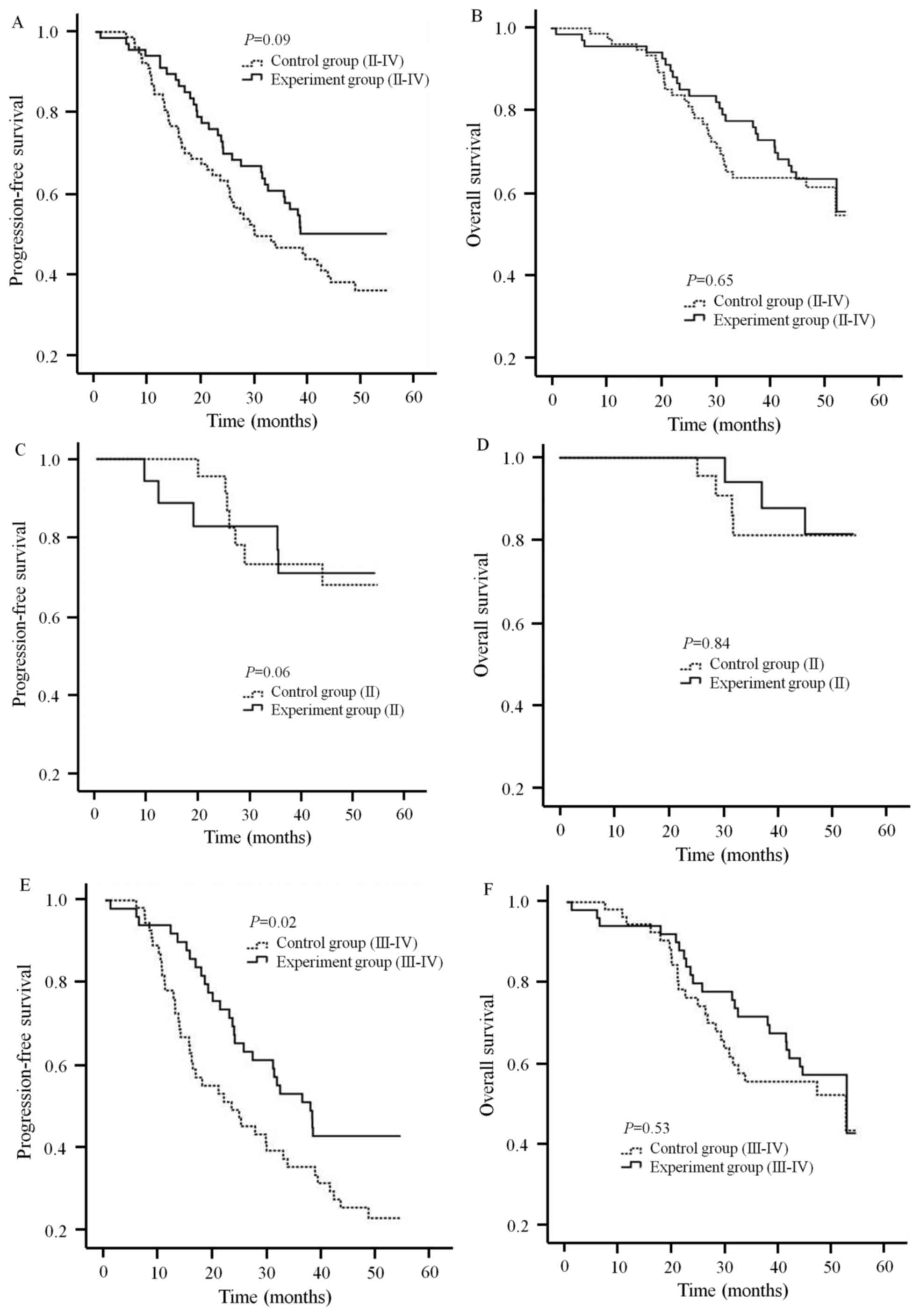
|
1
|
Weiderpass E and Labrèche F: Malignant
tumors of the female reproductive system. Saf Health Work.
3:166–180. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Heintz AP, Odicino F, Maisonneuve P, Quinn
MA, Benedet JL, Creasman WT, Ngan HY, Pecorellu S and Beller U:
Carcinoma of the ovary. FIGO 26th Annual Report on the Results of
Treatment in Gynecological Cancer. Int J Gynecol Obstet. 95 Suppl
1:S161–S192. 2006. View Article : Google Scholar
|
|
3
|
Bookman MA, Brady MF, McGuire WP, Harper
PG, Alberts DS, Friedlander M, Colombo N, Fowler JM, Argenta PA, De
Geest K, et al: Evaluation of new platinum-based treatment regimens
in advanced-stage ovarian cancer: A phase III trial of the
Gynecologic Cancer Intergroup. J Clin Oncol. 27:1419–1425. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bristow RE, Tomacruz RS, Armstrong DK,
Trimble EL and Montz FJ: Survival effect of maximal cytoreductive
surgery for advanced ovarian carcinoma during the platinum era: A
meta-analysis. J Clin Oncol. 20:1248–1259. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Thigpen T, duBois A, McAlpine J, DiSaia P,
Fujiwara K, Hoskins W, Kristensen G, Mannel R, Markman M, Pfisterer
J, et al: First-line therapy in ovarian cancer trials. Int J
Gynecol Cancer. 21:756–762. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Monk BJ and Coleman RL: Changing the
paradigm in the treatment of platinum-sensitive recurrent ovarian
cancer: From platinum doublets to nonplatinum doublets and adding
antiangiogenesis compounds. Int J Gynecol Cancer. 19 Suppl
2:S63–S67. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Monk BJ, Alberts DS, Burger RA, Fanta PT,
Hallum AV III, Hatch KD and Salmon SE: In vitro phase II comparison
of the cytotoxicity of a novel platinum analog, nedaplatin (254-S),
with that of cisplatin and carboplatin against fresh, human
cervical cancers. Gynecol Oncol. 71:308–312. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Alberts DS, Fanta PT, Running KL, Adair JL
Jr, Garcia DJ, Liu-Stevens R and Salmon SE: In vitro phase II
comparison of the cytotoxicity of a novel platinum analog,
nedaplatin (254-S), with that of cisplatin and carboplatin against
fresh, human ovarian cancers. Cancer Chemother Pharmacol.
39:493–497. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Uchida N, Yoshida H, Yamada H, Wada T,
Daikatsu K, Ikeuchi I, Maekawa R, Sugita K and Yoshioka T:
Combination chemotherapy with nedaplatin and cyclophosphamide in
human ovarian cancer model. Jpn J Cancer Res. 90:887–894. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Uchida N, Yamada H, Maekawa R and Yoshioka
T: Combination chemotherapy of paclitaxel followed by nedaplatin
for human ovarian cancer. Gan To Kagaku Ryoho. 29:1943–1949.
2002.(In Japanese). PubMed/NCBI
|
|
11
|
Noda K, Ikeda M, Yakushiji M, Nishimura H,
Terashima Y, Sasaki H, Hata T, Kuramoto H, Tanaka K, Takahashi T,
et al: A phase II clinical study of cis-diammine glycolato
platinum, 254-S, for cervical cancer of the uterus. Gan To Kagaku
Ryoho. 19:885–892. 1992.(In Japanese). PubMed/NCBI
|
|
12
|
Inuyama Y, Miyake H, Horiuchi M, Hayasaki
K, Komiyama S and Ota K: A late phase II clinical study of
cis-diammine glycolato platinum, 254-S, for head and neck cancers.
Gan To Kagaku Ryoho. 19:871–877. 1992.(In Japanese). PubMed/NCBI
|
|
13
|
Kato T, Nishimura H, Yakushiji M, Noda K,
Terashima Y, Takeuchi S, Takamizawa H, Suzuki M, Arai M, Ota M, et
al: Phase II study of 254-S (cis-diammine glycolato platinum) for
gynecological cancer. Gan To Kagaku Ryoho. 19:695–701. 1992.(In
Japanese). PubMed/NCBI
|
|
14
|
Taguchi T, Wakui A, Nabeya K, Kurihara M,
Isono K, Kakegawa T and Ota K: A phase II clinical study of
cis-diammine glycolato platinum, 254-S, for gastrointestinal
cancers. 254-S Gastrointestinal Cancer Study Group. Gan To Kagaku
Ryoho. 19:483–488. 1992.(In Japanese).
|
|
15
|
Akaza H, Togashi M, Nishio Y, Miki T,
Kotake T, Matsumura Y, Yoshida O and Aso Y: Phase II study
ofcis-diammine(glycolato)platinum, 254-S, in patients with advanced
germ-cell testicular cancer, prostatic cancer, and
transitional-cell carcinoma of the urinary tract. 254-S Urological
Cancer Study Group. Cancer Chemother Pharmacol. 31:187–192. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fukuda M, Shinkai T, Eguchi K, Sasaki Y,
Tamura T, Ohe Y, Kojima A, Oshita F, Hara K and Saijo N: Phase II
study of (glycolate-O,O,)diammineplatinum(II), a novel platinum
complex, in the treatment of non-small-cell lung cancer. Cancer
Chemother Pharmacol. 26:393–396. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lebwohl D and Canetta R: Clinical
development of platinum complexes in cancer therapy: An historical
perspective and an update. Eur J Cancer. 34:1522–1534. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hong WS, Min YI, Kim HT, Cho YB, Kim KH
and Kim DK: Antitumor activity of five new platinum complexes
having a glycolate leaving ligand. J Korean Med Sci. 10:269–274.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Weiss RB and Christian MC: New cisplatin
analogues in development. A review. Drugs. 46:360–377. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Paik ES, Lee YY, Lee EJ, Choi CH, Kim TJ,
Lee JW, Bae DS and Kim BG: Survival analysis of revised 2013 FIGO
staging classification of epithelial ovarian cancer and comparison
with previous FIGO staging classification. Obstet Gynecol Sci.
58:124–134. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Calvert AH, Newell DR, Gumbrell LA,
O'Reilly S, Burnell M, Boxall FE, Siddik ZH, Judson IR, Gore ME and
Wiltshaw E: Carboplatin dosage: Prospective evaluation of a simple
formula based on renal function. J Clin Oncol. 7:1748–1756. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cockcroft DW and Gault MH: Prediction of
creatinine clearance from serum creatinine. Nephron. 16:31–41.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Giantonio BJ, Catalano PJ, Meropol NJ,
O'Dwyer PJ, Mitchel EP, Alberts SR, Schwartz MA and Benson AB:
Bevacizumab in combination with oxaliplatin, fluorouracil, and
leucovorin (FOLFOX4) for previously treated metastatic colorectal
cancer: Result from the Eastern Cooperative Oncology Group Study
E3200. J Clin Oncol. 25:1539–1544. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Aghajanian C, Blank SV, Goff BA, Judson
PL, Teneriello MG, Husain A, Sovak MA, Yi J and Nycum LR: OCEANS: A
randomized, double-blinded, placebo-controlled phase III trial of
chemotherapy with or without bevacizumab in patients with
platinum-sensitive recurrent epithelial ovarian, primary
peritoneal, or fallopian tube cancer. J Clin Oncol. 30:2039–2045.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jemal A, Murray T, Ward E, Samuels A,
Tiwari RC, Ghafoor A, Feuer EJ and Thun MJ: Cancer statistics,
2005. CA Cancer J Clin. 55:10–30. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Piccart MJ, Bertelsen K, Stuart G, Cassidy
J, Mangioni C, Simonsen E, James K, Kaye S, Vergote I, Blom R, et
al: Long-term follow-up confirms a survival advantage of the
paclitaxel-cisplatin regimen over the cyclophosphamide-cisplatin
combination in advanced ovarian cancer. Int J Gynecol Cancer. 13
Suppl 2:S144–S148. 2003. View Article : Google Scholar
|
|
27
|
Ozols RF, Bundy BN, Greer BE, Fowler JM,
Clarke-Pearson D, Burger RA, Mannel RS, DeGeest K, Hartenbach EM
and Baergen R; Gynecologic Oncology Group, : Phase III trial of
carboplatin and paclitaxel compared with cisplatin and paclitaxel
in patients with optimally resected stage III ovarian cancer: A
Gynecologic Oncology Group study. J Clin Oncol. 21:3194–3200. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
du Bois A, Lück HJ, Meier W, Adams HR,
Möbus V, Costa S, Bauknecht T, Richter B, Warm M, Schröder W, et
al: A randomized clinical trial of cisplatin/paclitaxel versus
carboplatin/paclitaxel as first-line treatment of ovarian cancer. J
Natl Cancer Inst. 95:1320–1329. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Neijt JP, Engelholm SA, Tuxen MK, Sorensen
PG, Hansen M, Sessa C, de Swart CA, Hirsch FR, Lund B and van
Houwelingen HC: Exploratory phase III study of paclitaxel and
cisplatin versus paclitaxel and carboplatin in advanced ovarian
cancer. J Clin Oncol. 18:3084–3092. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
McGuire WP, Hoskins WJ, Brady MF, Kucera
PR, Partridge EE, Look KY, Clarke-Pearson DL and Davidson M:
Cyclophosphamide and cisplatin compared with paclitaxel and
cisplatin in patients with stage III and stage IV ovarian cancer.
New Engl J Med. 334:1–6. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li Y and Zhang Q: A Weibull multi-state
model for the dependence of progression-free survival and overall
survival. Stat Med. 34:2497–2513. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Markman M, Kennedy A, Webster K, Elson P,
Peterson G, Kulp B and Belinson J: Clinical features of
hypersensitivity reactions to carboplatin. J Clin Oncol.
17:11411999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sasaki Y, Shinkai T, Eguchi K, Tamura T,
Ohe Y, Ohmori T and Saijo N: Prediction of the antitumor activity
of new platinum analogs based on their ex vivo pharmacodynamics as
determined by bioassay. Cancer Chemother Pharmacol. 27:263–270.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Michikami H, Minaguchi T, Ochi H, Onuku M,
Okada S, Matsumoto K, Satoh T, Oki A and Yoshikawa H: Safety and
efficacy of substituting nedaplatin after carboplatin
hypersensitivity reactions in gynecologic malignancies. J Obstet
Gynaecol Res. 39:330–335. 2013. View Article : Google Scholar : PubMed/NCBI
|