Introduction
Leukemia is a malignant tumor of the blood.
Excessive proliferation, blocked differentiation, and disorder of
apoptosis are features of malignant cells of leukemia that arrest
at various stages of differentiation of bone marrow cells. The main
clinical manifestations of this disease include anemia, hemorrhage,
fever, and organ infiltration (1,2). Acute
myeloid leukemia (AML) is a hematopoietic stem/progenitor cell
malignant disease. Primitive and immature myeloid cell hyperplasia
in bone marrow and peripheral blood constitute the primary feature,
and clinical manifestations include anemia, bleeding, infection,
fever, organ invasion and metabolic abnormalities (2). Most AML cases are acute with poor
prognosis. If AML is not treated in time, it often becomes life
threatening. The disease accounts for 30% of childhood leukemia
(3–5).
The focus of current research was to investigate the pathogenesis
of leukemia and achieve the greatest degree of treatment for
leukemia. In recent years, it has been found that angiogenesis
plays an important role in leukemia, which affects the sensitivity
of the body to chemotherapy. Some investigators consider that
anti-angiogenesis therapy is to become a new treatment of leukemia.
Angiogenesis is a complex process. It has been confirmed that many
factors can regulate angiogenesis, such as phosphatase and tension
homolog (PTEN), IL-1, IL-6, IL-8, cyclooxygenase-2, and matrix
metalloproteinase-7 (MMP-7) (6–10).
PTEN is encoded by phosphatase and tension homolog
gene (10). Additionally, PTEN is one
of the many genes that inhibit cell proliferation (11). It was previously confirmed that MMP-7
is highly expressed in gastric, colon, lung and ovarian cancer
(12–15), and closely associated with
angiogenesis. Other authors have identified the presence of MMP-7
in tumors of the blood system (16).
Specifically, the role of MMP-7 in the treatment of AML and its
association with angiogenesis is not well known, and the
measurement of the level of MMP-7 in the serum of AML patients is
rare in China (17).
On the basis of previous studies, the current study
examined the expression level of MMP-7 in patients with AML and its
clinical significance, in order to provide a theoretical basis for
the targeted treatment of AML.
Subjects and methods
Subjects
In total, 80 patients with AML who were treated at
the Department of Hematology of Zhongnan Hospital of Wuhan
University (Wuhan, China) from September 2013 to June 2014, were
randomly selected (3 cases of M0, 38 cases of M2, 5 cases of M4, 14
cases of M5), including 19 cases of relapse, 20 cases of primary
untreated patients, 21 cases of patients with incomplete remission,
and 20 cases of complete remission. The diagnosis and treatment of
patients were according to a previous study (2). The exclusion criteria were as follows:
Heart, liver and kidney dysfunction, infection, autoimmune diseases
and other malignancies. There were 42 male and 21 female subjects,
6–72 years of age, with a median age of 36 years. Twenty subjects
with normal physical examination were included in the control
group; 15 male and 5 female subjects, aged 18–50 years, with a
median age of 35 years. The study was approved by the Ethics
Committee of Zhongnan Hospital of Wuhan University and informed
consents were signed by the patients and/or guardians.
Instruments
Instruments used in the experiments included
GeneAmp® 9700 PCR system (Applied Biosystems, Foster
City, CA, USA), Centrifuge 5415D, PB3002-S Electronic Balance
(Mettler Toledo, Columbus, OH, USA), 96-well plate, TL-5.0 Desk
Centrifuge, Micropipette (Eppendorf, Hamburg Germany), NanoDrop1000
Spectrophotometer and SpeedVac Vacuum Centrifugal Concentration
system (both from Thermo Fisher Scientific, Inc., Waltham, MA,
USA), NimbleGen HX1 Mixer and NimbleGen Elution system (both from
Roche NimbleGen, Inc., Madison, WI, USA), pH meter (Φ71; Bio-Rad
Laboratories, Inc, Hercules, CA, USA), Electric Thermostatic Water
Bath Box (WS2-280-79) and Vortex (both from Beijing Medical
Equipment Factory, Beijing, China) and Magnetic Stirrer (JB-3;
Shanghai Leici Instrument Factory, Shanghai, China).
Main reagents
The reagents used were as follows: Enzyme-linked
immunosorbent assay (ELISA) kit for human MMP-7 (Shanghai Elisa
Biotech Co., Shanghai, China), and ELISA kit for human PTEN
(Booster Bioengineering Co., Ltd., Wuhan, China) (3). Supporting reagents used in the blood
cell analyzer were provided by Mindray (Shenzhen, China).
Methods
Baseline characteristics
The medical history of 80 AML patients and 20
healthy control subjects was obtained. Clinicopathological
characteristics including sex, age, height, weight, smoking
history, drinking history, history of radiation exposure, blood
glucose and blood pressure were recorded.
ELISA
For detection, the samples, and standard samples
were incubated with horseradish peroxidase (HRP)-labeled
antibodies. The plate was then thoroughly cleaned. Substrate TMB
was used to develop color. Under the catalytic action of
peroxidase, TMB became blue. Absorbance was measured at 450 nm. The
specific concentrations of the samples were then determined.
Sample processing method
Whole blood samples were obtained and kept at room
temperature for 2 h or placed at 4°C overnight. Serum samples were
centrifuged at 1,650 × g for 20 min. A certain volume of
supernatant was extracted. The specimens were preserved at −20 or
−80°C until use. Repeated freeze-thaw was strictly avoided.
To obtain plasma, EDTA or heparin were selected as
the anticoagulant. Within 30 min after sample collection,
centrifugation was performed at 2–8°C, 1,650 × g for 30 min. The
specimens were preserved at −20 or −80°C until use. Repeated
freeze-thaw was strictly avoided.
For other biological samples or supernatant of cell
culture, samples were centrifuged at 1,650 × g for 20 min. After
extraction of a certain volume of supernatant, the test commenced.
The specimen was then stored at −20 or −80°C. Repeated freeze-thaw
was strictly avoided.
Determination of serum MMP-7 and PTEN
levels
Standard samples (50 µl) and the samples to be
tested were added to the well, followed by HRP-labeled antibodies
(100 µl). The ELISA plate was incubated in a 37°C incubator for 60
min. The antibodies were discarded and the plate was washed 3 times
with PBS. The substrate TMB (50 µl A + 50 µl B) was added and the
plate was incubated in the dark at 37°C for 15 min. The reaction
was stopped with 50 µl stop solution. The OD was measured at a
wavelength of 450 nm. The actual OD value was calculated by
measured OD value minus the OD value of blank well. A standard
curve was plotted, and the levels of MMP-7 and PTEN were
calculated.
Statistical analysis
The current study used SPSS 17.0 (SPSS, Inc.,
Chicago, IL, USA) for statistical analysis. Measurement data were
expressed as means ± standard deviation (SD) and compared using a
Student's t-test. The comparison among groups was analyzed by one
way of variance, and the Pearson correlation analysis was used to
analyze the correlation between the data. P<0.05 indicated that
the difference was statistically significant.
Results
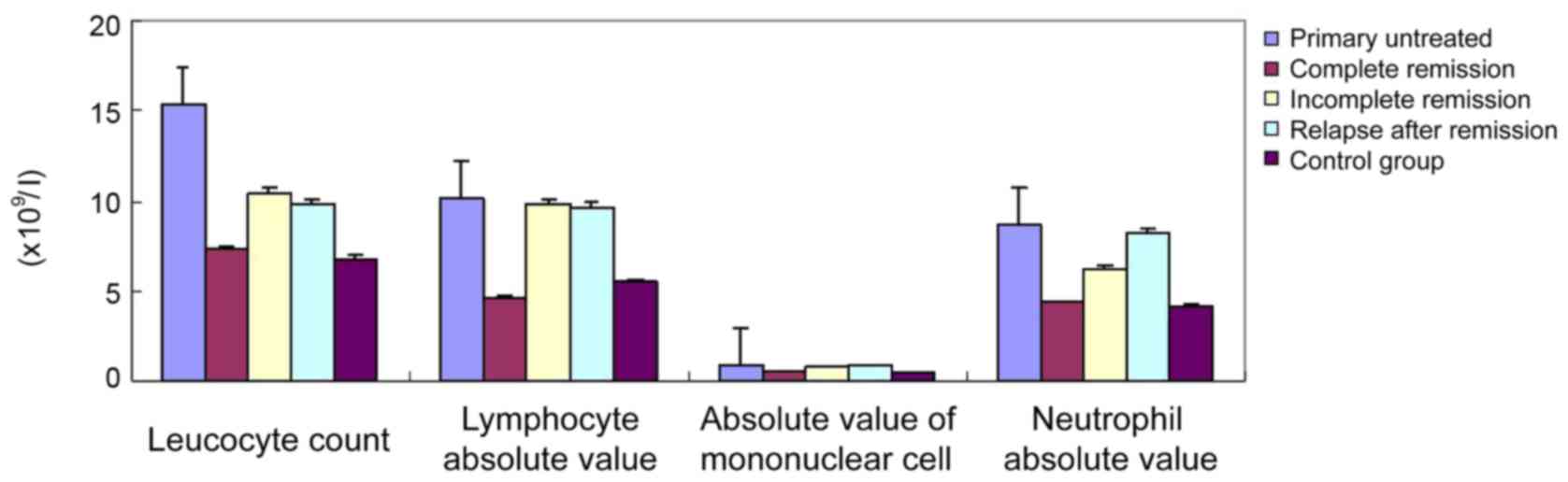
Comparison of baseline data between
AML patients and healthy control (mean ± SD)
The statistical analysis of the hemogram data of the
AML group and healthy control revealed that primary untreated
patients did not achieve complete remission, and the leucocyte
count (1×109/l), absolute value of lymphocyte
(1×109/l), monocyte absolute value (1×109/l),
and neutrophil absolute value (1×109/l) of the group of
relapse after remission were significantly higher (P<0.05) than
those of the complete remission group and the control group, while
the hemoglobin level, red blood cell count and platelet count were
not significantly different (Table I
and Fig. 1).
 | Table I.Eighty cases of acute myeloid leukemia
patients and 20 cases of normal blood index (mean ± standard
deviation). |
Table I.
Eighty cases of acute myeloid leukemia
patients and 20 cases of normal blood index (mean ± standard
deviation).
| Group | No. of cases (N) | Hemoglobin (g/l) | Red blood cell count
(1×1012/l) | Platelet count
(1×109/l) | Leucocyte count
(1×109/l) | Lymphocyte absolute
value (1×109/l) | Absolute value of
mononuclear cells (1×109/l) | Neutrophil absolute
value (1×109/l) |
|---|
| Primary
untreated | 20 | 9.7±4.64 | 3.5±6.4 | 101.2±23.5 | 15.4±8.6 | 10.2±2.5 | 0.92±0.75 | 8.74±3.26 |
| Complete
remission | 20 | 12.7±2.4 | 4.2±2.1 | 185.7±12.6 | 7.3±2.4 | 4.6±3.1 | 0.54±0.22 | 4.37±1.25 |
| Incomplete
remission | 21 | 10.7±1.4 | 4.3±1.7 | 199.4±21.7 | 10.4±1.4 | 9.8±1.3 | 0.82±0.33 | 6.27±2.14 |
| Relapse after
remission | 19 | 9.7±10.9 | 4.8±1.6 | 190.4±28.3 | 9.8±2.1 | 9.6±2.7 | 0.87±0.21 | 8.26±1.06 |
| Control group | 20 | 11.8±3.5 | 5.0±1.5 | 201.4±23.9 | 6.8±3.6 | 5.5±3.3 | 0.48±0.73 | 4.22±0.89 |
| F value | – | 0.86 | 0.97 | 0.97 | 2.24 | 1.02 | 1.22 | 1.05 |
| P-value |
| >0.05 | >0.05 | >0.05 | <0.05 | <0.05 | <0.05 | <0.05 |
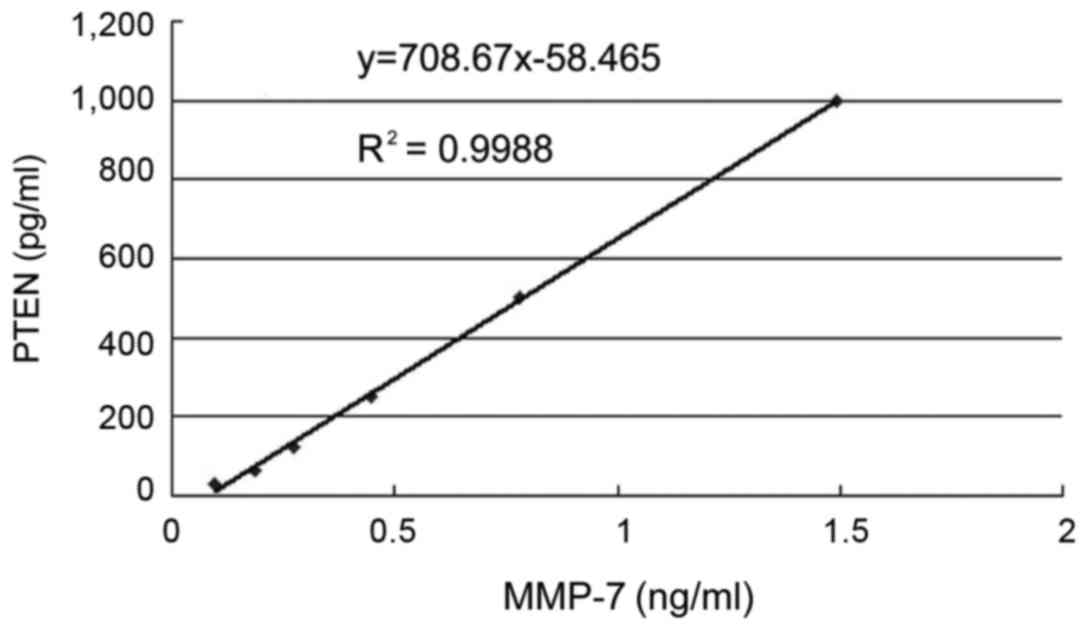
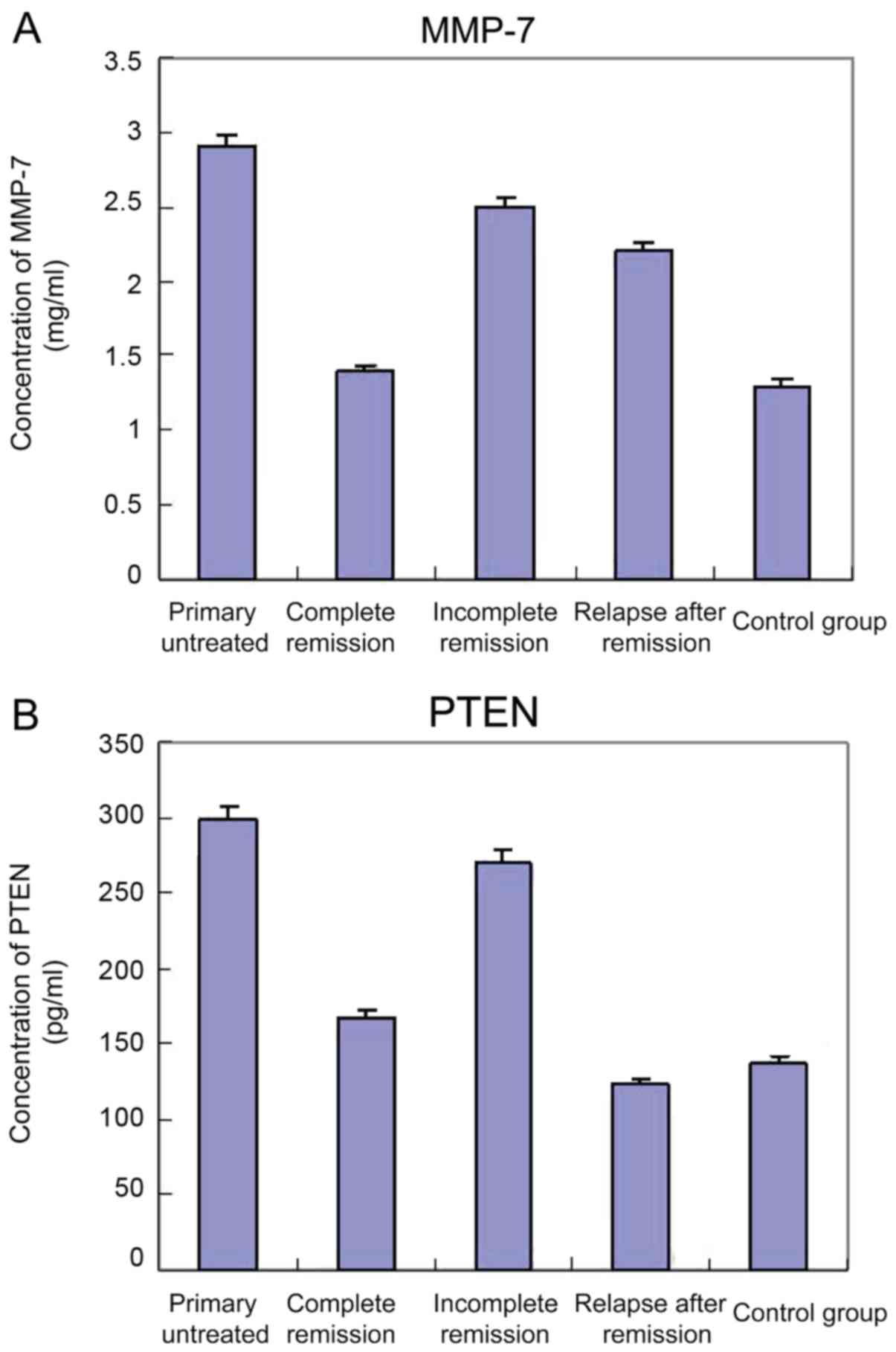
Serum MMP-7 levels in AML
patients
The levels of MMP-7 in 80 AML patients and 20
healthy control subjects were measured by ELISA. Compared with the
control group, the levels of serum MMP-7 were significantly
increased (P<0.05) in 20 patients with primary untreated AML,
while there was no significant difference in the PTEN levels when
compared with the incomplete remission group. Compared with the
control group and complete remission group, both the serum levels
of MMP-7 and PTEN in the samples of 21 patients with incomplete
remission were significantly increased (P<0.05). In addition,
the levels of MMP-7 in 19 patients of relapse after remission were
significantly higher than those of the complete remission group and
healthy control (P<0.05), and the serum PTEN levels were not
significantly altered. Serum MMP-7 and PTEN levels are shown in
Table II, and the standard curve is
shown in Fig. 2.
 | Table II.Serum MMP-7 and PTEN levels (mean ±
standard deviation). |
Table II.
Serum MMP-7 and PTEN levels (mean ±
standard deviation).
| Group | No. of cases | PTEN (pg/ml) | MMP-7 (ng/ml) |
|---|
| Primary
untreated | 20 | 298.45a,b | 2.887a,b |
| Complete
remission | 20 | 166.84 | 1.52 |
| Incomplete
remission | 21 | 270.81a,b | 2.50a,b |
| Relapse after
remission | 19 | 123.90a,b | 2.35a,b |
| Control | 20 | 137.55 | 1.45 |
Correlation analysis between MMP-7 and
PTEN
For the Pearson correlation analysis, MMP-7 was
considered the dependent variable, and PTEN the independent
variable. It was found that r=0.37, P=0.46, i.e., the levels of
MMP-7 and PTEN were not correlated (P>0.05) (Fig. 3). Thus, the serum levels of MMP-7 and
PTEN did not affect each other.
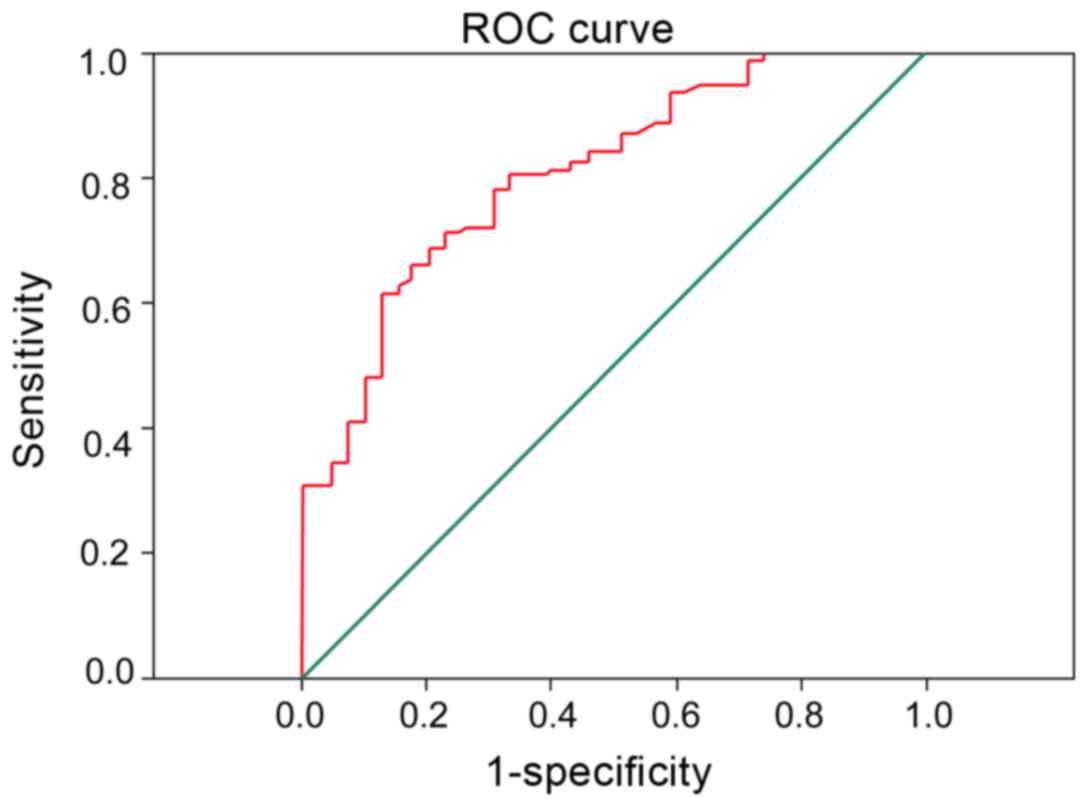
Combined diagnostic efficacy of PTEN
and MMP-7 in serum
To determine the significance of MMP-7 combined with
PTEN in the diagnosis of different stages of AML patients. It was
found that MMP-7 combined with PTEN significantly improved the
diagnostic performance of the different treatment stages of AML
patients, and the area under ROC curve was 0.902 (Fig. 4).
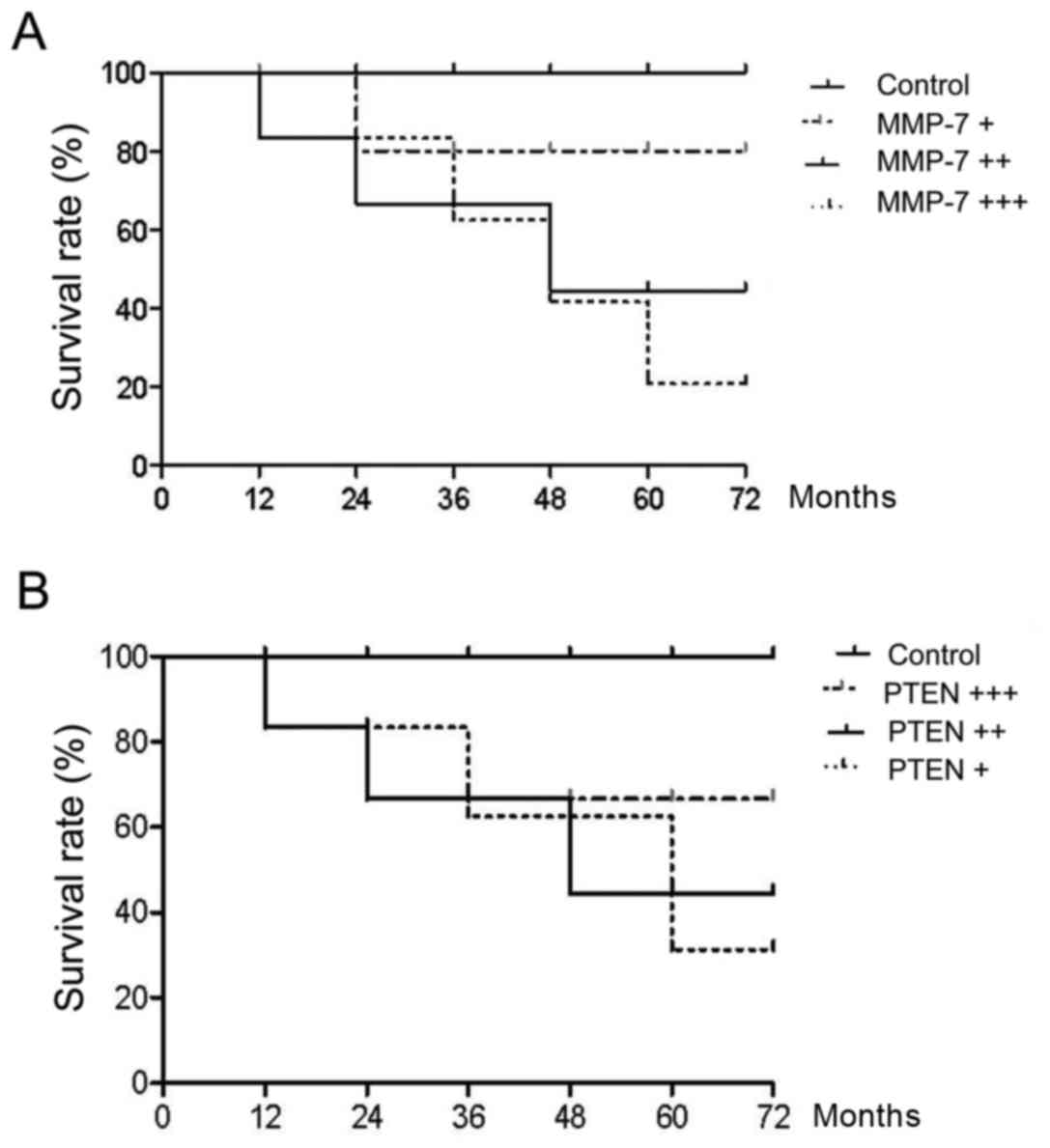
Comparison of survival rate
We followed up for 80 AMP patients for 3 months
after treatment. Both serum levels of PTEN and MMP-7 were detected.
Patient survival was compared. The results showed that after
complete remission, higher serum MMP-7 levels correlated with worse
prognosis and shorter survival while higher PTEN levels indicated
better prognosis (Fig. 5).
Discussion
Small blood vessels in the tissue surrounding the
tumor are invaded by germination and tumor tissues, known as tumor
angiogenesis. This phenomenon is very common in hematologic
malignancies. Previous findings showed that the former
neovascularization was significantly less in patients with
monoclonal immunoglobulin than patients with multiple myeloma
disease (18). At the same time, in
the treatment of multiple myeloma, thalidomide was widely used in
clinical practice and the drug resistance on neovascularization was
also an important mechanism. This conclusion was confirmed in the
corresponding research in patients with acute lymphoblastic
leukemia (ALL) (18,19).
Folkman and Hochberg suggested that the growth and
metastasis of solid tumors depended on the generation of new blood
vessels (19). A number of studies
showed the overexpression of angiogenic factors (VEGF, MMP-7, PDGF,
TGF-β, bFGF, PLGF, IGF) in solid tumors, and that inhibition of the
expression of angiogenic factors could be used in the treatment of
malignant tumors (20,21). However, studies on the expression of
the angiogenic factor gene in non-solid tumors and its
anti-angiogenesis therapy are limited (19). The role of neovascularization in
malignant hematologic diseases has been widely accepted. Many
clinical trials and experiments confirmed that patients with
multiple myeloma, lymphoma and leukemia were accompanied by
angiogenesis (22,23). Numerous newborn blood vessels in bone
marrow and the levels of angiogenic factors in patients showed an
increasing trend. These were closely related to the prognosis and
progression of the disease. By taking child patients with ALL as
the research focus and comparing them with patients without bone
marrow metastasis of lymphoma and patients with solid tumors, McKee
et al (24) investigated
newborn blood vessels in the bone marrow of the patients and found
that the blood vessel density of ALL patients was significantly
higher than that of patients without bone marrow metastasis of
lymphoma and patients with solid tumors. At the same time, the
researchers who built the three-dimensional model of the blood
vessels in the bone marrow found that in the micro-vascular
imaging, the leukemia and control groups, respectively, showed
arborization and single-straight shape (25). By detection of urinary BFGF and
fibroblast growth factor, the researchers found that urinary BFGF
levels in children with disease onset were significantly higher
than those after complete remission group (25). Results of the present study showed
that in primary untreated AML patients, serum MMP-7 was
significantly higher than that in the healthy controls. By
contrast, the level of MMP-7 was decreased significantly in the
complete remission group compared with the control group.
Differences were not statistically significant (P>0.05). This
was similar to the results of previous studies, which undertook
research on the bone marrow from 20 adult AML patients, and
compared the above samples with those of normal subjects (25). Those authors eventually found that
angiogenesis in the bone marrow of AML patients was significantly
increased in comparison with the control group.
However, we also found that in the diagnosis of AML,
MMP-7 exhibited sensitivity without specificity. This was because
other types of leukemia may also express MMP-7 to a certain degree
(26). Therefore, we further detected
the serum level of PTEN in patients. Previous studies found that
PTEN may be used as a marker for the diagnosis of some solid
tumors, especially in the evaluation of metastasis and prognosis of
malignant tumor (27). Previous
findings showed that the level of PTEN was significantly increased
in the early stage of cervical cancer, although the level of PTEN
was significantly decreased with the increasing degree of
malignancy (27). PTEN played a role
by competing with tyrosine kinase as a tumor suppressor and played
a regulatory role by preventing cell growth and mitosis (28). However, the underlying mechanism
remains to be clarified. In human solid tumors, this role has also
not been confirmed. Our study found that in primary untreated AML
patients, both MMP-7 and PTEN levels were significantly increased,
while in patients with relapse after remission, MMP-7 levels were
significantly increased, while PTEN levels were normal or even low.
Given these reasons, we hypothesized that in the early stage of
cancer, MMP-7 was activated by certain factors to promote the
occurrence of carcinogenesis. At the same time, the tumor
suppressor gene PTEN was also activated by inhibiting tumor cell
mitosis to suppress tumor cell proliferation and distant
metastasis. However, during disease progression, the proto-oncogene
was activated by various signaling pathways and the release of
inflammatory cytokines, which may inhibit the PTEN signaling
pathway. As a result, the protective effect of tumor suppressor
gene PTEN was weakened, and tumor metastasis was promoted, albeit
this hypotheseis remains to be confirmed (27–29).
To sum up, PTEN and MMP-7 exert a synergistic effect
in the generation of new blood vessels. Detection of the expression
level of PTEN and MMP-7 in AML patients at different stages of
treatment was clinically significant for the diagnosis of AML and
targeted therapy.
References
|
1
|
Gallogly MM and Lazarus HM: Midostaurin:
An emerging treatment for acute myeloid leukemia patients. J Blood
Med. 7:73–83. 2016.PubMed/NCBI
|
|
2
|
Grain A, Sirvent A, Strullu M, Françoise
M, Mohty M, Guillaume T, Chevallier P and Rialland F: Sustained
responses after clofarabine-based sequential allogeneic stem cell
transplantation in children with high-risk, relapse and/or
refractory acute myeloid leukemia or juvenile myelomonocytic
leukemia: A study on behalf of the French society of bone marrow
transplantation or cell therapy (SFGM-TC). Leuk Lymphoma.
57:2937–2941. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yung KW, Yung TT, Chung CY, Tong GT, Liu
Y, Henderson J, Welbeck D and Oseni S: Principles of cancer
staging. Asian Pac J Surg Oncol. 1:1–16. 2015.
|
|
4
|
Sterling B, Cole R, Jen KK and Shieh JS:
Surgical oncology in the elderly. Asian Pac J Surg Oncol. 1:83–100.
2015.
|
|
5
|
Lee R, Yeung AW, Hong SE, Brose MS and
Michels DL: Principles of medical oncology. Asian Pac J Surg Oncol.
1:39–46. 2015.
|
|
6
|
Buchanan J and Tirado CA: A
t(16;21)(p11;q22) in acute myeloid leukemia (AML) resulting in
fusion of the FUS/TLS and ERG genes: A review of the literature. J
Assoc Genet Technol. 42:24–33. 2016.PubMed/NCBI
|
|
7
|
Presta M, Dell'Era P, Mitola S, Moroni E,
Ronca R and Rusnati M: Fibroblast growth factor/fibroblast growth
factor receptor system in angiogenesis. Cytokine Growth Factor Rev.
16:159–178. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hajime M, Shuichi Y, Makoto N, Masanori Y,
Ikuko K, Atsushi K, Mutsuo S and Keiichi T: Inhibitory effect of
4-methylesculetin on hyaluronan synthesis slows the development of
human pancreatic cancer in vitro and in nude mice. Int J Cancer.
120:2704–2709. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
de Bont ES, Rosati S, Jacobs S, Kamps WA
and Vellenga E: Increased bone marrow vascularization in patients
with acute myeloid leukaemia: A possible role for vascular
endothelial growth factor. Br J Haematol. 113:296–304. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Milella M, Falcone I, Conciatori F, Cesta
Incani U, Del Curatolo A, Inzerilli N, Nuzzo CM, Vaccaro V, Vari S,
Cognetti F and Ciuffreda L: PTEN: Multiple functions in human
malignant tumors. Front Oncol. 5:242015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hopkins BD, Hodakoski C, Barrows D, Mense
SM and Parsons RE: PTEN function: The long and the short of it.
Trends Biochem Sci. 39:183–190. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yonemura Y, Endou Y, Fujita H, Fushida S,
Bandou E, Taniguchi K, Miwa K, Sugiyama K and Sasaki T: Role of
MMP-7 in the formation of peritoneal dissemination in gastric
cancer. Gastric Cancer. 3:63–70. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kioi M, Yamamoto K, Higashi S, Koshikawa
N, Fujita K and Miyazaki K: Matrilysin (MMP-7) induces homotypic
adhesion of human colon cancer cells and enhances their metastatic
potential in nude mouse model. Oncogene. 22:8662–8670. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Safranek J, Pesta M, Holubec L, Kulda V,
Dreslerova J, Vrzalova J, Topolcan O, Pesek M, Finek J and Treska
V: Expression of MMP-7, MMP-9, TIMP-1 and TIMP-2 mRNA in lung
tissue of patients with non-small cell lung cancer (NSCLC) and
benign pulmonary disease. Anticancer Res. 29:2513–2517.
2009.PubMed/NCBI
|
|
15
|
Chang MC, Chen CA, Chen PJ, Chiang YC,
Chen YL, Mao TL, Lin HW, Lin Chiang WH and Cheng WF: Mesothelin
enhances invasion of ovarian cancer by inducing MMP-7 through
MAPK/ERK and JNK pathways. Biochem J. 442:293–302. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Koskensalo S, Mrena J, Wiksten JP,
Nordling S, Kokkola A, Hagström J and Haglund C: MMP-7
overexpression is an independent prognostic marker in gastric
cancer. Tumour Biol. 31:149–155. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mitsiades N, Yu WH, Poulaki V, Tsokos M
and Stamenkovic I: Matrix metalloproteinase-7-mediated cleavage of
Fas ligand protects tumor cells from chemotherapeutic drug
cytotoxicity. Cancer Res. 61:577–581. 2001.PubMed/NCBI
|
|
18
|
Sanna M, Caocci G, Vacca A, Piras E, Orrù
F and La Nasa G: Daunorubicin, cytarabine, and cladribine regimen
plus radiotherapy and donor lymphocyte infusion for extramedullary
relapse of acute myeloid leukemia after hematopoietic stem cell
transplantation. Case Rep Hematol. 2013:2580282013.PubMed/NCBI
|
|
19
|
Folkman J and Hochberg M: Self-regulation
of growth in three dimensions. J Exp Med. 138:745–753. 1973.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Poon RT, Fan ST and Wong J: Clinical
implications of circulating angiogenic factors in cancer patients.
J Clin Oncol. 19:1207–1225. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jones LW, Fels DR, West M, Allen JD,
Broadwater G, Barry WT, Wilke LG, Masko E, Douglas PS, Dash RC, et
al: Modulation of circulating angiogenic factors and tumor biology
by aerobic training in breast cancer patients receiving neoadjuvant
chemotherapy. Cancer Prev Res (Phila). 6:925–937. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hussong JW, Rodgers GM and Shami PJ:
Evidence of increased angiogenesis in patients with acute myeloid
leukemia. Blood. 95:309–313. 2000.PubMed/NCBI
|
|
23
|
Mineo M, Garfield SH, Taverna S, Flugy A,
De Leo G, Alessandro R and Kohn EC: Exosomes released by K562
chronic myeloid leukemia cells promote angiogenesis in a
Src-dependent fashion. Angiogenesis. 15:33–45. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
McKee C, Perez-Cruet M, Chavez F and
Chaudhry GR: Simplified three-dimensional culture system for
long-term expansion of embryonic stem cells. World J Stem Cells.
7:1064–1077. 2015.PubMed/NCBI
|
|
25
|
Bieker R, Padró T, Kramer J, Steins M,
Kessler T, Retzlaff S, Herrera F, Kienast J, Berdel WE and Mesters
RM: Overexpression of basic fibroblast growth factor and autocrine
stimulation in acute myeloid leukemia. Cancer Res. 63:7241–7246.
2003.PubMed/NCBI
|
|
26
|
Cheng HW, Li CW, Chan KY, Au HY, Chan PF,
Sin YC, Szeto Y and Sham MK: End-of-life characteristics and
palliative care provision for elderly patients suffering from acute
myeloid leukemia. Support Care Cancer. 23:111–116. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Burgucu D, Guney K, Sahinturk D, Ozbudak
IH, Ozel D, Ozbilim G and Yavuzer U: Tbx3 represses PTEN and is
over-expressed in head and neck squamous cell carcinoma. BMC
Cancer. 12:4812012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li L, Wang LX, Xu GL, Yang F, Gao QL, Niu
H, Shi B and Jiang X: Bio-informatics analysis of renal carcinoma
gene matrix metalloproteinase-7. Indian J Cancer. 53:13–18. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mieszało K, Ławicki S and Szmitkowski M:
The utility of metalloproteinases (MMPs) and their inhibitors
(TIMPs) in diagnostics of gynecological malignancies. Pol Merkur
Lekarski. 40:193–197. 2016.(In Polish). PubMed/NCBI
|