Introduction
Esophageal squamous cell carcinoma (ESCC) is the
most malignant lesion in China, particularly in Linzhou, Henan
(1). Treatment of ESCC is largely
useless due to the occurrence of invasion and metastasis in the
early stage. However, the mechanism of invasion and metastasis of
ESCC has been poorly elucidated (2).
Epithelial-to-mesenchymal transition (EMT) now is
always viewed as a key event that occurs during cancer invasion and
metastasis. However, current understanding of the alterations that
give rise to the occurrence of EMT is limited. MicroRNAs
(miRNA/miRs) are small non-coding RNA molecules that may directly
regulate target gene expression by binding to their 3′-untranslated
regions (3′-UTRs) (3,4). Furthermore, miRNAs have been
demonstrated to serve notable functions in tumor invasion and
metastasis by functioning as EMT suppressors or inducers (5,6). miR-106b
is a type of miRNA transcribed from the miR-106b-25 cluster located
on chromosome 7 (7). Yau et al
(8) reported that miR-106b
overexpressed in hepatocellular carcinoma cells and that the
overexpression of miR-106b may promote cell migration and
metastasis by activating the EMT process. Zhou et al
(9) additionally demonstrated that
miR-106b contributed as an EMT inducer in breast cancer. However,
whether miR-106b participates in the invasion and metastasis
process of ESCC by inducing EMT and the mechanism of how miR-106b
induces EMT in ESCC had not been fully explored until now.
miRNAs participate in the occurrence and development
of a cancer by regulating the post-transcriptional process of their
target gene. Several genes have been verified to be the targets of
miR-106b, including transforming growth factor-β type II receptor,
cyclin dependent kinase inhibitor 1A, DLC-1 Rho GTPase activating
protein, signal transducer and activator of transcription 3 and
mitogen-activated protein kinase 14 (7,10–12). Phosphatase and tensin homolog (PTEN),
a key negative regulator of the phosphoinositide 3-kinase
(PI3K)/protein kinase B (Akt) pathway, is also a direct target of
miR-106b (13). One previous study
had reported that PTEN served as a tumor-suppressing gene in cancer
invasion and metastasis (14).
Additionally, PTEN is a key factor in inducing EMT (15). However, whether miR-106b regulates the
expression and activity of PTEN in ESCC has never been previously
elucidated, to the best of our knowledge.
In the present study, it was determined that the
expression of miR-106b was substantially higher in ESCC tissues and
cell lines compared with non-tumorous tissues and cells.
Furthermore, it was demonstrated that the upregulation of miR-106b
was significantly associated with ESCC tissues lymph node
metastasis and that the reduction of miR-106b expression in ESCC
cell lines may inhibit cell metastasis, invasion and proliferation.
Further mechanism exploration revealed that PTEN was a direct
target of miR-106b. Therefore, miR-106b may participate in the
invasion and metastasis of ESCC via PTEN mediated EMT.
Materials and methods
Patients
Samples of ESCC tissues and adjacent normal mucosa
in paraffin-embedded blocks were collected from 32 patients who
were undergoing an esophagectomy in the First Affiliated Hospital
of Zhengzhou University (Henan, China). The mean age of the 32
patients (14 females and 18 males) was 60.7±8.1 years (range,
40–78). None of the patients had received radiation therapy or
chemotherapy prior to surgery. All specimens were dissected,
transferred and frozen immediately in liquid nitrogen at 196°C for
RNA extraction. The present study was approved by the Ethics
Committee of Zhengzhou University (Henan, China) and written
informed consent was obtained from all patients prior to
surgery.
Cell lines, cell culture and cell
transfection
Immoral embryonic esophageal epithelium cell line
SHEE and the esophageal squamous cell carcinoma cell lines EC-1 and
EC9706 were all maintained at 37°C and 5% CO2, in
RPMI-1640 medium with 10% fetal calf serum (Hangzhou Sijiqing
Bioengineering Material Co., Hangzhou, China), supplemented with
100 µg/ml streptomycin and 100 U/ml penicillin G. Transfection was
generally performed on cells that were at ~65% density. miR-106b
inhibitor purchased from GE Healthcare Dharmacon, Inc. (Lafayette,
CO, USA) was transfected into EC-1 or EC9706 cells by using
Lipofectamine® 2000 at room temperature for 6 h
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA).
EC-1 or EC9706 cells transfected with scrambled miRNA purchased
from GE Healthcare Dharmacon, Inc., or blank EC-1 or EC9706 cells
were used respectively as negative or blank controls. For mRNA and
protein assays, cells were all collected after 48 h of
transfection. PTEN siRNA designed and synthesized by Zhengzhou
Chuangsheng Company Co., Ltd. (Zhengzhou, China) were transfected
together with miR-106b inhibitor or scrambled miRNA into EC-1 or
EC9706 cells by using Lipofectamine® 2000 at room
temperature for 6 h. EC-1 or EC9706 cells transfected individually
with PTEN siRNA or blank EC-1 cells were used as the control.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
assay
Total RNA was extracted using either an miRNeasy kit
(Qiagen, Inc., Valencia, CA, USA) or TriReagent (Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) at room temperature for 10 min
according to the manufacturer's protocols from tissues lysates or
cell samples and reverse transcribed to cDNA with
TaqMan® miRNA Reverse Transcription kit (Applied
Biosystems; Thermo Fisher Scientific, Inc.) or tranScript
First-strand cDNA Synthesis SuperMix kit (Qiagen, Inc.). The
relative expression levels of miR-106b were determined using the
TaqMan® MicroRNA Assay (Applied Biosystems; Thermo
Fisher Scientific, Inc.) protocol specific for miR-106b and were
normalized to that of internal control U6 by using the
2−ΔΔCq method (16) While
the relative expression levels of PTEN were determined using the
STBR Premix Ex Taq™ kit (Qiagen GmbH, Hilden, Germany) with the
following primers: PTEN sense, ACTGGACCACCTAATTGCTGT and antisense,
GCAAACTTTTACACTGGCAGC; E-cadherin forward, CTCAAAGCCCAGAATCCCCA and
reverse, CGGTTTTCTGTGCACACCTG; vimentin forward,
TCCGCACATTCGAGCAAAGA and reverse, ATTCAAGTCTCAGCGGGCTC. The
relative amount of mRNA was normalized by using β-actin.
Experiments were performed in triplicate. The thermal cycle for
miRNA was: 94°C for 3 min; 95°C for 15 sec; 65°C 40 times for 40
sec; and 62°C for 40 sec. For others the thermal cycle was: 95°C
for, 10 min; 94°C for 40 sec; 56°C for 35sec; 72°C 30 times for 60
sec; and 72°C for 10 min.
Western blot assay
The protein contents of tissues or cell lysates were
measured using a BCA kit (Beyotime Institute of Biotechnology,
Shanghai, China). Then suitable amount of total protein of each
sample (10–20 µg) was separated by 10% SDS-PAGE. Following vertical
electrophoretic separation, proteins were shifted to polyvinylidene
fluoride (PVDF) membranes by wet transfer. Subsequently, the PVDF
membranes were blocked with 5% fat-free milk for 1 h at room
temperature, and then incubated with rabbit anti-PTEN antibodies
(dilution 1:1,000; cat no. AF1426) rabbit anti-E-cadherin (dilution
1:500; cat. no. AF0138) and rabbit anti-vimentin (dilution 1:1,000;
cat. no. AF1975) (all from Beyotime Institute of Biotechnology) at
4°C overnight. Following washing the membranes for three times in
tris-buffered saline with Tween-20 (TBST), the membranes were
incubated with HRP-conjugated secondary antibody (dilution 1:5,000;
cat. no. A0208; Beyotime Institute of Biotechnology) at room
temperature for 1 h. Subsequent to washing three times with TBST
again, exposure and development were performed using an ECL
Chemiluminescence Detection kit (GE Healthcare Life Sciences,
Little Chalfont, UK) and the densities were measured using the
GS-900™ Calibrated Densitometer with ImageLab 4.1 software from
Bio-Rad Laboratories, Inc. (Hercules, CA, USA). All of the assays
were performed in triplicate.
Boyden chamber invasion assay
A Boyden chamber invasion assay was performed as
previously described (17). Briefly,
the upper chamber was coated with Matrigel were filled with EC-1 or
EC9706 cells suspended in serum-free RPMI-1640 medium, while the
lower chamber was loaded with RPMI-1640 medium with 10% fetal calf
serum. EC-1 or EC9706 cells were allowed to invade through the
filter for 48 h at 37°C and the cells on the upper sides were
removed using cotton swabs. The cells that invaded to the lower
side were subjected to H&E staining at room temperature for 7
min and calculated in five random fields per membrane under
inverted microscopy at ×200 magnification. Migration experiments
were performed in triplicate.
Wound healing assay
EC-1 (5×105) or EC9706 cells
(5×105) were plated into 12-well plates and grew to 70%
confluence. Then, a constant wide wound (1 mm) was created using a
plastic tip on monolayer cultured EC-1 or EC9706 cells. A total of
48 h after the migration of EC-1 or EC9706 cells, the average
migrating distance was measured under an inverted microscope and
photographed at ×200 magnification.
Cell Counting kit-8 (CCK-8) assay
The proliferation ability of EC-1 or EC9706 cells
was measured using a CCK-8 kit according to the manufacturer's
instructions. In briefly, EC-1 or EC9706 cells were seeded into
96-well plates at a density of 5×103 cells/well and
incubated for 48 h at room temperature. Subsequently, 10 µl CCK-8
solution was added to the well and cultured for another 1 h at room
temperature. The optical density was calculated by measuring the
absorbance at 450 nm. All of the experiments were performed in
triplicate.
Target prediction
The prediction of the 3′UTR of PTEN as a binding
target of miR-106b was checked by using PicTar (pictar.mdc-berlin.de) and Targetscan (www.targetscan.org).
Luciferase activity assay
Pmir-REPORT system (Promega Corporation, Madison,
WI, USA) was used to determine whether PTEN was a direct target of
miR-106b. 3′UTR of the PTEN gene was amplified, sub-cloned into the
pmirGLO luciferase reporter vector and amplified in DH5α. The
resulting reporter vector was designated as pmirGLO-PTEN-WT. A
site-directed mutagenesis kit (Stratagene; Agilent Technologies,
Inc., Santa Clara, CA, USA) was used to produce the mutant type of
PTEN 3′UTR luciferase reporter vector (pmirGLO-PTEN-MUT), according
to the manufacturer's protocol. The luciferase reporter vectors
were verified by sequencing. miR-106b inhibitor or scrambled miRNA,
100 ng pmirGLO-PTEN-WT or pmirGLO-PTEN-MUT was co-transfected into
EC-1 cells in 6-well plates using Lipofectamine® 2000 at
room temperature. After 48 h, luciferase activity was measured
using a Dual-Luciferase Reporter Assay system (Promega
Corporation).
Statistical analysis
Statistical analysis was performed using SPSS 17.0
(SPSS, Inc., Chicago, IL, USA). Multi-group comparisons were
performed using a one-way analysis of variance test with a least
significant difference test used for post-hoc comparisons.
P<0.05 was considered to indicate a statistically significant
difference. And the data were presented as the mean ± the standard
deviation.
Results
miR-106b was upregulated in ESCC
tissues and cell lines
To evaluate the expression levels of miR-106b in
ESCC tissues and adjacent normal mucosa tissues, RT-qPCR was
performed. The results revealed that the expression levels of
miR-106b in ESCC tissues were significantly increased compared with
those in adjacent normal mucosa tissue (P<0.01; Fig. 1A). miR-106b has been reported to serve
a notable function in cancer invasion and metastasis, thus the
association between miR-106b and the clinicopathological parameters
of patients with ESCC was analyzed. As was expected, the
upregulation of miR-106b was significantly associated with lymph
node metastasis (P<0.05; Fig. 1B).
Then, the expression levels of miR-106b were examined in ESCC cell
lines EC-1 and EC9706. They were revealed to be significantly
higher, compared with that in the immortal embryonic esophageal
epithelium cell line SHEE (P<0.05). These findings suggest that
miR-106b may promote tumorigenesis in ESCC (Fig. 1C).
Downregulation of miR-106b expression
may inhibit the invasion, metastasis and proliferation ability of
ESCC cells
To further elucidate the function of miR-106b in
ESCC invasion and metastasis, miR-106b inhibitor was transfected
into EC-1 or EC9076 cells to investigate the effects of the
downregulation of miR-106 on the invasion and metastasis ability of
ESCC cell lines. EC-1 cells transfected with scrambled miRNA and
vacant EC-1 cells were used as negative and blank controls,
respectively. The results of RT-qPCR revealed that miR-106b
inhibitor significantly decreases miR-106b expression compared with
those in control groups 48 h after transfection in EC-1 cells
(P<0.01; Fig. 2A).
Then, the function of miR-106b in ESCC cells was
evaluated by measuring the cell invasion and metastasis ability of
EC-1 cells which were transfected with miR-106b inhibitor. EC-1
cells transfected with scrambled miRNA or vacant EC-1 cells were
used as negative and blank controls, respectively. Compared with
the control groups, the number of EC-1 cells transfected with
miR-106b inhibitor that traversed the Matrigel were significantly
lower (P<0.05; Fig. 2B). The
results of a wound-healing assay further indicated the function of
miR-106b in the invasion and metastasis of EC-1 cells. The wound
healing ability of EC-1 cells transfected with miR-106b inhibitor
was significantly decreased compared with those in control groups
(P<0.01; Fig. 2C).
The increase of the invasion and metastasis ability
of cancer cells is usually closely associated with an increase of
the proliferation ability. Thus, the effects of miR-106b inhibitor
on the proliferation ability of EC-1 cells were examined by using a
CCK-8 assay. The results revealed that the downregulation of
miR-106b inhibited cell proliferation significantly compared with
control groups at 48 and 72 h (Fig.
2D), indicative that the expression change of miR-106b may
alter the proliferation of ESCC cells.
Then similar results were obtained in EC9706 cells
(Fig. 2E-H). Thus, it may be
concluded that miR-106b is an important factor in the invasion and
metastasis process of ESCC cells.
Downregulation of miR-106b expression
could inhibit the induction of EMT in ESCC cells
Previous evidence has reported that miRNAs promote
invasion and metastasis through mediating EMT (18). In order to evaluate the function of
miR-106b in inducing EMT in ESCC, we examined the mRNA and protein
expression of the epithelial marker E-cadherin and the mesenchymal
marker vimentin in EC-1 cells. As presented in Fig. 3A and B, with the downregulation of
miR-106b in EC-1 cells, the mRNA and protein expression levels of
E-cadherin were significantly increased compared with those in
control groups (P<0.05), whilst the expression levels of
vimentin were significantly decreased compared with the control
groups (P<0.05). Additionally, similar results were revealed in
EC9706 cells (Fig. 3C and D). Thus,
miR-106b was concluded to be capable of advancing invasion and
metastasis by regulating EMT in ESCC cells. Due to its predominant
function in ESCC EMT, miR-106b may be used as a target to limit
EMT-associated invasion and metastasis.
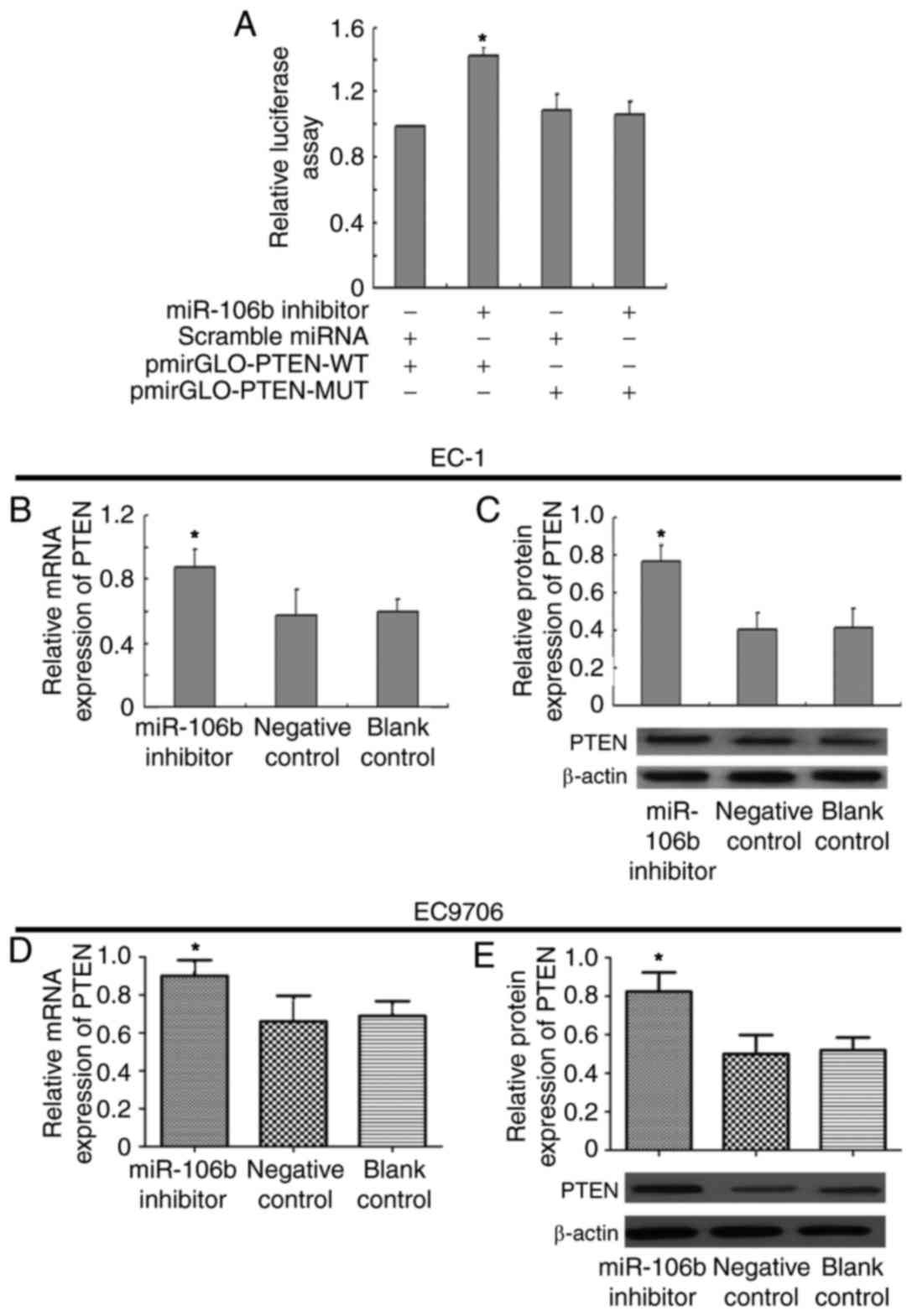
PTEN was directly targeted by miR-106b
via the 3′-UTR
Although the major function of miR-106b in the
occurrence and development of ESCC had already been elucidated, the
mechanism by which miR-106b promoted invasion and metastasis had
yet to be determined in ESCC cell lines. PTEN, a tumor suppressor,
is known to be a negative regulator of the PI3K-Akt pathway. Loss
or downregulation of PTEN has been identified in numerous different
cancer types, including ESCC, and has been revealed to be
associated with the invasive properties of cancer cells. By using
PicTar and TargetScan software, miR-106b was revealed to have a
putative biding site in the 3′-UTR of PTEN. In order to validate
whether PTEN was a functional target of miR-106b, miR-106b
inhibitor or scrambled miRNA was co-transfected with
pmirGLO-PTEN-WT or pmirGLO-PTEN-MUT in ESCC cells. As presented in
Fig. 4A, ESCC cells with miR-106b
inhibitor and pmirGLO-PTEN-WT co-transfection presented a
significant increase in reporter activity compared with ESCC cells
with scrambled miRNA and pmirGLO-PTEN-WT co-transfection
(P<0.05). Furthermore, ESCC cells co-transfected with miR-106b
inhibitor and pmirGLO-PTEN-MUT or scrambled miRNA with
pmirGLO-PTEN-MUT demonstrated no statistically significant change
in reporter activity (Fig. 4A). Thus,
the luciferase activity of the wild type PTEN-3′-UTR but not the
mutated PTEN-3′-UTR reporter was reduced by miR-106b in ESCC cells.
In order to further verify the regulatory effects of miR106b on
PTEN, RT-qPCR and western blot analysis were used to check the
expression levels of PTEN. The results revealed that with the
downregulation of miR-106b in EC-1 or EC9706 cells, the mRNA and
protein expression of PTEN were significantly increased (P<0.05;
Fig. 4B-E). However, the mRNA and
protein expression of PTEN in EC-1 or EC9706 cells transfected with
scrambled miRNA demonstrated no significant difference compared
with vacant EC-1 or EC9706 cells (Fig.
4B-E). These results indicate that miR-106b may regulate the
expression of PTEN in ESCC cells.
Downregulation of PTEN partially
increased the invasion, metastasis and proliferation ability of
ESCC cells
PTEN has been reported to participate in invasion
and metastasis (19). In order to
evaluate whether miR-106b may participate in the invasion and
metastasis of ESCC by regulating the expression of PTEN, PTEN siRNA
was chemically synthesized and co-transfected into EC-1 or EC9706
cells with miR-106b inhibitor. PTEN siRNA co-transfected with
scrambled miRNA, PTEN siRNA transfected into EC-1 or EC9706 cells
alone and vacant EC-1 or EC9706 cells were used as the controls. In
EC-1 cells, the expression levels of PTEN were significantly lower
in the PTEN siRNA transfected group compared with the untransfected
group (P<0.01; Fig. 5A).
Additionally, the expression levels of PTEN were significantly
lower in the PTEN siRNA and miR-106b inhibitor co-transfected group
compared with the miR-106b inhibitor alone transfected group
(P<0.05; Fig. 5A). A Boyden
chamber invasion assay and a wound healing assay were used again to
analyze the invasion and metastasis ability of EC-1 cells between
different groups. A significant difference was identified in the
PTEN siRNA and miR-106b inhibitor transfected group in EC-1 cells
in invasion and metastasis, compared with the miR-106b inhibitor
alone transfected group (P<0.05; Fig.
5B and C). In addition, the downregulation of PTEN may
partially rescue the proliferation ability of EC-1 or EC9706 cells
evaluated by using a CCK-8 assay, as a significant difference was
identified in the PTEN siRNA transfected group (P<0.01) and the
PTEN siRNA and miR-106b inhibitor transfected group (P<0.05) in
EC-1 cells (Fig. 5D). In EC9706
cells, downregulation of PTEN also rescued the invasion and
metastasis of EC9706 cells inhibited by miR-106b inhibitor, the
only difference was that no significant difference was revealed in
the proliferation ability of EC9706 cells (Fig. 5E-H). Altogether, miR-106b is
potentially a key regulator in the occurrence and development
process of ESCC that may occur via regulating the expression of
PTEN.
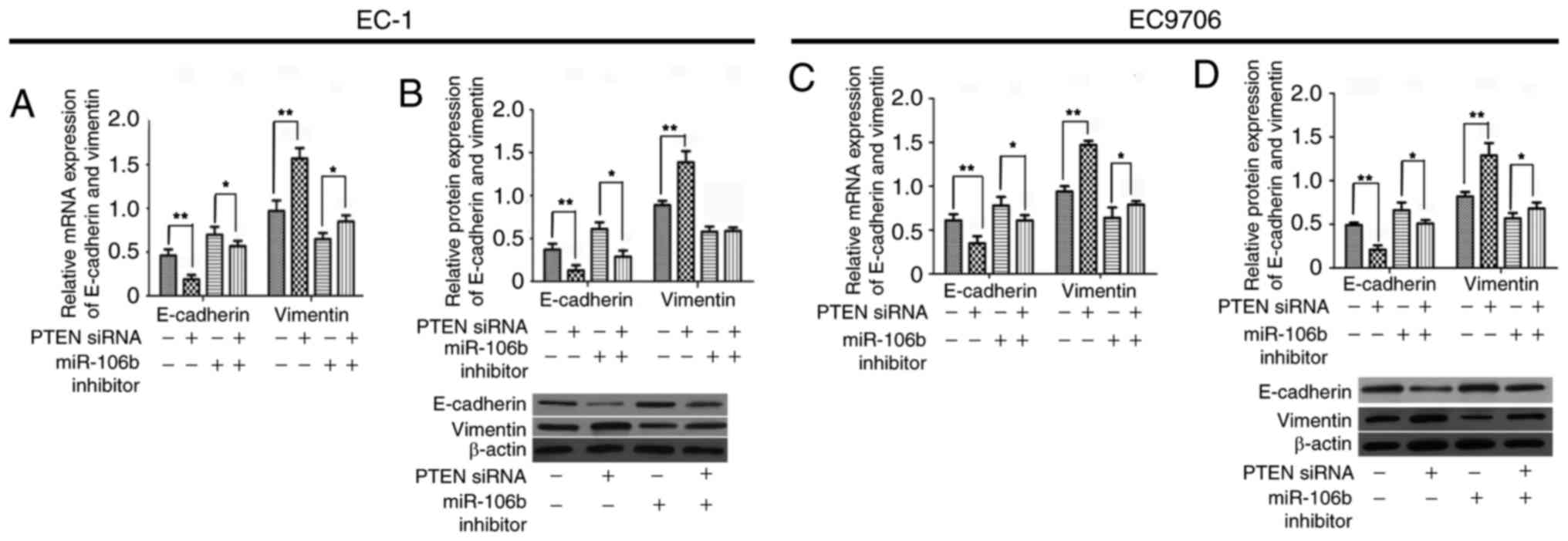
Downregulation of PTEN partially
rescued the induction of the EMT of ESCC cells
miRNAs modulate EMT by impacting the expression of
receptors, signaling pathways and specific ligands. PTEN serves a
notable function in tumor invasion and migration, and its deletion
and mutation is also present in numerous different cancer types and
is associated with EMT. As had been previously suggested, miR-106b
regulates the expression of PTEN by binding to its promoter.
However, whether PTEN contributes to the EMT induced by miR-106b in
ESCC had yet to be elucidated. In the present study, it was
revealed that downregulation of PTEN in EC-1 or EC9706 cells
partially rescued the EMT inhibited by decreased expression of
miR-106b. The mRNA and protein expression levels of E-cadherin in
EC-1 cells were significantly downregulated and levels of vimentin
were significantly upregulated in cells transfected with PTEN siRNA
alone compared with untransfected cells (P<0.01) and in cells
transfected with PTEN siRNA and miR-106b inhibitor compared with
cells transfected with miR-106b inhibitor alone (P<0.05;
Fig. 6A and B). Identical results
were revealed in EC9706 cells (Fig. 6C
and D). Collectively, the results of the present study
demonstrate that miR-106b induces EMT via suppressing the
expression of PTEN.
Discussion
miRNAs, small non-coding RNAs of ~22 nucleotides in
length, have been demonstrated to be associated with the occurrence
and development of human cancer types where they may function as
tumor suppressors or oncogenes (3–6,20,21).
Previous studies have demonstrated the abnormal expression of a
number of types of miRNAs in ESCC (22,23).
miR-106b is a member of the miR-106b-25 cluster and it has been
demonstrated to be upregulated in a number of different types of
cancer, including prostate cancer, hepatocellular cancer, gastric
cancer, laryngeal carcinoma and breast cancer (24–28).
However, its function and mechanism in ESCC have remained
unclear.
In the present study, the expression levels of
miR-106b in ESCC tissues were revealed to be significantly higher
compared with in adjacent normal mucosa. Consistent with the data
that was investigated in ESCC tissues, it was further verified that
the expression levels of miR-106b in the ESCC cell lines EC-1 and
EC9706 were also significantly higher compared with in the immoral
embryonic esophageal epithelium cell line SHEE, suggesting that the
upregulation of miR-106b may promote the occurrence of ESCC.
Furthermore, significant associations were also identified between
the expression levels of miR-106b and advanced stage and lymph node
metastasis, indicating that the expression of miR-106b also served
a notable function in ESCC invasion and metastasis.
To further elucidate the function of miR-106b in the
occurrence and development of ESCC, a miR-106b inhibitor and
scrambled miRNA were synthesized individually and transfected into
EC-1 or EC9706 cells using Lipofectamine® 2000 for 48 h,
and vacant EC-1 or EC9706 cells were used as the blank control. As
expected, the expression levels of miR-106b decreased significantly
in EC-1 or EC9706 cells transfected with miR-106b inhibitor
compared with those transfected with scrambled miRNA or vacant EC-1
or EC9706 cells. With the drop of the expression of miR-106b, the
invasion, metastasis and proliferation ability of EC-1 or EC9706
cells also dropped significantly compared with those transfected
with scrambled miRNA and vacant EC-1 or EC9706 cells. These results
further validate the hypothesis that miR-106b may function as an
oncogene and serves an important function in the promotion of
invasion, metastasis and proliferation in ESCC cells. EMT serves an
integral function in cancer metastasis and emerging evidence
reveals that miRNAs are involved in cancer metastasis by regulating
EMT (29). In the present study, it
was demonstrated that by the downregulation of miR-106b, the mRNA
and protein expression of E-cadherin were increased significantly
whilst the expression level of vimentin was decreased
significantly. Therefore, it was concluded that miR-106b induces
EMT in ESCC cells, and miR-106b potentially participates in the
metastasis process of ESCC cells by inducing EMT.
PTEN, in previous years, has been widely viewed as a
tumor suppressor gene, which regulates survival, growth, invasion,
metastasis and proliferation (30,31). PTEN
may regulate PI3K signaling by dephosphorylating the lipid
signaling intermediate phosphatidylinositol (3,4,5)-trisphosphate, and a clear link between
the PI3K pathway and cancer was established in the 1980s (32). miRNA always operated by binding to the
3′UTRs of their target genes and a number of previous reports have
confirmed that miR-106b serves its part in colorectal cancer,
gastric cancer and hepatocellular carcinoma (33,34). In
the present study, it was demonstrated that miR-106b inhibition may
result in the increased expression of PTEN in ESCC cells compared
with those in control cells. Using cell transfection and luciferase
reporter assay, it was additionally revealed that PTEN was a direct
target of miR-106b. It was revealed that the depletion of PTEN by
siRNA in EC-1 or EC9706 cells transfected with miR-106b inhibitor
may partially reverse the effects on invasion, metastasis and
proliferation caused by miR-106b downregulation. Furthermore, the
depletion of PTEN partially rescued EMT inhibited by miR-106b
inhibitor. These results further demonstrated that miR-106b
promoted the occurrence and development of ESCC via targeting PTEN
mediated EMT.
To conclude, the results of the present study
suggest that miR-106b was upregulated in ESCC tissues and cell
lines, particularly in ESCC tissues with lymph node metastasis.
Furthermore, it was demonstrated that function of miR-106b in ESCC
was dependent on regulating PTEN mediated EMT. miR-106b may be a
potential therapeutic target for the treatment of ESCC.
Acknowledgements
The present study was supported by the Science and
Technology Project of the Zhengzhou Municipal Science and
Technology Bureau (grant no. 141PPTGG438).
References
|
1
|
Qi B, Lu JG, Yao WJ, Chang TM, Qin XG, Ji
YH, Wang TY, Liu SG, Li HC, Liu YZ and Zhao BS: Downregulation of
microRNA-382 is associated with poor outcome of esophageal squamous
cell carcinoma. World J Gastroenterol. 21:6884–6891. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li X, Wu Z, Mei Q, Li X, Guo M, Fu X and
Han W: Long non-coding RNA HOTAIR, a driver of malignancy, predicts
negative prognosis and exhibits oncogenic activity in oesophageal
squamous cell carcinoma. Br J Cancer. 109:2266–2278. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Iorio MV and Croce CM: MicroRNA
dysregulation in cancer: Diagnostics, monitoring and therapeutics.
A comprehensive review. EMBO Mol Med. 4:143–159. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schetter AJ, Okayama H and Harris CC: The
Role of microRNAs in Colorectal Cancer. Cancer J. 18:244–252. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tie J, Pan Y, Zhao L, Wu K, Liu J, Sun S,
Guo X, Wang B, Gang Y, Zhang Y, et al: mir-218 inhibits invasion
and metastasis of gastric cancer by targeting the Robo1 receptor.
PLoS Genet. 6:e10008792010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li X, Liu X, Xu W, Zhou P, Gao P, Jiang S,
Lobie PE and Zhu T: c-MYC-regulated miR-23a/24-2/27a cluster
promotes mammary carcinoma cell invasion and hepatic metastasis by
targeting Sprouty2. J Biol Chem. 288:18121–18133. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang GJ, Li JS, Zhou H, Xiao HX, Li Y and
Zhou T: MicroRNA-106b promotes colorectal cancer cell migration and
invasion by directly targeting DLC1. J Exp Clin Cancer Res.
34:732015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yau WL, Lam CS, Ng L, Chow AK, Chan ST,
Chan JY, Wo JY, Ng KT, Man K, Poon RT and Pang RW: Over-expression
of miR-106b promotes cell migration and metastasis in
hepatocellular carcinoma by activating epithelial-mesenchymal
transition process. PLoS One. 8:e578822013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhou Y, Hu Y, Yang M, Jat P, Li K,
Lombardo Y, Xiong D, Coombes RC, Raguz S and Yagüe E: The
miR-106b~25 cluster promotes bypass of doxorubicin-induced
senescence and increase in motility and invasion by targeting the
E-cadherin transcriptional activator EP300. Cell Death Differ.
21:462–474. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang H, Liu J, Zong Y, Xu Y, Deng W, Zhu
H, Liu Y, Ma C, Huang L, Zhang L and Qin C: miR-106b aberrantly
expressed in a double transgenic mouse model for Alzheimer's
disease targets TGF-β type II receptor. Brain Res. 1357:166–174.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ivanovska I, Ball AS, Diaz RL, Magnus JF,
Kibukawa M, Schelter JM, Kobayashi SV, Lim L, Burchard J, Jackson
AL, et al: MicroRNAs in the miR-106b family regulate p21/CDKN1A and
promote cell cycle progression. Mol Cell Biol. 28:2167–2174. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dai F, Liu T, Zheng S, Liu Q, Yang C, Zhou
J, Chen Y, Sheyhidin I and Lu X: miR-106b promotes migration and
invasion through enhancing EMT via downregulation of Smad 7 in
Kazakh's esophageal squamous cell carcinoma. Tumour Biol.
37:14595–14604. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zheng L, Zhang Y, Liu Y, Zhou M, Lu Y,
Yuan L, Zhang C, Hong M, Wang S and Li X: miR-106b induces cell
radioresistance via the PTEN/PI3K/AKT pathways and p21 in
colorectal cancer. J Transl Med. 13:2522015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li Y, Cui J, Zhang CH, Yang DJ, Chen JH,
Zan WH, Li B, Li Z and He YL: High-expression of DJ-1 and loss of
PTEN associated with tumor metastasis and correlated with poor
prognosis of gastric carcinoma. Int J Med Sci. 10:1689–1697. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yan T, Jiang X, Guo X, Chen W, Tang D,
Zhang J, Zhang X, Zhang D, Zhang Q, Jia J and Huang Y: Electric
field-induced suppression of PTEN drives epithelial-to-mesenchymal
transition via mTORC1 activation. J Dermatol Sci. 85:96–105. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang T, Xuan X, Li M, Gao P, Zheng Y, Zang
W and Zhao G: Astragalus saponins affect proliferation, invasion
and apoptosis of gastric cancer BGC-823 cells. Diagn Pathol.
8:1792013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Harazono Y, Muramatsu T, Endo H, Uzawa N,
Kawano T, Harada K, Inazawa J and Kozaki K: miR-655 is an
EMT-suppressive MicroRNA targeting ZEB1 and TGFBR2. PLoS One.
8:e627572013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Qi Q, Ling Y, Zhu M, Zhou L, Wan M, Bao Y
and Liu Y: Promoter region methylation and loss of protein
expression of PTEN and significance in cervical cancer. Biomed Rep.
2:653–658. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang W and Luo YP: MicroRNAs in breast
cancer: Oncogene and tumor suppressors with clinical potential. J
Zhejiang Univ Sci B. 16:18–31. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu M and Mo YY: The Akt-associated
microRNAs. Cell Mol Life Sci. 69:3601–3612. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mei LL, Qiu YT, Zhang B and Shi ZZ:
MicroRNAs in esophageal squamous cell carcinoma: Potential
biomarkers and therapeutic targets. Cancer Biomark. 19:1–9. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sakai NS, Samia-Aly E, Barbera M and
Fitzgerald RC: A review of the current understanding and clinical
utility of miRNAs in esophageal cancer. Semin Cancer Biol.
23:512–521. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hudson RS, Yi M, Esposito D, Glynn SA,
Starks AM, Yang Y, Schetter AJ, Watkins SK, Hurwitz AA, Dorsey TH,
et al: MicroRNA-106b-25 cluster expression is associated with early
disease recurrence and targets caspase-7 and focal adhesion in
human prostate cancer. Oncogene. 32:4139–4147. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li BK, Huang PZ, Qiu JL, Liao YD, Hong J
and Yuan YF: Upregulation of microRNA-106b is associated with poor
prognosis in hepatocellular carcinoma. Diagn Pathol. 9:2262014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang Q, Zhang RW, Sui PC, He HT and Ding
L: Dysregulation of non-coding RNAs in gastric cancer. World J
Gastroenterol. 21:10956–10981. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cai K, Wang Y and Bao X: miR-106b promotes
cell proliferation via targeting RB in laryngeal carcinoma. J Exp
Clin Cancer Res. 30:732011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ni X, Xia T, Zhao Y, Zhou W, Wu N, Liu X,
Ding Q, Zha X, Sha J and Wang S: Downregulation of miR-106b induced
breast cancer cell invasion and motility in association with
overexpression of matrix metalloproteinase 2. Cancer Sci.
105:18–25. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ma F, Li W, Liu C, Li W, Yu H, Lei B, Ren
Y, Li Z, Pang D and Qian C: miR-23a promotes TGF-β1-induced EMT and
tumor metastasis in breast cancer cells by directly targeting CDH1
and activating Wnt/β-catenin signaling. Oncotarget. 8:69538–69550.
2017.PubMed/NCBI
|
|
30
|
Xu W, Yang Z, Zhou SF and Lu N:
Posttranslational regulation of phosphatase and tensin homolog
(PTEN) and its functional impact on cancer behaviors. Drug Des
Devel Ther. 8:1745–1751. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Madhunapantula SV and Robertson GP: The
PTEN-AKT3 Signaling Cascade as a Therapeutic Target in Melanoma.
Pigment Cell Melanoma Res. 22:400–419. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Georgescu MM: PTEN tumor suppressor
network in PI3K-Akt pathway control. Genes Cancer. 1:1170–1177.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ren P, Gong F, Zhang Y, Jiang J and Zhang
H: MicroRNA-92a promotes growth, metastasis, and chemoresistance in
non-small cell lung cancer cells by targeting PTEN. Tumour Biol.
37:3215–25. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zheng X, Li J, Peng C, Zhao J, Chi J, Meng
X, Yun X, Li D, Yu Y, Gao M and Li Y: MicroRNA-4 induces cisplatin
resistance by targeting PTEN in human tongue squamous cell
carcinoma. Oral Oncol. 51:998–1003. 2015. View Article : Google Scholar : PubMed/NCBI
|