Introduction
Cancer is a leading cause of mortality; ~8.2 million
people worldwide succumbed to cancer-associated mortality in 2012,
with lung cancer accounting for ~1.59 million cases of mortality,
or ~20% of the total (1). Lung cancer
cell metastasis is a primary cause of mortality and those that
experience it have a mortality rate of ≥90% (2). When it metastasizes, lung cancer has a
95% chance of affecting local lymph nodes and an 80% chance of
affecting other organs (3,4). Thus, in clinical terms, the overall
5-year survival rate from the time of diagnosis to mortality for
patients with lung cancer undergoing treatment is only 10–15%
(5). Cancer cell metastasis is a
complex multistep process involving invasion and migration
(6–8).
Matrix metalloproteinases (MMPs) are zinc/calcium-dependent
endopeptidases involved in the degradation of the extracellular
matrix, two examples of which are MMP-2 and −9 (7,9). MMP-2 is
a notable factor in the development of tumor metastasis (7,10). MMP-2
is highly expressed in malignant tumors and serves a key role in
cancer invasion and angiogenesis (7,11). The
inhibition of MMP-2 is, therefore, an effective strategy for
preventing tumor cell metastasis (11,12). The
NME/NM23 nucleoside diphosphate kinase 1 (NM23) gene was first
identified in the murine melanoma cell line exhibiting high
metastatic activity; increasing the expression of this gene may
also reduce the metastatic activity of tumor cells (13). When transfected with NM23 genes, a
variety of tumor cell types were reported to exhibit the inhibition
of metastatic properties, including migration, invasion, and
colonization (14–18). The NM23-H1 (NME1) gene was reported to
be the one closely associated with the metastasis of cancer cells,
including breast, lung and liver cancer (19–21).
Lindera aggregata (Sims) Kosterm is a
traditional Chinese herbal medicine often used in Asia. It has been
used to treat chest and abdominal pain, indigestion, regurgitation,
colds, hernia and frequent urination (22). Studies have demonstrated that L.
aggregata extract has antioxidant properties, and can inhibit
tumor cell growth and induce apoptosis (23–26). For
instance, Li et al (23)
identified that Lindera strychifolia extract was able to
inhibit the growth of lung cancer A549 and SBC-3 cell lines, and
induce cell apoptosis. Allografts also produced similar results;
L. strychifolia extract was able to inhibit the growth of
Lewis lung, A549 and SBC-3 cancer cells, and induce cancer cell
apoptosis (23). Isolinderalactone
(ILL) is a type of sesquiterpene compound obtained from the root
tuber of L. aggregata. Yen et al (27) verified that ILL could induce apoptosis
in the human breast cancer MDA-MB-231 cell line, possibly via the
inhibition of microRNA hsa-miR-30c expression and increasing the
expression of suppressor of cytokine signaling 3 (SOCS3). This in
turn inhibits the phosphorylation of signal transducer and
activator of transcription 3 (STAT3) and regulates the downstream
processing of STAT3 pathways, increasing B-cell lymphoma-2 (Bcl-2)
and Bcl-extra large protein expression, and inhibiting X-linked
inhibitor of apoptosis expression. A previous study also revealed
that sesquiterpene lactone compounds, including ILL, linderalactone
and linderane, were able to inhibit the proliferation of the A549
cancer cells, with ILL exhibiting the best inhibitory properties
(28). However, it remains unclear
whether ILL can inhibit lung cancer cell metastasis and the
associated mechanisms require further investigation.
The present study aimed to investigate the effects
of ILL on lung cancer A549 cell invasion and migration, as well as
the association between potential mechanisms, and the expression of
MMP-2 and NM23-H1 genes.
Materials and methods
Chemicals and reagents
ILL was purchased from Wuhan Chem Faces Biochemical
Co., Ltd. (Wuhan, China). RPMI-1640, minimum essential
medium-non-essential amino acids, Gluta MAX, trypsin, penicillin,
streptomycin, and sodium pyruvate were purchased from Gibco; Thermo
Fisher Scientific, Inc. (Waltham, MA, USA). Dimethyl sulfoxide
(DMSO) was purchased from Merck KGaA (Darmstadt, Germany).
Anti-β-catenin monoclonal antibody (mAb; cat. no. NBP1-54467),
anti-NM23 mAb (cat. no. NBP1-47398) and anti-E-cadherin mAb (cat.
no. NBP2-19051) were purchased from Novus Biologicals, LLC.
(Littleton, CO, USA). Anti-MMP-2 mAb (cat. no. 031129) and
anti-mouse immunoglobulin G (IgG)-horseradish peroxidase (HRP)
antibodies (cat. no. 140769-HRP) were purchased from United States
Biological (Salem, MA, USA). Anti-tissue inhibitor
metalloproteinase-2 (TIMP-2; cat. no. 5738) mAb was obtained from
Cell Signaling Technology, Inc. (Danvers, MA, USA). Transwell
inserts were acquired from Costar; Corning Incorporated (Corning,
NY, USA). Matrigel was purchased from BD Biosciences (Franklin
Lakes, NJ, USA). Curcumin (CUM) and protease inhibitor cocktail
(cat. no. S8820) were obtained from Sigma-Aldrich; Merck KGaA. All
chemicals used were of reagent grade or higher.
Cell culture
Human A549 lung cancer cells were obtained from the
Bioresource Collection and Research Center, Institute of Biological
Resources Conservation and Research (Hsinchu, Taiwan) and were
cultured in RPMI-1640 medium containing 10% (v/v) fetal bovine
serum (FBS; Gibco; Thermo Fisher Scientific, Inc.), 0.37% (w/v)
NaHCO3, penicillin (100 U/ml), and streptomycin (100
µg/ml) at 37°C in a humidified incubator under 5% CO2
and 95% air. An equal number (1×104/ml) of cells were
incubated for 24 h prior to the various treatments. Prior to
experimentation, the medium was removed, and the cells were washed
twice with PBS. Next, fresh RPMI-1640 medium (with 10% FBS)
containing various concentrations (1, 5, 10, and 20 µM) of ILL were
added andthe samples were incubated for 24 h. In addition, the
effects of CUM at a concentration of 10 µM were also evaluated and
used as a positive control, as CUM has been reported to inhibit the
migration and invasion of tumor cells by decreasing protein
expression and the activity of MMP-2 in tumor cells (29,30). Stock
solutions of ILL and CUM were dissolved in DMSO. Prior to use, the
compounds were diluted in 10% FBS in RPMI-1640 medium to the
desired concentrations at the time of addition. The highest
concentration of DMSO used did not exceed 0.1% (v/v) of the total
assay volume, which did not affect cell viability.
Cell growth analysis
Cell growth was assayed as described previously by
Yeh et al (31). A549 cells
were seeded in 6-well plates at a density of 1×105
cells/well and cultured at 37°C for 24 and 48 h. Various
concentrations of ILL werethen added to the cells to reach final
concentrations of 1, 5, 10, and 20 µM in the presence of FBS. The
control group contained 10% FBS. The cells were then cultured at
37°C, 5% CO2, and 95% air for 24 and 48 h, and the
trypan blue exclusion protocol was used to determine cell
viability, as described previously (32,33).
Briefly, an amount of 0.4% trypan blue dye solution equal to the
sample volume was added. After gentle mixing, samples were loaded
into a hemocytometer (cat. no. 065003, Marienfeld-Superior, Paul
Marienfeld GmbH & Co. KG, Lauda-Königshofen, Germany) for
counting under a microscope at ×100 magnification (33).
Cell migration assay
Tumor cell migration was assayed in transwell
chambers according to the methods reported by Repesh (34), with certain modifications as
previously described by Chuang et al (35). Briefly, transwell chambers with 6.5-mm
polycarbonate filters of 8-µm pore size were used. Following
preincubation with the tested compounds for ≥24 h, A549 cells
(2.5×105/ml) were suspended in 100 µl serum-free
RPMI-1640 medium and placed in the upper transwell chamber.
RPMI-1640 medium containing 10% FBS (300 µl) was placed in the
lower transwell chamber to serve as the source of chemoattractant.
The transwell was incubated for 12 h at 37°C. Subsequently, the
cells on the upper surface of the filter were completely wiped away
with a cotton swab. The cells on the lower surface of the filter
were fixed in methanolat 25°C for 10 min, stained with Giemsa at
25°C for 1 h, and counted under a microscope (Inverted Microscope;
ECLIPSE/TS100; Nikon Corporation, Tokyo, Japan; magnification,
×100). For each replicate, the tumor cells in 5 randomly selected
fields were determined, and the counts were averaged.
Cell invasion assay
Cell invasion was also assessed using transwell
chambers as described in the migration assay; however, each filter
was first coated with 100 µl of 1:20 diluted Matrigel in cold
RPMI-1640 medium (without chemoattractant) to form a thin
continuous film on the top of the filter. An aliquot (100 µl) of
serum-free RPMI-1640 containing 5×104 cells was added to
each of the triplicate wells in RPMI-1640 medium containing 10%
FBS, which served as a chemoattractant in the assay. Following
incubation at 37°C for 24 h, cells were stained and counted as
aforementioned. The number of cells invading the lower side of the
filter was measured as representative of invasion activity.
Western blotting
Expression levels of NM23-H1, MMP-2, E-cadherin,
β-catenin and, TIMP-2 proteins were determined by western blotting.
Western blot analysis was performed as described previously
(35). Briefly, the medium was
removed and cells were lysed with protein lysis buffer (50 mM
Tris-HCl buffer pH 7.4, 1 mM EDTA, 5% SDS, 1 mM
phenylmethylsulfonyl fluoride, 1X protease inhibitor cocktail). The
lysate was sonicated (frequency, 20 kHz), for 30 sec at 4°C
followed by centrifugation at 12,000 × g for 30 min at 4°C. An
amount of protein (40 µg per lane) from the supernatant was
resolved by 0.1% SDS-PAGE and transferred onto a nitrocellulose
membrane. Following blocking with Tris-buffered saline buffer (20
mM Tris-HCl, 150 mM NaCl, pH 7.4) containing 5% non-fat milk for 1
h at 25°C, the membrane was incubated at 4°C for 12 h with
anti-NM23-H1 mAb (1:500 dilution), anti-MMP-2 mAb (1:500 dilution),
anti-E-cadherin mAb (1:500 dilution), anti-β-catenin mAb (1:1,000
dilution) and, anti-TIMP-2 mAb (1:1,000 dilution) followed by
HRP-conjugated anti-mouse IgG (incubated at 25°C for 2 h; 1:5,000
dilution) and then visualized using an ECL Chemiluminescent
Detection kit (EMD Millipore, Billerica, MA, USA). The relative
expression levels of NM23-H1, MMP-2, E-cadherin, β-catenin and
TIMP-2 proteins were quantitated using Matrox Inspector version 2.1
software (Matrox Electronic Systems Ltd. Dorval, QC, Canada).
Gelatin zymography assay
MMP-2 activity was assayed using gelatin zymography
according to the method reported by Hwang et al (36), with certain modifications, as
previously described in a study by Chuang et al (35). The cells (5×104 cells/ml)
were pretreated with ILL or CUM in RPMI-1640 medium containing 10%
FBS for 24 h. Following two washes with PBS, the cells were
incubated in serum-free RPMI-1640 medium for 24 h. Subsequently,
the culture medium was harvested and stored at −20°C until use. For
the gelatin zymography assay, the culture medium was separated on a
10% SDS-PAGE gel containing 0.1% (w/v) gelatin. Following
electrophoresis, the gel was washed for 30 min at room temperature
in a solution containing 2.5% (v/v) Triton X-100, with two changes,
and then transferred to a reaction buffer for enzymatic reaction
containing 1% sodium azide (NaN3), 10 mM calcium
chloride (CaCl2), and 40 mM Tris-hydrochloride, pH 8.0,
at 37°C with agitation overnight (for 15 h). Finally, the gel was
stained at 25°C for 30 min with 0.25% (w/v) Coomassie blue in 10%
acetic acid and 50% methanol and de-stained with 10% acetic acid
and 50% methanolat 25°C for 2 h. The relative MMP-2 activities were
compared with control group andquantitated using Matrox Inspector
version 2.1 software.
Scavenging of DPPH radicals
The effect of ILL on DPPH radicals was studied by
employing the modified method described by Blois (37) with some modifications as previously
described in a study by Lin et al (38). Briefly, 0.1 ml 1 mM methanol solution
of DPPH (cat. no. D9132, Sigma-Aldrich; Merck KGaA) was incubated
with varying concentrations (1, 5, 10 and 100 µM) of ILL. After a
30 min incubation period at room temperature, the absorbance of the
resulting solution was read at 517 nm against a blank. The radical
scavenging activity was measured as a decrease in the absorbance of
DPPH and was calculated using the following equation: Scavenging
effect
(%)=[(1-absorbancesample)/absorbancecontrol]
×100.
Statistical analysis
Data were expressed as the mean ± standard deviation
and analyzed using one-way analysis of variance followed by
Duncan's multiple range test for comparisons of group means. The
correlation analysis was conducted using simple linear regression
analysis. Statistical analysis was performed using SPSS version 10
(SPSS, Inc. Chicago, IL, USA). P<0.05 was considered to indicate
a statistically significant difference.
Results
Effects of ILL on cell viability
To examine the effect of ILL on cell growth, the
numbers of treated A549 cells were counted. As presented in
Fig. 1, incubation of A549 cells with
1, 5 and 10 µM ILL for 24 h did not decrease cell viability.
However, cell viability significantly decreased at 48 h of
incubation with 5, 10 and 20 µM ILL. Neither ILL (1–10 µM) nor CUM
(10 µM) inhibited the proliferation of A549 cancer cells at 24 h of
incubation; thus, the following experiments were conducted after a
24 h incubation.
Effects of ILL on invasion and
migration of A549 cancer cells
The anti-invasive and anti-migratory activity of ILL
was investigated using transwell inserts, coated with Matrigel in
the case of the invasion assay. As presented in Fig. 2, ILL significantly inhibited the
invasion of A549 cancer cells in a dose-dependent manner, with a
maximum inhibition rate of 64% at 10 µM of ILL, compared with the
control (P<0.05). As a positive control, the addition of 10 µM
of CUM significantly inhibited the invasion of A549 cancer cells by
62% (P<0.05). Similar to the invasion assay results, ILL
inhibited the migration of A549 cells in a dose-dependent manner,
with a maximum inhibition of 50% at 10 µM of ILL, compared with the
control (P<0.05).
Effects of ILL on MMP-2 activity and
protein expression in A549 cells
The activity of MMP-2 was assayed using gelatin
zymography. As presented in Fig. 3,
ILL significantly inhibited MMP-2 activity in a dose-dependent
manner, with a maximum inhibition of 32% at 10 µM ILL, compared
with the control (P<0.05). The addition of 10 µM CUM
significantly inhibited MMP-2 activity by 31% (P<0.05). As
presented in Fig. 3, the results
demonstrated that ILL significantly decreased MMP-2 protein
expression levels in a dose-dependent manner (P<0.05). The
addition of ILL at 5 and 10 µM significantly inhibited MMP-2
protein expression by 26 and 58%, respectively (P<0.05). In
addition, CUM inhibited MMP-2 protein expression levels by 46% at
10 µM. These results were in accordance with those of the MMP-2
activity assay. Collectively, the data of the present study implied
that inhibition of MMP-2 protein expression may inhibit the
progression of the metastasis of A549 cancer cells.
Effects of ILL on NM23-H1 protein
expression in A549 cells
As presented in Fig.
4, ILL significantly up regulated the expression of NM23-H1
protein in a concentration-dependent manner. The addition of ILL at
5 and 10 µM significantly increased the protein expression of
NM23-H1 by 32 and 61%, respectively (P<0.05). In addition, CUM
increased the expression of NM23-H1 by 35% (P<0.05). However,
the level of TIMP-2 protein in A549 cancer cells was not
significantly different among the groups (P>0.05; data not
shown).
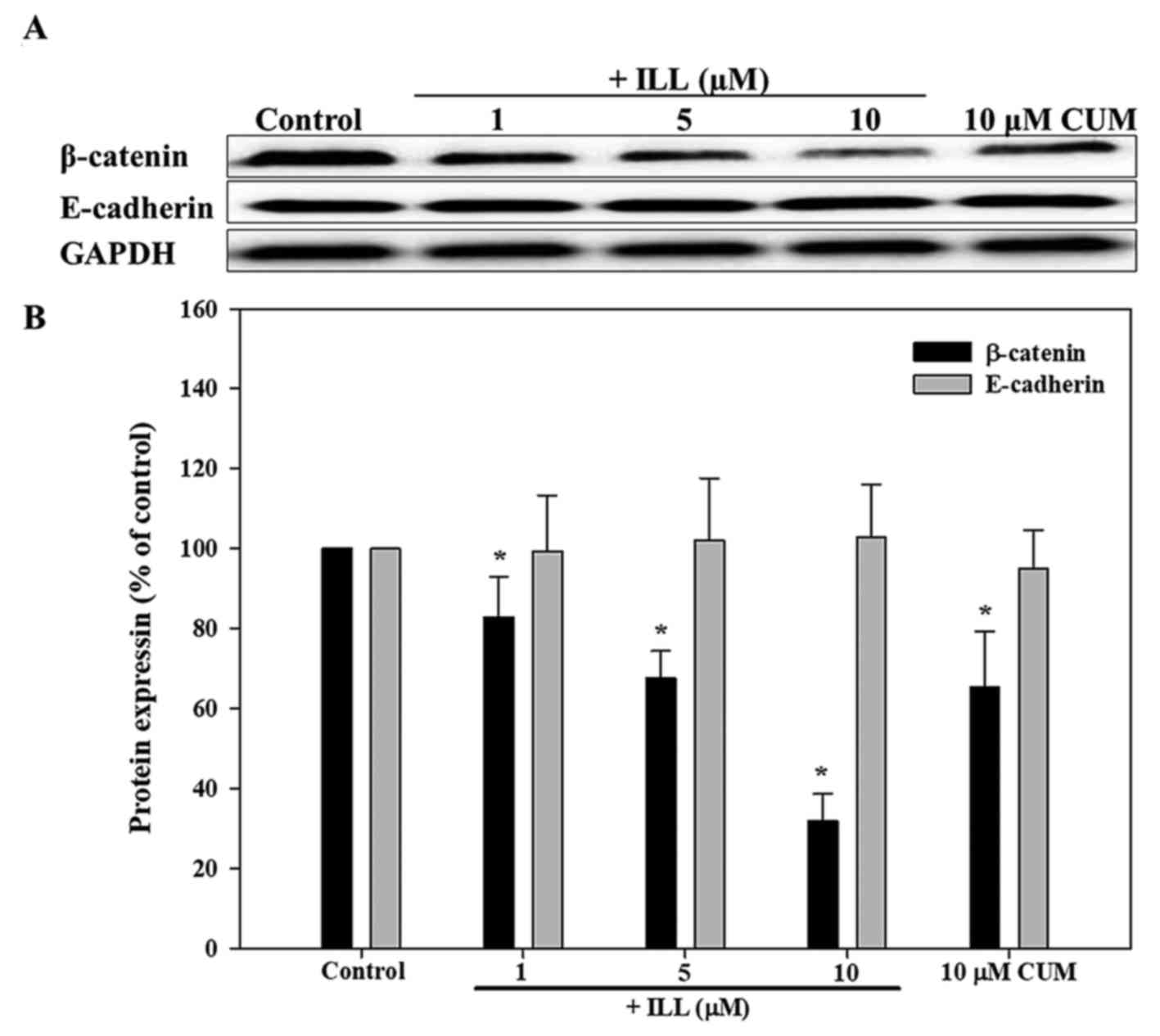
Effects of ILL on E-cadherin and
β-catenin protein expression in A549 cells
As presented in Fig.
5, ILL significantly decreased β-catenin protein expression
levels in a dose-dependent manner (P<0.05). The addition of 5
and 10 µM ILL significantly decreased the protein expression levels
of β-catenin by 32.5 and 68.1%, respectively (P<0.05). In
addition, CUM decreased the protein expression of β-catenin by
34.7% (P<0.05). However, the levels of E-cadherin protein in
A549 cancer cells were not significantly different between
groups.
Antioxidant activity of ILL
DPPH is widely used as a reagent to evaluate free
radical scavenging activity of antioxidants (39). The present study indicated that, at a
concentration of 1–10 µM, ILL did not significantly halt the
activity of DPPH radicals (data not shown).
Correlation of NM23-H1 protein with
MMP-2 activity and MMP-2 protein expression
NM23-H1 protein expression was negatively correlated
with MMP-2 activity (R2=0.69, P<0.001; Fig. 6A) and MMP-2 protein expression
(R2=0.74, P<0.001; Fig.
6B) in A549 cancer cells.
Discussion
Tumor cell metastasis is the leading cause of
mortality in patients with lung cancer (2,4). The
present study reported that ILL was able to inhibit the invasion
and migration of A549 cancer cells, exhibiting a dose-response
association. Numerous studies have reported that MMP-2 serves an
important role in the invasion and migration of tumor cells and
angiogenesis (6–8). Qian et al (12) reported that the expression level of
MMP-2 was an independent prognostic factor for patients with
non-small cell lung cancer, and was closely associated with
pathological grade, clinical stage and lymphatic metastasis.
Further analysis of MMP-2 expression and activity revealed that ILL
was able to significantly inhibit MMP-2 expression and activity,
again exhibiting a dose-response association. The inhibition trend
of MMP-2 was similar to that of its invasion and
migration-associated properties. The results of the present study
indicated that the ability of ILL to inhibit the invasion and
migration of A549 cells might be associated with reduced protein
expression and secretion of MMP-2 in lung cancer cells. TIMP-2 is
the endogenous inhibitor of MMP-2, and can combine extra cellularly
with MMP-2 to inhibit MMP-2 activity (40,41).
However, the present study did not observe any significant increase
in TIMP-2 protein expression brought about by ILL (data not shown).
It is thus speculated that the inhibition of MMP-2 by ILL was not
associated with any mechanisms involving TIMP-2 expression.
The gene encoding NM23-H1 is a suppressor of tumor
metastasis; research has revealed that it may reduce MMP-2 and −9
expression in various types of cancer cells (18–21,35,42,43).
Boissan et al (19) previously
reported that NM23-H1 silencing promotesthe extracellular matrix
invasion of HepG2 cells by up regulating numerous MMPs, including
MMP-2. The results of the present study demonstrated that ILL was
able to significantly increase NM23-H1 expression in a
dose-dependent manner. Additionally, NM23-H1 expression does not
affect the status of MMP-2 and −9 for certain tumor cells: Examples
include oral cancer cell lines (44),
cervical cancer (45) and gastric
cancer (46). It is speculated that
this may be associated with tumor cell type and the extent of
oncogenesis. Thus, the ILL treatment group exhibited a negative
correlation with respect to NM23-H1 protein expression
(R2=0.74, P<0.001) and MMP-2 protein expression
(R2=0.69, P<0.001) and activity; however, it remains
unclear whether the alterations in MMP-2 were correlated with the
alterations in NM23-H1.
Numerous studies have reported that NM23-H1 is
critical for control of cell-cell adhesion and cell migration
during the early stages of the invasive program in tumor cells
(19,43,47). Che
et al (43) transfected
NM23-H1 cDNA into the human non-small cell lung cancer L9981 cell
line, which is negative NM23 expression, and reported that, via
increasing β-catenin and E-cadherin protein expression, NM23-H1 may
simultaneously inhibit MMP-2 and vascular endothelial growth factor
protein expression, inhibiting cell mobility and metastasis. In
another previous study, it was reported that NM23-H1 silencing may
disrupt the cell-cell adhesion mediated by E-cadherin, resulting in
β-catenin nuclear translocation in HepG2 cells (19). β-catenin is an important component of
cell-cell adhesion (19,43,48).
However, the loss of cell-cell adhesion due to the activation of
β-catenin can increase the migratory potential of tumor cells
(48). In the present study, ILL
treatment significantly decreased β-catenin protein expression in a
dose-dependent manner. We therefore hypothesize that the mechanisms
of action of ILL, with respect to the reduction of A549 cancer cell
migration, may be associated with the inhibition of β-catenin by up
regulating the expression of NM23-H1 genes.
Recent evidence has also indicated that the
transcriptional factor forkhead box O3 (FOXO3) in A549 cancer cells
will reduce the expression of NM23-H1 (49). Additionally, Gong et al
(50) also reported that STAT3
activation was inhibited by NM23-H1. STAT3 may bind directly to the
STAT3 binding site on the NM23-H1 promoter and activate NM23-H1
expression, whereas its own expression may be inhibited, thereby
reducing the metastasis of A549 cancer cells. Yen et al
(27) also demonstrated that ILL is
able to reduce microRNA hsa-miR-30c expression, increase SOCS3
activation and inhibit STAT3 phosphorylation. In this manner, cell
apoptosis was induced in human breast cancer cells. In light of
these findings, further research is required to determine whether
the inhibition of A549 cancer cell metastasis by ILL is associated
with the regulation of STAT3 or FOXO3 activity by NM23-H1.
The production of reactive oxygen species (ROS) in
tumor cells may promote the invasion and migration of tumor cells
(51). Therefore, the inhibition of
cancer cell metastasis could be partially linked to the inhibition
of ROS production. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) analysis is
widely used to assess the effects of various antioxidants on the
efficiency of free radical scavenging (52). In this study, 1–10 µM of ILL did not
significantly reduce the activity of DDPH free radicals (data not
shown), and it is thus speculated that the inhibition of A549 lung
cancer cell metastasis may be not be associated with the clearance
of ROS.
On the basis of the aforementioned results and to
the best of our knowledge, the present study is the first to
confirm that ILL can significantly inhibit the invasion and
migration of A549 lung cancer cells; the possible molecular
mechanisms may constitute increases in NM23-H1 expression, and the
inhibition of MMP-2 activity and protein expression, as well as the
inhibition of β-catenin protein expression. However, further
investigation into the relevant mechanisms of metastasis is
required.
Acknowledgements
The present study was supported by the Ministry of
Science and Technology, Republic of China (Taiwan) (grant nos. MOST
103-2221-E-025-013 and 104-2221-E-025-014). The authors thank Mr.
Jia-Yi Ting (Department of Nutrition, Hungkuang University,
Taichung, Taiwan, R.O.C), Ms Wei-Zhu Wang (Department of Beauty
Science, National Taichung University of Science and Technology,
Taichung, Taiwan, R.O.C), Ms Yi-Yun Lin (Department of Nutrition,
Hungkuang University) and Ms Tsen-Lin Shih (Department of
Nutrition, Hungkuang University) for their technical support.
Glossary
Abbreviations
Abbreviations:
|
MMP-2
|
matrix metalloproteinase-2
|
|
ILL
|
isolinderalactone
|
|
FOXO3
|
transcriptional factor fork head box
O3
|
|
STAT3
|
signal transducer and activator of
transcription 3
|
|
CUM
|
curcumin
|
|
DMSO
|
dimethyl sulfoxide
|
|
Nm23-H1
|
NME/NM23 nucleoside diphosphate kinase
1
|
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mehlen P and Puisieux A: Metastasis: a
question of life or death. Nat Rev Cancer. 6:449–458. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ihde DC: Chemotherapy of lung cancer. N
Engl J Med. 327:1434–1441. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stewart DJ: Tumor and host factors that
may limit efficacy of chemotherapy in non-small cell and small cell
lung cancer. Crit Rev Oncol Hematol. 75:173–234. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Reka AK, Goswami MT, Krishnapuram R,
Standiford TJ and Keshamouni VG: Molecular cross-regulation between
PPAR-γ and other signaling pathways: Implications for lung cancer
therapy. Lung Cancer. 72:154–159. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fidler IJ and Kripke ML: Metastasis
results from preexisting variant cells within a malignant tumor.
Science. 197:893–895. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Halbersztadt A, Halon A, Pajak J,
Rabczynski J and St Gabrys M: The role of matrix metalloproteinases
in tumor invasion and metastasis. Ginekol Pol. 77:63–71.
2006.PubMed/NCBI
|
|
8
|
Chuang CH, Liu CH, Lu TJ and Hu ML:
Suppression of alpha-tocopherol ether-linked acetic acid in
VEGF-induced angiogenesis and the possible mechanisms in human
umbilical vein endothelial cells. Toxicol Appl Pharmacol.
281:310–316. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Birkedal-Hansen H: Proteolytic remodeling
of extracellular matrix. Curr Opin Cell Biol. 7:728–735. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liotta LA, Tryggvason K, Garbisa S, Hart
I, Foltz CM and Shafie S: Metastatic potential correlates with
enzymatic degradation of basement membrane collagen. Nature.
284:67–68. 1980. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liabakk NB, Talbot I, Smith RA, Wilkinson
K and Balkwill F: Matrix metalloprotease 2 (MMP-2) and matrix
metalloprotease 9 (MMP-9) type IV collagenases in colorectal
cancer. Cancer Res. 56:190–196. 1996.PubMed/NCBI
|
|
12
|
Qian Q, Wang Q, Zhan P, Peng L, Wei SZ,
Shi Y and Song Y: The role of matrix metalloproteinase 2 on the
survival of patients with non-small cell lung cancer: A systematic
review with meta-analysis. Cancer Invest. 28:661–669. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Steeg PS, Bevilacqua G, Kopper L,
Thorgeirsson UP, Talmadge JE, Liotta LA and Sobel ME: Evidence for
a novel gene associated with low tumor metastatic potential. J Nati
Cancer Inst. 80:200–204. 1988. View Article : Google Scholar
|
|
14
|
Leone A, Flatow U, King CR, Sandeen MA,
Margulies IM, Liotta LA and Steeg PS: Reduced tumor incidence,
metastatic potential, and cytokine responsiveness of
NM23-transfected melanoma cells. Cell. 65:25–35. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu F, Zhang Y, Zhang XY and Chen HL:
Transfection of the nm23-H1 gene into human hepatocarcinoma cell
line inhibits the expression of sialyl Lewis X, alpha 1,3
fucosyltransferase VII, and metastatic potential. J Cancer Cancer
Res Clin Oncol. 128:189–196. 2002. View Article : Google Scholar
|
|
16
|
Khan MH, Yasuda M, Higashino F, Haque S,
Kohgo T, Nakamura M and Shindoh M: Nm23-H1 suppresses invasion of
oral sqamous cell carcinoma-derived cell lines without modifying
matrix metalloproteinase-2 and matrix metalloproteinase-9
expression. Am J Pathol. 158:1785–1791. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yokdang N, Nordmeier S, Speirs K, Burkin
HR and Buxton IL: Blockade of extracellular NM23 or its endothelial
target slows breast cancer growth and metastasis. Integr Cancer Sci
Ther. 2:192–200. 2015.PubMed/NCBI
|
|
18
|
Boissan M and Lacombe ML: Nm23, an example
of a metastasis suppressor gene. Bull Cancer. 99:431–440.
2012.PubMed/NCBI
|
|
19
|
Boissan M, De Wever O, Lizarraga F, Wendum
D, Poincloux R, Chignard N, Desbois-Mouthon C, Dufour S,
Nawrocki-Raby B and Birembaut P: Implication of metastasis
suppressor NM23-H1in maintaining adherens junctions and limiting
the invasive potential of human cancer cells. Cancer Res.
70:7710–7722. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
You J, Chang R, Liu B, Zu L and Zhou Q:
Nm23-H1 was involved in regulation of KAI1 expression in
high-metastatic lung cancer cells L9981. J Thorac Dis. 8:1217–1226.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu YB, Gao SL, Chen XP, Peng SY, Fang HQ,
Wu YL, Peng CH, Tang Z, Xu B, Wang JW, et al: Expression and
significance of heparanase and NM23-H1 in hepatocellular carcinoma.
World J Gastroenterol. 11:1378–1381. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Luo Y, Liu M, Yao X, Xia Y, Dai Y, Chou G
and Wang Z: Total alkaloids from Radix Linderae prevent the
production of inflammatory mediators in
lipopolysaccharide-stimulated RAW 264.7 cells by suppressing
NF-kappaB and MAPKs activation. Cytokine. 46:104–110. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li YM, Ohno Y, Minatoguchi S, Fukuda K,
Ikoma T, Ohno T, Akao S, Takemura G, Gotou K and Fujiwara H:
Extracts from the roots of Lindera strychifolia induces apoptosis
in lung cancer cells and prolongs survival of tumor-bearing mice.
Am J Chin Med. 31:857–869. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gan LS, Zheng YL, Mo JX, Liu X, Li XH and
Zhou CX: Sesquiterpene lactones from the root tubers of Lindera
aggregata. J Nat Prod. 72:1497–1501. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lin CT, Chu FH, Chang ST, Chueh PJ, Su YC,
Wu KT and Wang SY: Secoaggregatalactone-A from Lindera aggregate
induces apoptosis in Human Hepatoma Hep G2 Cells. Planta Med.
73:1548–1553. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ohno T, Nagatsu A, Nakagawa M, Inoue M, Li
YM, Minatoguchi S, Mizukami H and Fujiwara H: New sesquiterpene
lactones from water extract of the root of Lindera strychnifolia
with cytotoxicity against the human small cell lung cancer cell,
SBC-3. Tetrahedron Lett. 46:8657–8660. 2005. View Article : Google Scholar
|
|
27
|
Yen MC, Shih YC, Hsu YL, Lin ES, Lin YS,
Tsai EM, Ho YW, Hou MF and Kuo PL: Isolinderalactone enhances the
inhibition of SOCS3 on STAT3 activity by decreasing miR-30c in
breast cancer. Oncol Rep. 35:1356–1364. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chang WA, Lin ES, Tsai MJ, Huang MS and
Kuo PL: Isolinderalactone inhibits proliferation of A549 human
non-small cell lung cancer cells by arresting the cell cycle at the
G0/G1 phase and inducing a Fas receptor and soluble Fas
ligand-mediated apoptotic pathway. Mol Med Rep. 9:1653–1659. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ahmad A, Sayed A, Ginnebaugh KR, Sharma V,
Suri A, Saraph A, Padhye S and Sarkar FH: Molecular docking and
inhibition of matrix metalloproteinase-2 by novel
difluorinatedbenzylidene curcumin analog. Am J Transl Res.
7:298–308. 2015.PubMed/NCBI
|
|
30
|
Kumar D, Kumar M, Saravanan C and Singh
SK: Curcumin: A potential candidate for matrix metalloproteinase
inhibitors. Expert Opin Ther Targets. 6:959–972. 2012. View Article : Google Scholar
|
|
31
|
Yeh SL, Yeh CL, Chan ST and Chuang CH:
Plasma rich in quercetin metabolites induces G2/M arrest
by upregulating PPAR-γ expression in human A549 lung cancer cells.
Planta Medica. 77:992–998. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pettit GR, Hoard MS, Doubek DL, Schmidt
JM, Pettit RK, Tackett LP and Chapuis JC: Antineoplastic agents
338. The cancer cell growth inhibitory. Constituents of Terminnalia
arjuna (Combretaceae). J Ethnopharmacol. 53:57–63. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tholudur A, Giron L, Alam K, Thomas T,
Garr E, Weatherly G, Kulowiec K, Quick M and Shepard S: Comparing
automated and manual cell counts for cell culture applications. Bio
Process Int. 28–34. 2006.
|
|
34
|
Repesh LA: A new in vitro assay for
quantitating tumor cell invasion. Invasion Metastasis. 9:192–208.
1989.PubMed/NCBI
|
|
35
|
Chuang CH, Yeh CL, Yeh SL, Lin ES, Wang LY
and Wang YH: Quercetin metabolites inhibit MMP-2 expression in A549
lung cancer cells by PPAR-γ associated mechanisms. J Nutr Biochem.
33:45–53. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hwang HJ, Park HJ, Chung HJ, Min HY, Park
EJ, Hong JY and Lee SK: Inhibitory effects of caffeic acid
phenethyl ester on cancer cell metastasis mediated by the
down-regulation of matrix metalloproteinase expression in human
HT1080 fibrosarcoma cells. J Nutr Biochem. 17:356–362. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Blois MS: Antioxidant determinations by
the use of a stable freeradical. Nature. 26:1199–1200. 1958.
View Article : Google Scholar
|
|
38
|
Lin ES, Yang CT, Chou HJ and Chang TT:
Screening of antioxidant activities by the edible Basidiomycete
Antrodia cinnamomea strains in submerged culture. J Food Biochem.
34:1141–1156. 2010. View Article : Google Scholar
|
|
39
|
Ak T and Gülçin I: Antioxidant and radical
scavenging properties of curcumin. Chem Biol Interact. 174:27–37.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Marino N, Nakayama J, Collins JW and Steeg
PS: Insights into the biology and prevention of tumor metastasis
provided by the Nm23 metastasis suppressor gene. Cancer Metastasis
Rev. 31:593–603. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Crocker SJ, Pagenstecher A and Campbell
IL: The TIMPs tango with MMPs and more in the central nervous
system. J Neurosci Res. 75:1–11. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ohba K, Miyata Y, Koga S, Kand S and
Kanetake H: Expression of nm23-H1 gene product in sarcomatous
cancer cells of renal cell carcinoma: Correlation with tumor stage
and expression of matrix metalloproteinase-2, matrix
metalloproteinase-9, sialyl Lewis X, and c-erbB-2. Urology.
65:1029–1034. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Che G, Chen J, Liu L, Wang Y, Li L, Qin Y
and Zhou Q: Transfection of NM23-H1 increased expression of
β-Catenin, E-Cadherin and TIMP-1 and decreased the expression of
MMP-2, CD44v6 and VEGF and inhibited the metastatic potential of
human non-small cell lung cancer cell line L9981. Neoplasma.
53:530–537. 2006.PubMed/NCBI
|
|
44
|
Khan MH, Yasuda M, Higashino F, Haque S,
Kohgo T, Nakamura M and Shindoh M: Nm23-H1 suppresses invasion of
oral sqamous cell carcinoma-derived cell line without modifying
matrix metalloproteinase-2 and matrix metalloproteinase-9
expression. Am J Pathol. 158:1785–1791. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wang PH, Yang SF, Chen GD, Han CP, Chen
SC, Lin LY and Ko JL: Human nonmetastatic clone 23 type 1 gene
suppresses migration of cervical cancer cells and enhances the
migration inhibition of fungal immunomodulatory protein from
Ganoderma tsugae. Reprod Sci. 14:475–485. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wang LB, Jiang ZN, Fan MY, Xu CY, Chen WJ
and Shen JG: Changes of histology and expression of MMP-2 and
nm23-H1 in primary and metastatic gastric cancer. World J
Gastroenterol. 14:1612–1616. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhao R, Gong L, Li L, Guo L, Zhu D, Wu Z
and Zhou Q: NM23-H1is a negative regulator of TGF-β1-dependent
induction of epithelial-mesenchymal transition. Exp Cell Res.
319:740–749. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Vaid M, Prasad R, Sun Q and Katiyar SK:
Silymarin targets β-catenin signaling in blocking
migration/invasion of human melanoma cells. PLoS One. 6:e230002011.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhang L, Li L, Wei H, Guo L, Ai C, Xu H,
Wu Z and Zhou Q: Transcriptional factor FOXO3 negatively regulates
the expression of NM23-H1 in non-small cell lung cancer. Thorac
Cancer. 7:9–16. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Gong L, Wu Z, Guo L, Li L, Zhao R, Zhu D
and Zhou Q: Metastasis suppressor Nm23-H1 inhibits STAT3 signaling
via a negative feedback mechanism. Biochem Biophys Res Commun.
434:541–546. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Szatrowski TP and Nathan CF: Production of
large amounts of hydrogen peroxide by human tumor cells. Cancer
Res. 51:794–798. 1991.PubMed/NCBI
|
|
52
|
Ak T and Gülçin I: Antioxidant and radical
scavenging properties of curcumin. Chem Biol Interact. 174:27–37.
2008. View Article : Google Scholar : PubMed/NCBI
|