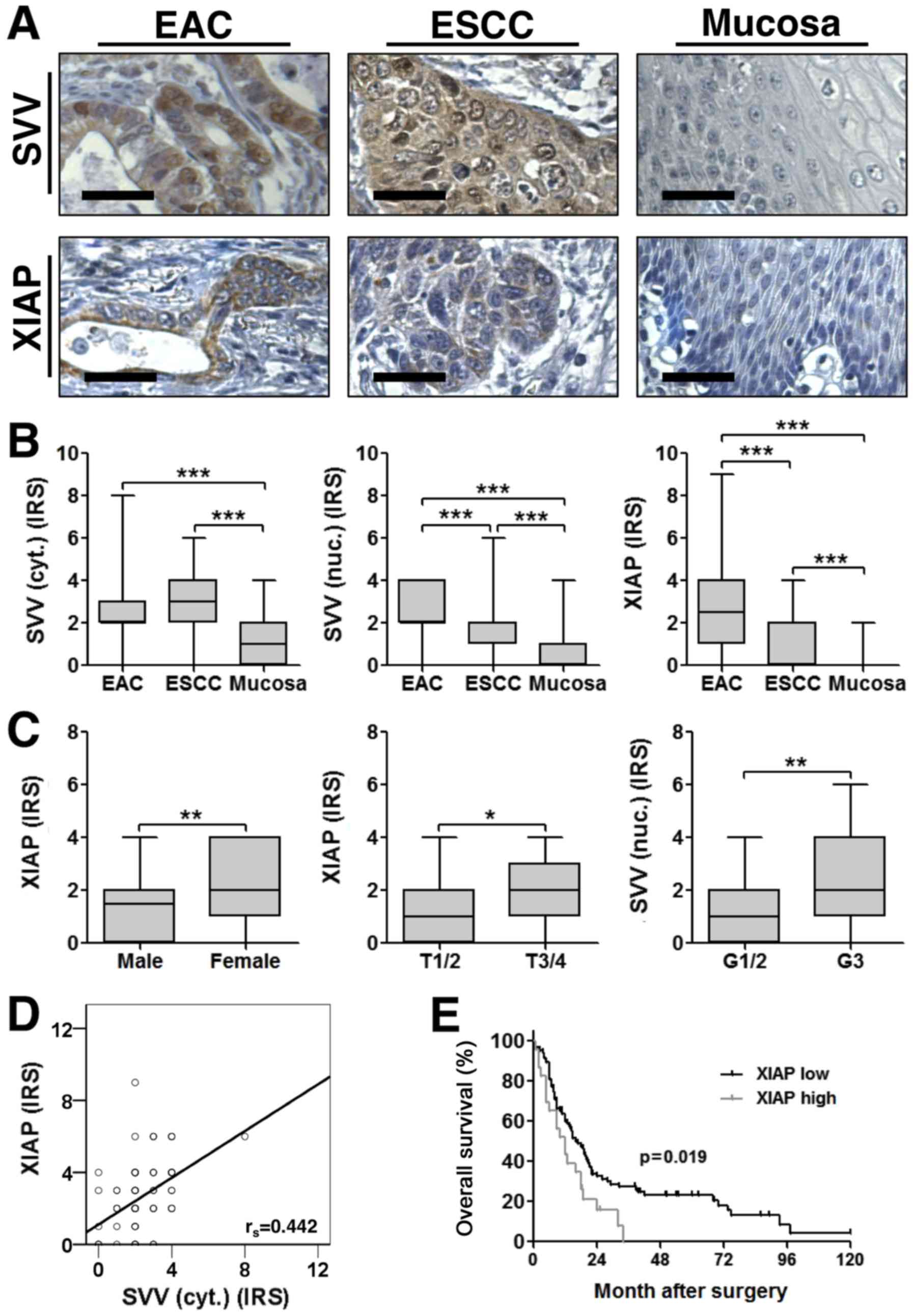 | Figure 1.(A) Representative images of
immunohistochemical staining for SVV (top) and XIAP (bottom) in
EAC, ESCC and tumor adjacent non-malignant mucosa. Images were
captured at ×400 magnification and scale bars indicate 25 µm. (B)
SVV and XIAP expression were significantly increased in esophageal
cancer tissue specimens, when compared to non-malignant mucosa.
Furthermore, nuclear SVV and XIAP expression levels were higher in
EAC when compared to ESCC (EAC, n=80; ESCC, n=114; NEM, n=73). (C)
IAP expression levels and their association with
clinicopathological variables. Boxplots display the median IRS with
the upper and lower quartile, as well as the maximum and minimum
stratified according to the respective clinicopathological
variable. [Sex: median IRS female=2 (n=30); median IRS male=1.5
(n=84); P=0.003; T-stage: median IRS T1/2=1 (n=39); median IRS
T3/4=2 (n=75); P=0.03; Grading: median IRS G1/2=1 (n=66); median
IRS G3=2 (n=48); P=0.005]. Data were analyzed using a two-tailed
nonparametric Mann-Whitney U test. *P<0.05, **P<0.01 and
***P<0.001, as indicated. (D) XIAP and cytoplasmic SVV
expression were positively correlated in corresponding EAC
(rs=0.442; P<0.001). (E) Kaplan-Meier curve represents the
prognostic value of XIAP expression in ESCC. SVV, survivin; EAC,
esophageal adenocarcinoma; ESCC, esophageal squamous-cell cancer;
XIAP, X-linked inhibitor of apoptosis protein; IRS,
immunoreactivity score. |
To further elucidate a correlation between survivin
or XIAP expression levels and clinicopathological variables, two
statistical approaches were used. First we compared the IRS across
groups for each clinicopathological parameter. This approach
revealed that high XIAP expression strongly correlated with female
gender and advanced tumor stages in ESCC patients. Furthermore,
high nuclear survivin expression levels were associated with poorly
differentiated (G3) ESCC (Fig. 1C).
In contrast, no significant correlation between survivin or XIAP
expression and clinicopathological variables became evident in EAC
patients. Next, by categorizing IAP expression into high (IRS>2)
or low (IRS≤2) we could confirm the correlation of high XIAP
expression and female gender as well as high nuclear survivin
expression and poorly differentiated (G3) ESCC (Tables II and III).
 | Table II.Associations between SVV and XIAP
expression, and clinicopathological variables in esophageal
adenocarcinoma. |
Table II.
Associations between SVV and XIAP
expression, and clinicopathological variables in esophageal
adenocarcinoma.
|
| XIAP | SVV (cyt.) | SVV (nuc.) |
|---|
|
|
|
|
|
|---|
|
| Low | High |
| Low | High |
| Low | High |
|
|---|
|
|
|
|
|
|
|
|
|
|
|
|---|
| Variable | n | % | n | % | P-value | n | % | n | % | P-value | n | % | n | % | P-value |
|---|
| Age |
|
<Median | 22 | 56.4 | 17 | 43.6 |
| 21 | 53.8 | 18 | 46.2 |
| 28 | 71.8 | 11 | 28.2 |
|
|
≥Median | 18 | 43.9 | 23 | 56.1 | 0.263 | 28 | 68.3 | 13 | 31.7 | 0.185 | 23 | 56.1 | 18 | 43.1 | 0.144 |
| Sex |
|
Men | 30 | 48.4 | 32 | 51.6 |
| 35 | 56.5 | 27 | 43.5 |
| 42 | 67.7 | 20 | 32.3 |
|
|
Women | 10 | 55.6 | 8 | 44.4 | 0.592 | 14 | 77.8 | 4 | 22.2 | 0.102 | 9 | 50.0 | 9 | 50.0 | 0.168 |
| Tumor stage |
|
T1/T2 | 24 | 54.5 | 20 | 45.5 |
| 24 | 54.5 | 20 | 45.5 |
| 27 | 61.4 | 17 | 38.6 |
|
|
T3/T4 | 13 | 39.4 | 20 | 60.6 | 0.188 | 23 | 69.7 | 10 | 30.3 | 0.177 | 21 | 63.6 | 12 | 36.4 | 0.839 |
| Lymph nodes |
|
Negative, N0 | 11 | 42.3 | 15 | 57.7 |
| 15 | 57.7 | 11 | 42.3 |
| 17 | 65.4 | 9 | 34.6 |
|
|
Positive, N+ | 26 | 51.0 | 25 | 49.0 | 0.471 | 32 | 62.7 | 19 | 37.3 | 0.667 | 31 | 60.8 | 20 | 39.2 | 0.694 |
| Metastasis |
| M0 | 34 | 47.9 | 37 | 52.1 |
| 43 | 60.6 | 28 | 39.4 |
| 45 | 63.4 | 26 | 36.6 |
|
| M1 | 3 | 50.0 | 3 | 50.0 | 0.921 | 4 | 66.7 | 2 | 33.3 | 0.768 | 3 | 50.0 | 3 | 50.0 | 0.516 |
| Grading |
|
G1/G2 | 7 | 33.3 | 14 | 66.7 |
| 13 | 61.9 | 8 | 38.1 |
| 10 | 47.6 | 11 | 52.4 |
|
|
G3/G4 | 28 | 52.8 | 25 | 47.2 | 0.130 | 32 | 60.4 | 21 | 39.6 | 0.903 | 36 | 67.9 | 17 | 32.1 | 0.104 |
| Resection
margins |
|
Negative, R0 | 36 | 48.0 | 39 | 52.0 |
| 46 | 61.3 | 29 | 38.7 |
| 47 | 62.7 | 28 | 37.7 |
|
|
Positive, R1 | 1 | 50.0 | 1 | 50.0 | 0.955 | 1 | 50.0 | 1 | 50.0 | 0.746 | 1 | 50.0 | 1 | 50.0 | 0.715 |
 | Table III.Associations between SVV expression,
and clinicopathological variables in esophageal squamous-cell
carcinoma. |
Table III.
Associations between SVV expression,
and clinicopathological variables in esophageal squamous-cell
carcinoma.
|
| XIAP | SVV (cyt.) | SVV (nuc.) |
|---|
|
|
|
|
|
|---|
|
| Low | High |
| Low | High |
| Low | High |
|
|---|
|
|
|
|
|
|
|
|
|
|
|
|---|
| Variable | n | % | n | % | P-value | n | % | n | % | P-value | n | % | n | % | P-value |
|---|
| Age |
|
<Median | 48 | 84.2 | 9 | 15.8 |
| 23 | 40.4 | 34 | 59.6 |
| 42 | 73.7 | 15 | 26.3 |
|
|
≥Median | 42 | 73.7 | 15 | 26.3 | 0.168 | 28 | 49.1 | 29 | 50.9 | 0.346 | 47 | 82.5 | 10 | 17.5 | 0.258 |
| Sex |
|
Men | 71 | 84.5 | 13 | 15.5 |
| 36 | 42.9 | 48 | 57.1 |
| 64 | 76.2 | 20 | 23.8 |
|
|
Women | 19 | 63.3 | 11 | 36.7 | 0.015 | 15 | 50.0 | 15 | 50.0 | 0.499 | 25 | 83.3 | 5 | 16.7 | 0.417 |
| Tumor stage |
|
T1/T2 | 34 | 87.2 | 5 | 12.8 |
| 18 | 46.2 | 21 | 53.8 |
| 28 | 71.8 | 11 | 28.2 |
|
|
T3/T4 | 56 | 74.7 | 19 | 25.3 | 0.120 | 33 | 44.0 | 42 | 56.0 | 0.826 | 61 | 81.3 | 14 | 18.7 | 0.243 |
| Lymph nodes |
|
Negative, N0 | 34 | 87.2 | 5 | 12.8 |
| 18 | 46.2 | 21 | 53.8 |
| 32 | 82.1 | 7 | 17.9 |
|
|
Positive, N+ | 56 | 74.7 | 19 | 25.3 | 0.120 | 33 | 44.0 | 42 | 56.0 | 0.826 | 57 | 76.0 | 18 | 24.0 | 0.459 |
| Metastasis |
| M0 | 89 | 79.5 | 23 | 20.5 |
| 50 | 44.6 | 62 | 55.4 |
| 87 | 77.7 | 25 | 22.3 |
|
| M1 | 1 | 50.0 | 1 | 50.0 | 0.311 | 1 | 50.0 | 1 | 50.0 | 0.880 | 2 | 100.0 | 0 | 0.0 | 0.450 |
| Grading |
|
G1/G2 | 54 | 81.8 | 12 | 18.2 |
| 32 | 48.5 | 34 | 51.5 |
| 57 | 86.4 | 9 | 13.6 |
|
|
G3/G4 | 36 | 75.0 | 12 | 25.0 | 0.378 | 19 | 39.6 | 29 | 60.4 | 0.345 | 32 | 66.7 | 16 | 33.3 | 0.012 |
| Resection
margins |
|
Negative, R0 | 90 | 78.9 | 24 | 21.1 |
| 51 | 44.7 | 63 | 55.3 |
| 89 | 78.1 | 25 | 21.9 |
|
|
Positive, R1 | 0 | 0.0 | 0 | 0.0 |
| 0 | 0.0 | 0 | 0.0 |
| 0 | 0.0 | 0 | 0.0 |
|
XIAP is an independent negative
prognostic marker in ESCC
Univariate survival analysis of EAC patients, using
Kaplan-Meier curves and log-rank test revealed that the presence of
lymph node and distant metastases were significantly associated
with poor overall survival. However, neither survivin, nor XIAP
expression levels correlated with poor prognosis in EAC patients
(Table IV). Moreover, multivariate
logistic regression analysis confirmed the presence of distant
metastasis as independent negative prognostic factor in EAC
patients (Table V).
 | Table IV.Overall survival of esophageal cancer
patients: Univariate analysis. |
Table IV.
Overall survival of esophageal cancer
patients: Univariate analysis.
|
| Adenocarcinoma | Squamous cell
carcinoma |
|---|
|
|
|
|
|---|
| Variable | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age | 1.369 | 0.756–2.478 | 0.279 | 1.092 | 0.713–1.671 | 0.683 |
| Sex | 0.901 | 0.421–1.930 | 0.781 | 1.328 | 0.819–2.154 | 0.243 |
| T 1/2 vs. T
3/4 | 1.339 | 0.748–2.399 | 0.306 | 1.491 | 0.957–2.323 | 0.072 |
| N0 vs. N+ | 2.173 | 1.097–4.305 | 0.018 | 1.348 | 0.865–2.102 | 0.181 |
| M0 vs. M+ | 12.91 | 2.571–64.82 | <0.001 | 3.445 | 0.833–14.240 | 0.065 |
| G 1/2 vs. G
3/4 | 1.582 | 0.759–3.297 | 0.199 | 1.285 | 0.843–1.959 | 0.237 |
| XIAP high vs.
low | 0.931 | 0.524–1.653 | 0.798 | 1.798 | 1.087–2.973 | 0.019 |
| SVV (cyt.) high vs.
low | 1.222 | 0.687–2.175 | 0.478 | 0.870 | 0.571–1.326 | 0.513 |
| SVV (nuc.) high vs.
low | 0.841 | 0.460–1.539 | 0.559 | 1.066 | 0.651–1.744 | 0.797 |
 | Table V.Overall survival of patients with
esophageal cancer: Multivariate analysis. |
Table V.
Overall survival of patients with
esophageal cancer: Multivariate analysis.
|
| Adenocarcinoma | Squamous cell
carcinoma |
|---|
|
|
|
|
|---|
| Variable | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| M0 vs. M+ | 18.264 | 3.290–101.4 | 0.001 | / | / | / |
| XIAP, high vs.
low | / | / | / | 1.798 | 1.087–2.973 | 0.022 |
In contrast to these findings, univariate analysis
in the group of ESCC patients revealed that high levels of XIAP
expression were significantly associated with a poor prognosis
(Fig. 1E; Table IV). Importantly, multivariate
logistic regression analysis confirmed high XIAP expression levels
as an independent negative prognostic factor in our cohort of ESCC
patients (Table V).
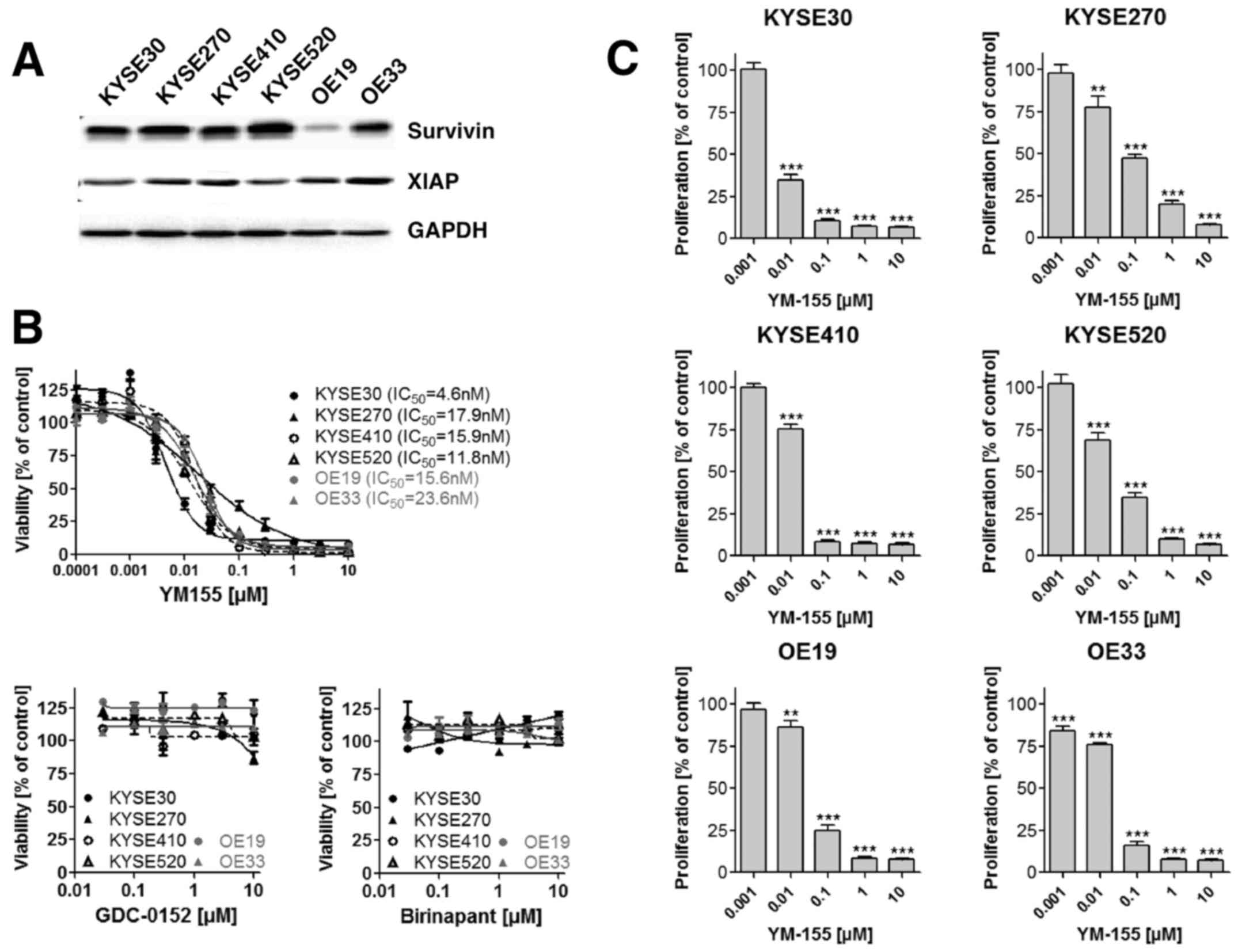
In vitro effects of survivin and XIAP
directed therapy in esophageal cancer cells
A compilation of cell lines originating from human
esophageal cancer comprising four ESCC (KYSE30, KYSE270, KYSE410
and KYSE520) and two EAC (OE19 and OE33) cell lines were analyzed
for survivin and XIAP expression using Western blot analysis. As
shown in Fig. 2A survivin and XIAP
expression were detectable in all esophageal cancer cell lines
independent of their histological subtype. To explore the effect of
a small molecule mediated inhibition of survivin and XIAP on
esophageal cancer cell viability, we incubated ESCC as well as EAC
cell lines with increasing concentrations of the survivin
antagonist YM155 and the XIAP antagonists Birinapant and GDC-0152.
YM155 decreased the cell viability dose dependently in all
investigated cell lines, with IC50 values ranging
between 4.6 and 23.6 nM (Fig. 2B). In
contrast, XIAP inhibitors Birinapant and GDC-0152 exhibited no
measurable effect on cancer cell viability (Fig. 2B). As only YM155 demonstrated in
vitro cell growth inhibitory effects, we focused our further
analysis on this small molecule survivin inhibitor. Comparable to
the effect observed on cell viability, YM155 treatment
significantly reduced cell proliferation of ESCC and EAC cells as
measured by BrdU incorporation (Fig.
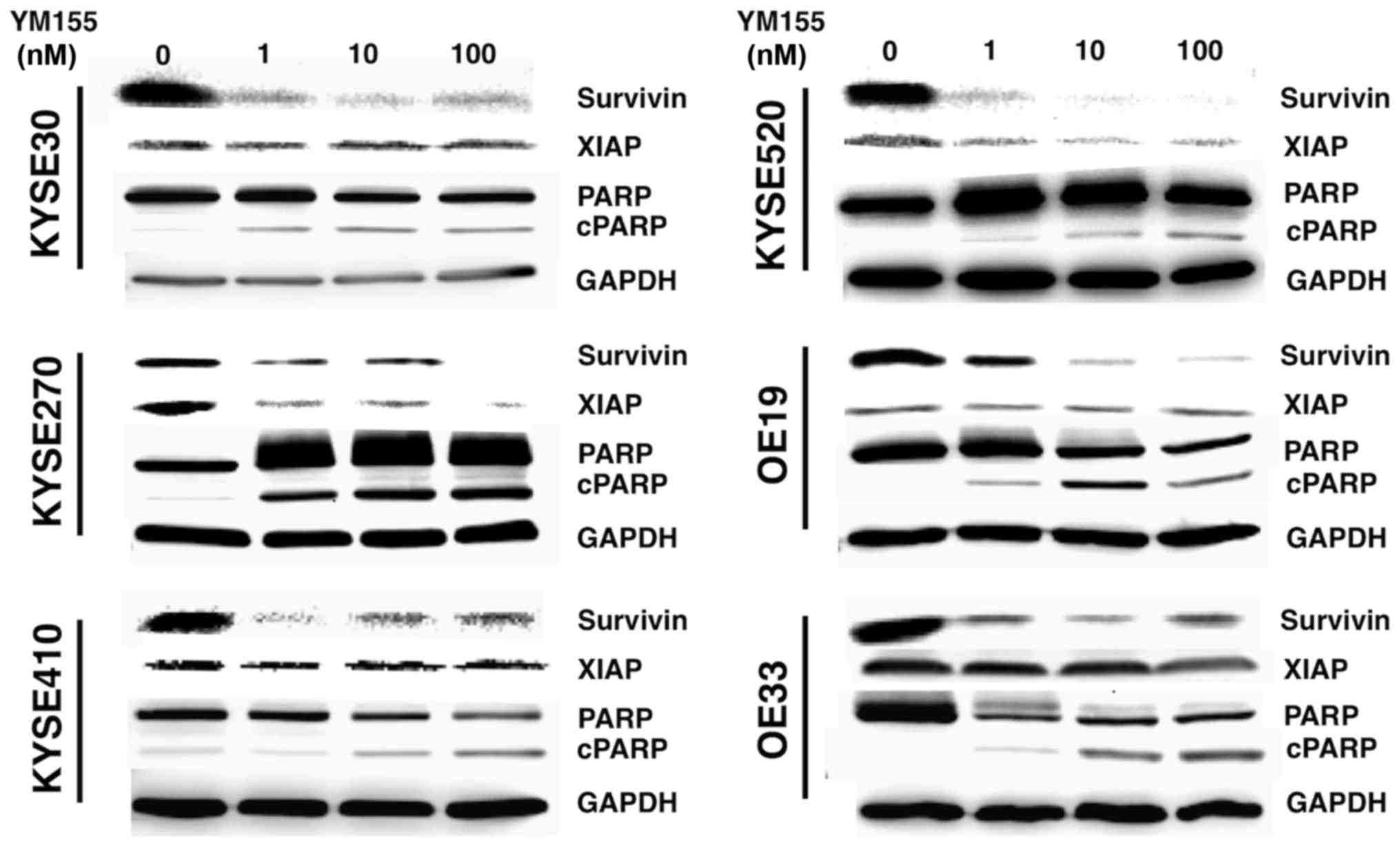
2C). Since YM155 has been demonstrated to execute its
anti-tumor effects through inhibition of survivin mRNA
transcription, we analyzed survivin protein expression in YM155
treated cells using Western blot analysis. As expected, YM155
treatment decreased survivin protein levels in all esophageal
cancer cell lines accompanied by a PARP cleavage, indicating
apoptotic cell death (Fig. 3). Of
note, YM155 also induced a decrease in XIAP expression levels in
KYSE270 and KYSE 520 cell lines (Fig.
3).
Discussion
Given their importance in therapy-resistance and
tumor progression, IAP family members survivin and XIAP display
promising biomarkers and novel druggable targets for innovative
anti-cancer therapies (14,28). Notably, both IAPs act synergistically
in many respects, as they stabilize each other and realize their
antiapoptotic and pro-metastatic functions by direct interaction
(13,20). Published data on survivin and XIAP
expression in esophageal cancer are in part controversial or even
incomplete and none of the studies reported so far has analyzed
survivin and XIAP expression in the same cohort of esophageal
cancer patients. In ESCC survivin expression could be correlated
with clinicopathological parameters and was linked to poor survival
in the majority of published studies (29–36). In
contrast, the prognostic impact of XIAP expression and its
association to pathological variables in ESCC patients has not yet
been adequately investigated. To date only one published study
exists, demonstrating a correlation of high XIAP expression and
poor survival in a collective of ESCC patients treated with
adjuvant radiotherapy after radical esophagectomy (37). Moreover, the value of survivin and
XIAP expression in EAC patients is even less clear and available
data more limited. To the best of our knowledge, studies
investigating XIAP expression in EAC patients have not been
published yet and only two studies analyzing survivin expression
exist. However, the results of these two studies are contradictory.
Whereas, Malhotra et al (38)
demonstrated an association between high survivin expression and an
increased risk of death, Rosato et al (29) detected no prognostic relevance for
survivin expression in their collective of 56 EAC patients.
Furthermore, both studies did not comprehensively analyze
correlations between survivin expression and clinicopathological
parameters.
Consistent with, previous reports we could show that
cytoplasmic and nuclear survivin, as well as XIAP expression are
significantly increased in EAC and ESCC tissue specimens, when
compared to non-malignant tumor adjacent mucosa (32,38–40). This
cancer specific expression pattern represents an important basis
for the use of both proteins in targeted therapies. Another
interesting finding of our analysis was the observation that
expression levels of cytoplasmic survivin and XIAP were
significantly correlated in EAC specimens, indicating the important
role of their intermolecular cooperation. Interestingly, Dohi et
al (20,41) could demonstrate that survivin and XIAP
interaction predominantly takes place inside the mitochondria and
cytoplasm. Particularly non-phosphorylated, mitochondrial survivin
stabilizes XIAP and protects it from polyubiquitination and
subsequent proteosomal degradation (20,41).
Whereas in EAC patients a correlation between
survivin or XIAP expression levels and clinicopathological
parameters became not evident, in ESCC high XIAP expression was
associated with female gender and advanced tumor stages. In
addition, nuclear survivin was linked to poorly differentiated (G3)
ESCC. Consistent with our findings, Zhou et al (37) detected high XIAP expression levels in
advanced ESCC and Takeno et al (30) revealed that increased survivin
expression levels were associated with poorly differentiated
esophageal cancer. Thus, suggesting a potential role of both IAPs
in ESCC progression.
Although a prognostic value of survivin expression
in esophageal cancer has been reported by several studies (29–33,36,38),
there exist some conflicting data (29,42,43). In
this context, our analysis revealed no significant correlation
between survivin expression and overall survival in ESCC or EAC
patients. In contrast to survivin, much less effort has been spent
in investigating the prognostic value of XIAP expression in
esophageal cancer. To date, only Zhou et al (37) reported that high levels of XIAP
expression were associated with poor outcome in ESCC patients. Of
note, our analysis verified the prognostic relevance of XIAP in
ESCC patients. However, for EAC patients a prognostic relevance of
XIAP became not evident.
To elucidate the potential of an IAP directed
therapy in esophageal cancer we made use of the most advanced
small-molecule survivin inhibitor YM155, known to repress survivin
promoter activity by disrupting proteins like SP1 and interleukin
enhancer-binding factor-3 (44,45). In
this context, Qin et al (46)
and Zhao et al (47) published
promising results concerning the antitumor effects of YM155
treatment in ESCC cell lines. However, to the best of our
knowledge, for the first time we report also effects of YM155 in
EAC. We could show that the small molecule survivin inhibitor
effectively reduces survivin expression, cell viability and
proliferation in esophageal cancer cell lines, irrespective of
their histologic subtype. In addition, we detected considerable
PARP cleavage in all investigated EAC and ESCC cell lines after
YM155 treatment, indicating apoptotic cell death (48). Consistent with our results, Qin et
al (46) demonstrated decreased
cell viability and survivin expression as well as a significant
PARP cleavage in YM155 treated ESCC cells. Moreover, they reported
that the small-molecule survivin inhibitor enhanced
radiosensitization by the abrogation of G2 checkpoint
and the inhibition of homologous recombination repair (46). In contrast to our findings, Zhao et
al (47) observed that YM155 did
not trigger apoptosis, but induced parthanatos, a cell death
depending on PARP-1 hyper-activation in ESCC cell lines KYSE410 and
KYSE150 (47). However, the key
message of both published studies investigating the effects of
YM155 treatment in esophageal cancer so far is that YM155 kills
esophageal cancer cells and represents a promising tool for novel
therapeutic approaches in esophageal cancer patients.
In addition, we tested the effects of the small
molecule XIAP antagonists Birinapant and GDC-0152. Both compounds
mimic the effects of the second mitochondrial-derived activator of
caspases (Smac), which acts as an endogenous antagonist of XIAP,
cIAP1 and cIAP2 (49–51). In contrast to YM155, both small
molecule IAP antagonists failed to achieve cytotoxic effects on
esophageal cancer cells in vitro. Although smac mimetics
have been shown to promote cell death by competing with caspases
for binding to the BIR domains of XIAP, cIAP1 or cIAP2 (52–54), these
IAP-antagonizing compounds turned out to exhibit single agent
activity only in a small subset of tumor cells (54,55). This
observation might be explained by different mechanisms contributing
to Smac mimetic resistance in malignant cells including a tumor
necrosis factor α (TNFα) mediated up-regulation of cIAP2, the
inability to form a ripoptosome complex or a defective PI3K
signaling pathway (56,57).
In conclusion, our analysis of survivin and XIAP
protein expression in esophageal cancer tissue specimens revealed
that only XIAP may be regarded as a prognostic marker in ESCC but
not in EAC. In addition, small-molecule survivin inhibitor YM155
induced impressive cytotoxic effects on esophageal cancer cells
in vitro even at nanomolar concentrations. Unfortunately
small molecule XIAP antagonists did not exhibit single agent
activity in our experiments. However, as they might be effective in
combination therapies with other chemotherapeutics or radiation, we
suggest that future studies should investigate the efficacy of Smac
mimetic-based combination therapies in esophageal cancer.
Despite the limitations of the study including its
retrospective design and the lack of in vivo experiments,
our findings underline the potential role of survivin and XIAP in
the oncogenesis of esophageal cancer and provide a rationale for
future clinical studies investigating the therapeutic efficacy of
IAP directed therapies in esophageal cancer patients.
References
|
1
|
Ferlay J, Soerjomataram I, Ervik M,
Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D and
Bray F: GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality
Worldwide: IARC CancerBase No. 11 (Internet). International Agency
for Research on Cancer; Lyon: 2013, http://globocan.iarc.frFebruary 28–2017
|
|
2
|
Lepage C, Rachet B, Jooste V, Faivre J and
Coleman MP: Continuing rapid increase in esophageal adenocarcinoma
in England and Wales. Am J Gastroenterol. 103:2694–2699. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pohl H and Welch HG: The role of
overdiagnosis and reclassification in the marked increase of
esophageal adenocarcinoma incidence. J Natl Cancer Inst.
97:142–146. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Eslick GD: Epidemiology of esophageal
cancer. Gastroenterol Clin North Am. 3817–25. (vii)2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Crane LM, Schaapveld M, Visser O, Louwman
MW, Plukker JT and van Dam GM: Oesophageal cancer in The
Netherlands: Increasing incidence and mortality but improving
survival. Eur J Cancer. 43:1445–1451. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Stavrou EP, McElroy HJ, Baker DF, Smith G
and Bishop JF: Adenocarcinoma of the oesophagus: Incidence and
survival rates in New South Wales, 1972–2005. Med J Aust.
191:310–314. 2009.PubMed/NCBI
|
|
7
|
Le Bras GF, Farooq MH, Falk GW and Andl
CD: Esophageal cancer: The latest on chemoprevention and state of
the art therapies. Pharmacol Res. 113:236–244. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pennathur A, Gibson MK, Jobe BA and
Luketich JD: Oesophageal carcinoma. Lancet. 381:400–412. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Howlader N, Noone AM, Krapcho M, Miller D,
Bishop K, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, et
al: SEER cancer statistics review, 1975–2013. National Cancer
Institute; Bethesda, MD: 2016, http://seercancergov/csr/1975_2013/February
28–2017
|
|
10
|
Mariette C, Balon JM, Piessen G, Fabre S,
Van Seuningen I and Triboulet JP: Pattern of recurrence following
complete resection of esophageal carcinoma and factors predictive
of recurrent disease. Cancer. 97:1616–1623. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nakagawa S, Kanda T, Kosugi S, Ohashi M,
Suzuki T and Hatakeyama K: Recurrence pattern of squamous cell
carcinoma of the thoracic esophagus after extended radical
esophagectomy with three-field lymphadenectomy. J Am Coll Surg.
198:205–211. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mita AC, Mita MM, Nawrocki ST and Giles
FJ: Survivin: Key regulator of mitosis and apoptosis and novel
target for cancer therapeutics. Clin Cancer Res. 14:5000–5005.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mehrotra S, Languino LR, Raskett CM,
Mercurio AM, Dohi T and Altieri DC: IAP regulation of metastasis.
Cancer Cell. 17:53–64. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fulda S and Vucic D: Targeting IAP
proteins for therapeutic intervention in cancer. Nat Rev Drug
Discov. 11:109–124. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Altieri DC: New wirings in the survivin
networks. Oncogene. 27:6276–6284. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Altieri DC: Survivin-The inconvenient IAP.
Semin Cell Dev Biol. 39:91–96. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dohi T, Beltrami E, Wall NR, Plescia J and
Altieri DC: Mitochondrial survivin inhibits apoptosis and promotes
tumorigenesis. J Clin Invest. 114:1117–1127. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Reed JC and Bischoff JR: BIRinging
chromosomes through cell division-and survivin' the experience.
Cell. 102:545–548. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fulda S: Regulation of cell migration,
invasion and metastasis by IAP proteins and their antagonists.
Oncogene. 33:671–676. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dohi T, Okada K, Xia F, Wilford CE, Samuel
T, Welsh K, Marusawa H, Zou H, Armstrong R, Matsuzawa S, et al: An
IAP-IAP complex inhibits apoptosis. J Biol Chem. 279:34087–34090.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
McShane LM, Altman DG, Sauerbrei W, Taube
SE, Gion M and Clark GM; Statistics Subcommittee of the NCI-EORTC
Working Group on Cancer Diagnostics, : REporting recommendations
for tumour MARKer prognostic studies (REMARK). Br J Cancer.
93:387–391. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schmelzle M, Dizdar L, Matthaei H, Baldus
SE, Wolters J, Lindenlauf N, Bruns I, Cadeddu RP, Kröpil F, Topp
SA, et al: Esophageal cancer proliferation is mediated by
cytochrome P450 2C9 (CYP2C9). Prostaglandins Other Lipid Mediat.
94:25–33. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dizdar L, Oesterwind KA, Riemer JC, Werner
TA, Mersch S, Möhlendick B, Schutte SC, Verde PE, Raba K, Topp SA,
et al: Preclinical assesement of survivin and XIAP as prognostic
biomarkers and therapeutic targets in gastroenteropancreatic
neuroendocrine neoplasia. Oncotarget. 8:8369–8382. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Werner TA, Tamkan-Ölcek Y, Dizdar L,
Riemer JC, Wolf A, Cupisti K, Verde PE, Knoefel WT and Krieg A:
Survivin and XIAP: Two valuable biomarkers in medullary thyroid
carcinoma. Br J Cancer. 114:427–434. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Remmele W and Stegner HE: Recommendation
for uniform definition of an immunoreactive score (IRS) for
immunohistochemical estrogen receptor detection (ER-ICA) in breast
cancer tissue. Pathologe. 8:138–140. 1987.(In German). PubMed/NCBI
|
|
26
|
Shimada Y, Imamura M, Wagata T, Yamaguchi
N and Tobe T: Characterization of 21 newly established esophageal
cancer cell lines. Cancer. 69:277–284. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Krieg A, Mersch S, Boeck I, Dizdar L,
Weihe E, Hilal Z, Krausch M, Möhlendick B, Topp SA, Piekorz RP, et
al: New model for gastroenteropancreatic large-cell neuroendocrine
carcinoma: Establishment of two clinically relevant cell lines.
PLoS One. 9:e887132014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tamm I, Richter S, Oltersdorf D, Creutzig
U, Harbott J, Scholz F, Karawajew L, Ludwig WD and Wuchter C: High
expression levels of x-linked inhibitor of apoptosis protein and
survivin correlate with poor overall survival in childhood de novo
acute myeloid leukemia. Clin Cancer Res. 10:3737–3744. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rosato A, Pivetta M, Parenti A, Iaderosa
GA, Zoso A, Milan G, Mandruzzato S, Del Bianco P, Ruol A, Zaninotto
G and Zanovello P: Survivin in esophageal cancer: An accurate
prognostic marker for squamous cell carcinoma but not
adenocarcinoma. Int J Cancer. 119:1717–1722. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Takeno S, Yamashita S, Takahashi Y, Ono K,
Kamei M, Moroga T and Kawahara K: Survivin expression in
oesophageal squamous cell carcinoma: Its prognostic impact and
splice variant expression. Eur J Cardiothorac Surg. 37:440–445.
2010.PubMed/NCBI
|
|
31
|
Xia H, Chen S, Huang H and Ma H: Survivin
over-expression is correlated with a poor prognosis in esophageal
cancer patients. Clin Chim Acta. 446:82–85. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Grabowski P, Kühnel T, Mühr-Wilkenshoff F,
Heine B, Stein H, Höpfner M, Germer CT and Scherübl H: Prognostic
value of nuclear survivin expression in oesophageal squamous cell
carcinoma. Br J Cancer. 88:115–119. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mega S, Miyamoto M, Li L, Kadoya M,
Takahashi R, Hase R, Kaneko H, Shichinohe T, Kawarada Y, Itoh T, et
al: Immunohistochemical analysis of nuclear survivin expression in
esophageal squamous cell carcinoma. Dis Esophagus. 19:355–359.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhu H, Wang Q, Hu C, Zhang W, Quan L, Liu
M, Xu N and Xiao Z: High expression of survivin predicts poor
prognosis in esophageal squamous cell carcinoma following
radiotherapy. Tumour Biol. 32:1147–1153. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hui MK, Lai KK, Chan KW, Luk JM, Lee NP,
Chung Y, Cheung LC, Srivastava G, Tsao SW, Tang JC and Law S:
Clinical correlation of nuclear survivin in esophageal squamous
cell carcinoma. Med Oncol. 29:3009–3016. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hsu KF, Lin CK, Yu CP, Tzao C, Lee SC, Lee
YY, Tsai WC and Jin JS: Cortactin, fascin, and survivin expression
associated with clinicopathological parameters in esophageal
squamous cell carcinoma. Dis Esophagus. 22:402–408. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhou S, Ye W, Shao Q, Qi Y, Zhang M and
Liang J: Prognostic significance of XIAP and NF-κB expression in
esophageal carcinoma with postoperative radiotherapy. World J Surg
Oncol. 11:2882013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Malhotra U, Zaidi AH, Kosovec JE, Kasi PM,
Komatsu Y, Rotoloni CL, Davison JM, R C, Irvin, Hoppo T, et al:
Prognostic value and targeted inhibition of survivin expression in
esophageal adenocarcinoma and cancer-adjacent squamous epithelium.
PLoS One. 8:e783432013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang S, Ding F, Luo A, Chen A, Yu Z, Ren
S, Liu Z and Zhang L: XIAP is highly expressed in esophageal cancer
and its downregulation by RNAi sensitizes esophageal carcinoma cell
lines to chemotherapeutics. Cancer Biol Ther. 6:973–980. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Nemoto T, Kitagawa M, Hasegawa M, Ikeda S,
Akashi T, Takizawa T, Hirokawa K and Koike M: Expression of IAP
family proteins in esophageal cancer. Exp Mol Pathol. 76:253–259.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Dohi T, Xia F and Altieri DC:
Compartmentalized phosphorylation of IAP by protein kinase A
regulates cytoprotection. Mol Cell. 27:17–28. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Dabrowski A, Filip A, Zgodziński W,
Dabrowska M, Polańska D, Wójcik M, Zinkiewicz K and Wallner G:
Assessment of prognostic significance of cytoplasmic survivin
expression in advanced oesophageal cancer. Folia Histochem
Cytobiol. 42:169–172. 2004.PubMed/NCBI
|
|
43
|
Warnecke-Eberz U, Hokita S, Xi H, Higashi
H, Baldus SE, Metzger R, Brabender J, Bollschweiler E, Mueller RP,
Dienes HP, et al: Overexpression of survivin mRNA is associated
with a favorable prognosis following neoadjuvant radiochemotherapy
in esophageal cancer. Oncol Rep. 13:1241–1246. 2005.PubMed/NCBI
|
|
44
|
Yamauchi T, Nakamura N, Hiramoto M, Yuri
M, Yokota H, Naitou M, Takeuchi M, Yamanaka K, Kita A, Nakahara T,
et al: Sepantronium bromide (YM155) induces disruption of the
ILF3/p54(nrb) complex, which is required for survivin expression.
Biochem Biophys Res Commun. 425:711–716. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Cheng Q, Ling X, Haller A, Nakahara T,
Yamanaka K, Kita A, Koutoku H, Takeuchi M, Brattain MG and Li F:
Suppression of survivin promoter activity by YM155 involves
disruption of Sp1-DNA interaction in the survivin core promoter.
Int J Biochem Mol Biol. 3:179–197. 2012.PubMed/NCBI
|
|
46
|
Qin Q, Cheng H, Lu J, Zhan L, Zheng J, Cai
J, Yang X, Xu L, Zhu H, Zhang C, et al: Small-molecule survivin
inhibitor YM155 enhances radiosensitization in esophageal squamous
cell carcinoma by the abrogation of G2 checkpoint and suppression
of homologous recombination repair. J Hematol Oncol. 7:622014.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhao N, Mao Y, Han G, Ju Q, Zhou L, Liu F,
Xu Y and Zhao X: YM155, a survivin suppressant, triggers
PARP-dependent cell death (parthanatos) and inhibits esophageal
squamous-cell carcinoma xenografts in mice. Oncotarget.
6:18445–18459. 2015.PubMed/NCBI
|
|
48
|
Kaufmann SH, Desnoyers S, Ottaviano Y,
Davidson NE and Poirier GG: Specific proteolytic cleavage of poly
(ADP-ribose) polymerase: An early marker of chemotherapy-induced
apoptosis. Cancer Res. 53:3976–3985. 1993.PubMed/NCBI
|
|
49
|
Benetatos CA, Mitsuuchi Y, Burns JM,
Neiman EM, Condon SM, Yu G, Seipel ME, Kapoor GS, Laporte MG,
Rippin SR, et al: Birinapant (TL32711), a bivalent SMAC mimetic,
targets TRAF2-associated cIAPs, abrogates TNF-induced NF-κB
activation, and is active in patient-derived xenograft models. Mol
Cancer Ther. 13:867–879. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Flygare JA, Beresini M, Budha N, Chan H,
Chan IT, Cheeti S, Cohen F, Deshayes K, Doerner K, Eckhardt SG, et
al: Discovery of a potent small-molecule antagonist of inhibitor of
apoptosis (IAP) proteins and clinical candidate for the treatment
of cancer (GDC-0152). J Med Chem. 55:4101–4113. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Du C, Fang M, Li Y, Li L and Wang X: Smac,
a mitochondrial protein that promotes cytochrome c-dependent
caspase activation by eliminating IAP inhibition. Cell. 102:33–42.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Liu Z, Sun C, Olejniczak ET, Meadows RP,
Betz SF, Oost T, Herrmann J, Wu JC and Fesik SW: Structural basis
for binding of Smac/DIABLO to the XIAP BIR3 domain. Nature.
408:1004–1008. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wu G, Chai J, Suber TL, Wu JW, Du C, Wang
X and Shi Y: Structural basis of IAP recognition by Smac/DIABLO.
Nature. 408:1008–1012. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Fulda S: Smac mimetics as IAP antagonists.
Semin Cell Dev Biol. 39:132–138. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Bai L, Smith DC and Wang S: Small-molecule
SMAC mimetics as new cancer therapeutics. Pharmacol Ther.
144:82–95. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Petersen SL, Peyton M, Minna JD and Wang
X: Overcoming cancer cell resistance to Smac mimetic induced
apoptosis by modulating cIAP-2 expression. Proc Natl Acad Sci USA.
107:pp. 11936–11941. 2010; View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Maas C, Tromp JM, van Laar J, Thijssen R,
Elias JA, Malara A, Krippner-Heidenreich A, Silke J, van Oers MH
and Eldering E: CLL cells are resistant to smac mimetics because of
an inability to form a ripoptosome complex. Cell Death Dis.
4:e7822013. View Article : Google Scholar : PubMed/NCBI
|