Introduction
Inhibition of angiogenesis is a promising strategy
for breast and lung cancer therapy (1,2). It has
been demonstrated that combined therapy with angiogenesis
inhibitors may significantly enhance the treatment effect and
extend survival in breast and lung cancer patients (3,4). However,
inhibitors of angiogenesis tend to be expensive and are not
effective in a proportion of cancer patients (5). In addition, the tumor volume changes are
much slower than blood supply inhibition. Conventional tools [X-ray
and computed tomography (CT)] rely on tumor volume change to
evaluate the effect of tumor, which is not suitable for
antiangiogenic drugs (6). Therefore,
it is critical that a reliable tool is identified to monitor and
assess the treatment effect of inhibiting angiogenesis therapy.
Imaging tools have been used to monitor cancer
therapy for decades (7,8). In this area, nuclear medicine imaging
techniques are promising. 99mTc-3PRGD2
single-photon emission computed tomography (SPECT)/CT has been
developed as an imaging modality for evaluating tumor vascular
status (9,10). 99mTc-3PRGD2 is a
radiolabeled dimeric arginylglycylaspartic acid (RGD) peptide that
is being investigated for measurement of the expression of integrin
αvβ3 (11).
Integrin αvβ3 is widely distributed in newly
generated vessels. Additionally, this subtype of integrin has been
documented as being associated with tumor angiogenesis and
metastasis (12). Therefore, the
degree of expression of integrin αvβ3 may
reflect the status of tumor angiogenesis.
Bevacizumab is a recombinant humanized monoclonal
antibody that blocks angiogenesis by inhibiting vascular
endothelial growth factor-A (VEGF-A); it has been used to treat
breast and lung cancer since 2004, and has shown promising
therapeutic results (3). The present
study determined the utility of imaging the uptake of
99mTc-3PRGD2 by tumors as a biomarker for
anti-angiogenic treatment with bevacizumab in a lung cancer A549
cell xenograft model, which has high-to-moderate vessel density and
exhibits integrin αvβ3 expression, and a PC-3
prostate cancer model, which has low vessel density and also
exhibits integrin αvβ3 expression. Following
the present study, how changes in tumor uptake of
99mTc-3PRGD2 affect the tumor response to
antiangiogenic treatment can be better understood before it can be
used clinically to monitor investigational therapy.
Materials and methods
Animal models and treatment
protocol
All animal experiments were performed in accordance
with the protocol approved by the Institutional Animal Care and Use
Committee at Jilin University (Changchun, China). The A549 and PC-3
cell lines were obtained from the American Type Culture Collection
(Manassas, VA, USA). These cells were cultured in F-12 medium
(Gibco, Life Technologies, Grand Island, NY, USA) supplemented with
10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) and 1% penicillin and streptomycin (Gibco; Thermo
Fisher Scientific, Inc.) solution at 37°C in a humidified
atmosphere of 5% CO2. Cells were grown as a monolayer
and were harvested or passaged when they reached 90% confluence to
maintain exponential growth. A total of 30 male Athymic nu/nu mice
were obtained from the Department of Experimental Animals (Peking
University) at 4–5 weeks of age. Mice were housed under standard
laboratory conditions (temperature, 20–24°C; relative humidity,
50–60%, 12/12 h light/dark cycle) and had food and water available
ad libitum. Each mouse was implanted subcutaneously near the
shoulder with 5×106 cells. At 4 weeks after inoculation
with A549 and PC-3 cells, the mice were divided into three groups,
with 9 or 10 mice in each group. All the groups were size-matched,
with an average tumor volume of 180±90 mm3 1 day before
baseline SPECT/CT imaging. Vehicle (0.15% hydroxypropylmethyl
cellulose, 2% ethanol, 5% Tween 80, 20% PEG 400 and 73% saline) or
bevacizumab (Genentech, San Francisco, CA, USA) was injected
intraperitoneally. The treatment protocol of each group was: A549
Bevacizumab group (A549 model, n=10), 1 mg bevacizumab twice a week
from day 0 after baseline imaging; A549 Vehicle group (A549 model,
n=10), vehicle with a dose of 1 mg twice a week from day 0 after
baseline imaging; and PC-3 Bevacizumab group (PC-3 model, n=9), 1
mg bevacizumab twice a week from day 0 after baseline imaging.
Preparation of
99mTc-3PRGD2 and small animal SPECT/CT
Na99mTcO4 was obtained from a
commercial 99Mo-99mTc generator (Beijing Atom
High Tech Co., Ltd., Beijing, China). The kit for preparation of
99mTc-3PRGD2 was formulated by containing 20
µg/ml of HYNIC-3P4-RGD2, 5 mg of TPPTS, 6.5
mg of tricine, 40 mg of mannitol, 38.5 mg of disodium succinate
hexahydrate and 12.7 mg of succinic acid. Then 1 ml of
Na99mTcO4 solution (1110–1850 MBq) in saline
was added to each kit vial followed by 20 min incubation at 100°C
(11,13). The radiochemical purity of the
prepared 99mTc-3PRGD2 was >90%. Helical CT
and SPECT scans of rats were obtained using a SPECT/CT system
(NanoScan; Mediso Medical Imaging Systems, Budapest, Hungary).
Longitudinal SPECT/CT imaging was performed at baseline (−1), 5,
and 15 days after treatment initiation. At 1 h prior to SPECT/CT
imaging, 37.0–44.4 MBq 99mTc-3P-RGD2 in
0.1–0.2 ml of saline was administered intravenously via the lateral
tail vein. Animals were anesthetized with 3% isoflurane inhalation,
which was maintained at 1.5% for the duration of scanning, and then
placed in a prone position in an air-warmed chamber. For
radioactivity quantification, the regions of interest were drawn
manually to cover the entire tumor, based on a transverse view of
the CT image. For tumor delineation with SPECT, a threshold of ≥50%
of the maximum pixel value on the SPECT image was chosen. Tumor
volume and radioactivity counts were generated using NanoScan Image
Processing software (version 3.306; PMOD Technologies LLC, Zürich,
Switzerland) and the amount of radioactivity in each tumor was
calculated. The tumor uptake of 99mTc-3PRGD2
was expressed as the percent-injected dose (%ID) and %ID/g.
Reference regions of interest were drawn over muscle as background
radioactivity for tumor-to-muscle (T/M) ratio calculations.
Tumor immunostaining
Immunofluorescence staining was performed to
determine the location and expression of integrin
αvβ3. Tumors were sectioned into two pieces
for immunostaining and hematoxylin and eosin (H&E) staining.
Once tumors were harvested, the tumor sections for immunostaining
were immediately snap-frozen in optical cutting temperature
solution (99% purity, Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany). Tumors were then cut into 5-mm sections. Following
thorough drying at room temperature, slides were fixed with
ice-cold acetone for 10 min, and air-dried for 20 min at room
temperature. The sections were then blocked with 10% goat serum
(Abcam, Cambridge, MA, USA) for 30 min at room temperature and then
incubated with rat anti-integrin β3 antibody (1:100;
cat. no. 181720; BD Biosciences, Franklin Lakes, NJ, USA) and rat
anti-CD31 antibody (1:100; cat. no. 551262; BD Biosciences) for 1 h
at room temperature. The β3 antibody was chosen to
represent αvβ3 as the only other integrin
with an αβ3 subunit besides αvβ3
is expressed on platelets. The majority of β3 in the
tumor sections is likely to be in the vasculature and tumor cells.
After incubating with Cy3-conjugated goat anti-rat (1:100; cat. no.
115-165-003; Jackson ImmunoResearch Europe Ltd., Newmarket, UK) and
fluoresceinisothiocyanate-conjugated goat anti-rat secondary
antibodies (1:100; cat. no. 115-095-003; Jackson ImmunoResearch
Europe Ltd.) at room temperature (25°C) for 4 h, the sections were
washed with PBS. Fluorescence was visualized with a Nikon
fluorescence microscope at ×200 magnification (Nikon Eclipse E600;
Nikon Corporation, Tokyo, Japan).
H&E staining
Histopathological analysis was performed by H&E
staining of tumors according to previously published methods
(14). Briefly, all the tissues were
fixed in 10% neutral buffered formalin at room temperature (25°C)
for 4 h. Tissues were embedded in paraffin and 4-mm sections were
deparaffinized and rehydrated using a graded alcohol series.
Sections were stained with H&E at room temperature (25°C) for
20 min to evaluate the morphology and then examined under a light
microscope. Aperio's Image Scope v10.1.3.2028 Viewer (Leica
Mircosystems, GmbH, Wetzlar, Germany) was used to visualize the
whole-slide digital scans and capture images in 30 fields of view
for analysis.
Statistical analysis
All data were expressed as the mean ± standard
error. Statistical analyses were performed by two-way analysis of
variance followed by the Newman-Keuls test for multiple comparisons
to compare treatment groups. P<0.05 was considered to indicate a
statistically significant difference. A one-way analysis of
variance and Newman-Keuls post-hoc test was performed to determine
alterations over time. SPSS 19.0 software package (IBM Corp.,
Armonk, NY, USA) was used for linear and nonlinear regression
analysis.
Results
Tumor volume in bevacizumab-treated
tumor models
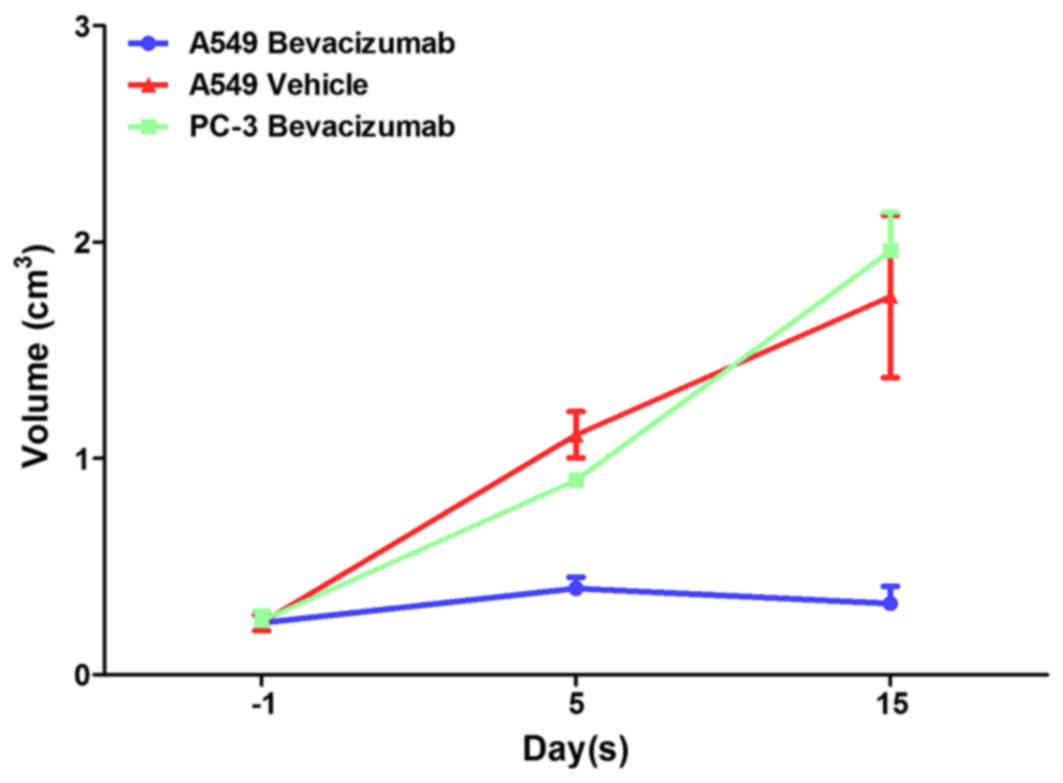
Fig. 1 depicts the
tumor volumes for A549 Bevacizumab, A549 Vehicle and PC-3
Bevacizumab groups. In the PC-3 Bevacizumab and A549 Vehicle group,
the tumor volume increased at 5 and 15 days after therapy. The
difference in tumor volume was not significantly different prior to
and following treatment in the A549 Bevacizumab group
(P>0.05).
Tumor uptake in bevacizumab-treated
tumor models
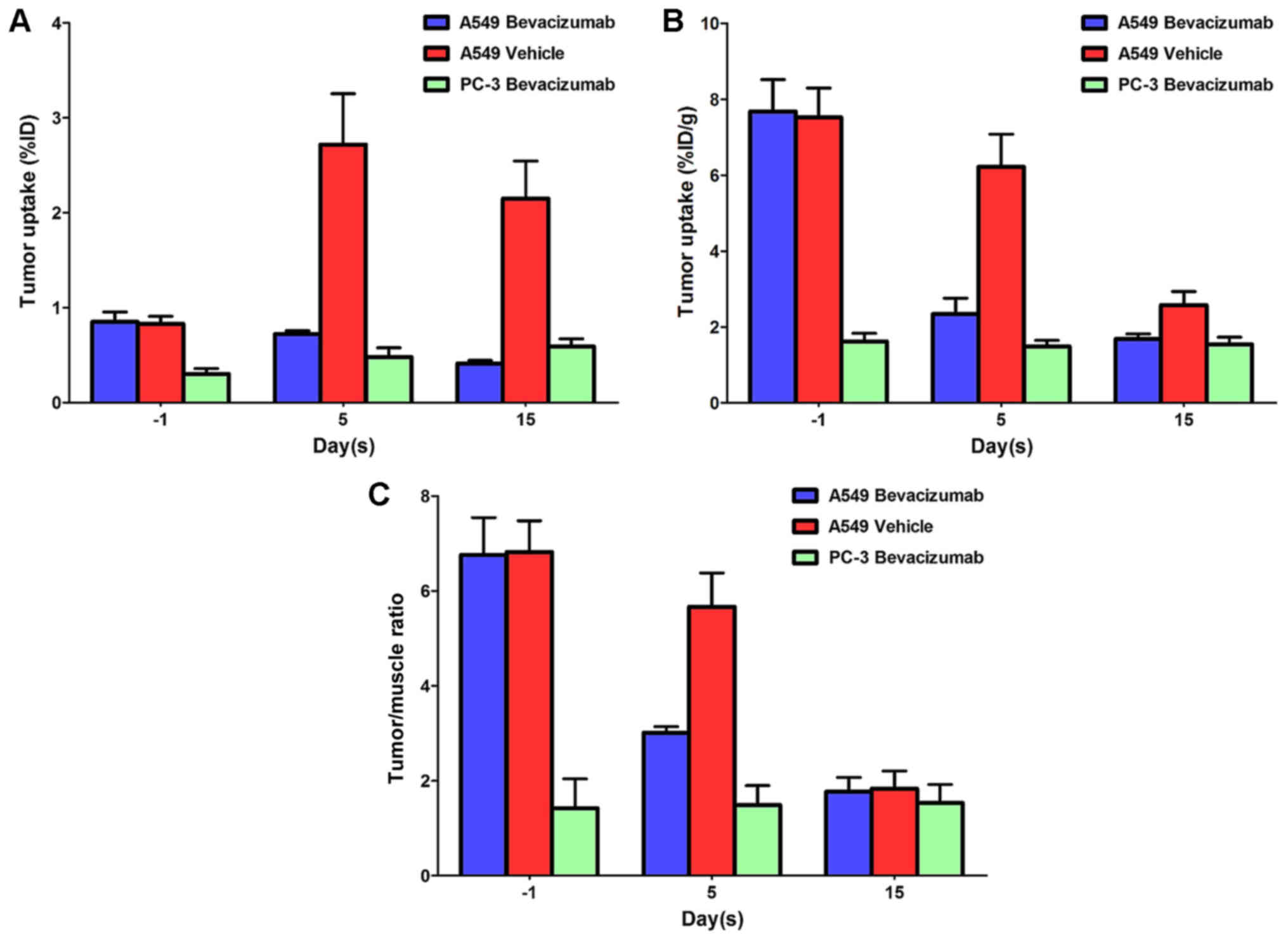
Fig. 2 compared the
%ID tumor uptake (Fig. 2A), %ID/g
tumor uptake (Fig. 2B) and T/M ratios
(Fig. 2C) of
99mTc-3PRGD2 in the A549 Bevacizumab, A549
Vehicle and PC-3 Bevacizumab groups. For the PC-3 Bevacizumab
group, a lower tumor uptake was observed throughout the study
compared with the other 2 groups, and there was no significant
alteration prior to and following bevacizumab therapy. After 5 and
15 days therapy, the %ID/g value for A549 Bevacizumab group
declined. However, for the A549 Vehicle group, the %ID value
increased 5 days after injection, but the %ID/g tumor uptake
decreased after 5 and 15 days therapy as the weight of the tumor
increased. For the A549 Bevacizumab and A549 Vehicle groups, the
T/M ratio decreased 5 and 15 days after injection. Further SPECT/CT
studies confirmed that bevacizumab-treated tumors have persistent
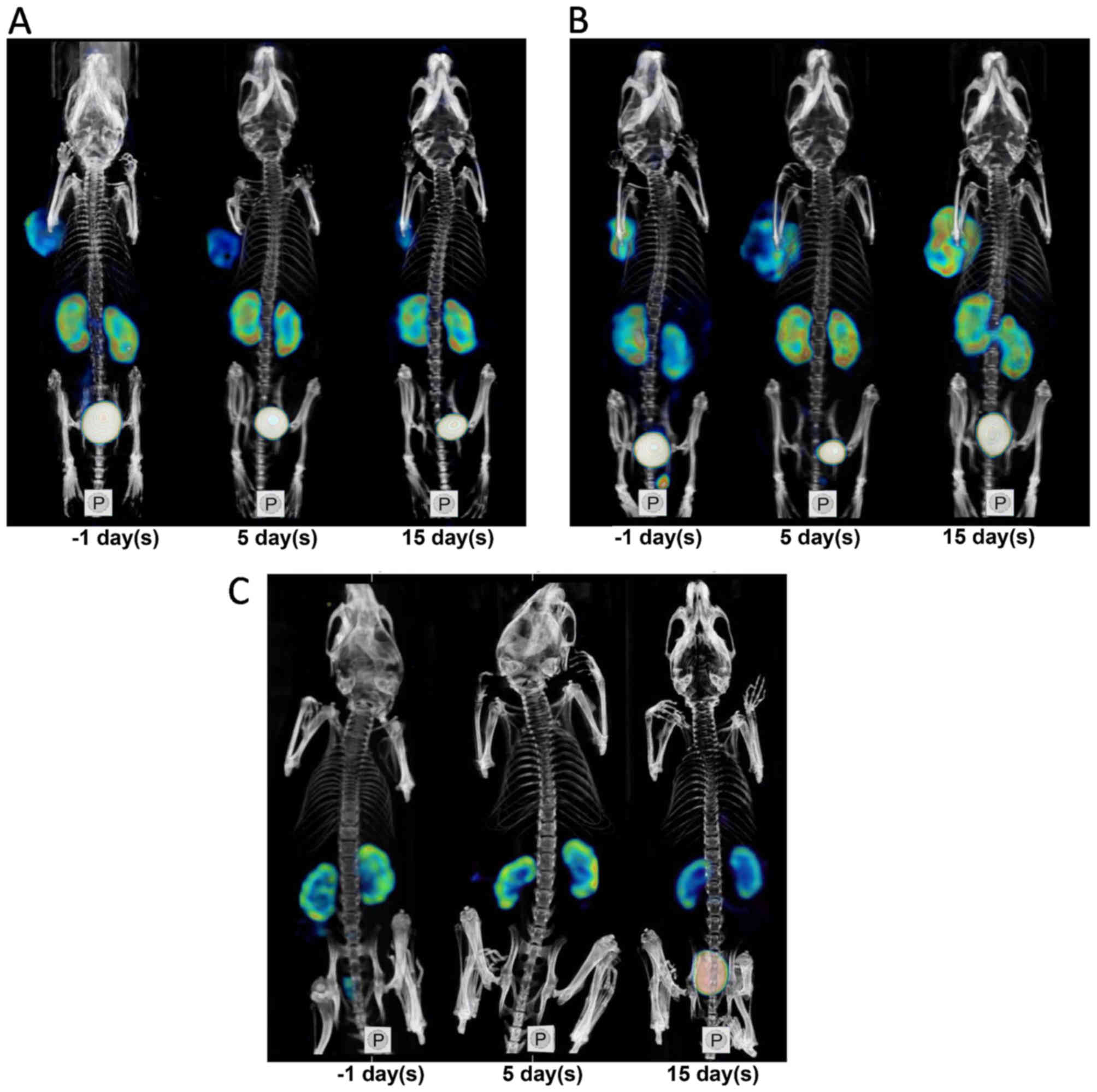
tumor uptake decrease. Fig. 3
depicted SPECT/CT images for decreased uptake of
99mTc-3PRGD2 of tumors in the A549
Bevacizumab group after treatment, increased uptake in the A549
Vehicle group, and low uptake of 99mTc-3PRGD2
before and after treatment in the PC-3 Bevacizumab group.
Change in microvessel density (MVD)
following bevacizumab treatment
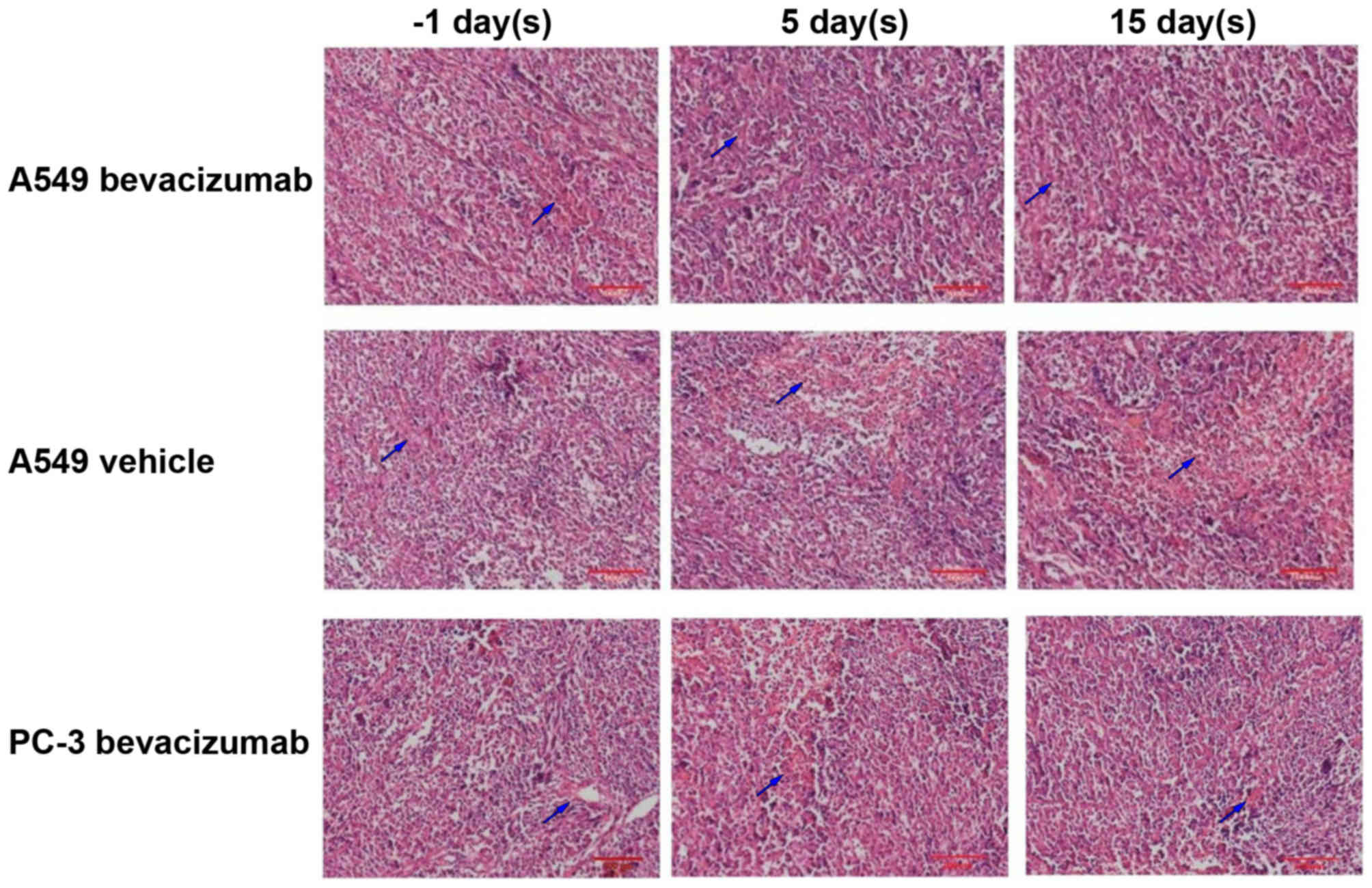
Fig. 4 depicts
selected histological slices (H&E stained) of tumor tissues
from animals before and after 5 and 15 days of A549 Bevacizumab,
A549 Vehicle and PC-3 Bevacizumab groups. The PC-3 model exhibited
low MVD throughout the study. Prior to bevacizumab therapy, the MVD
of the A549 model was moderately high. After 15 days therapy, the
MVD of the A549 model was significantly lower than before
therapy.
Change in integrin
αvβ3 following bevacizumab treatment
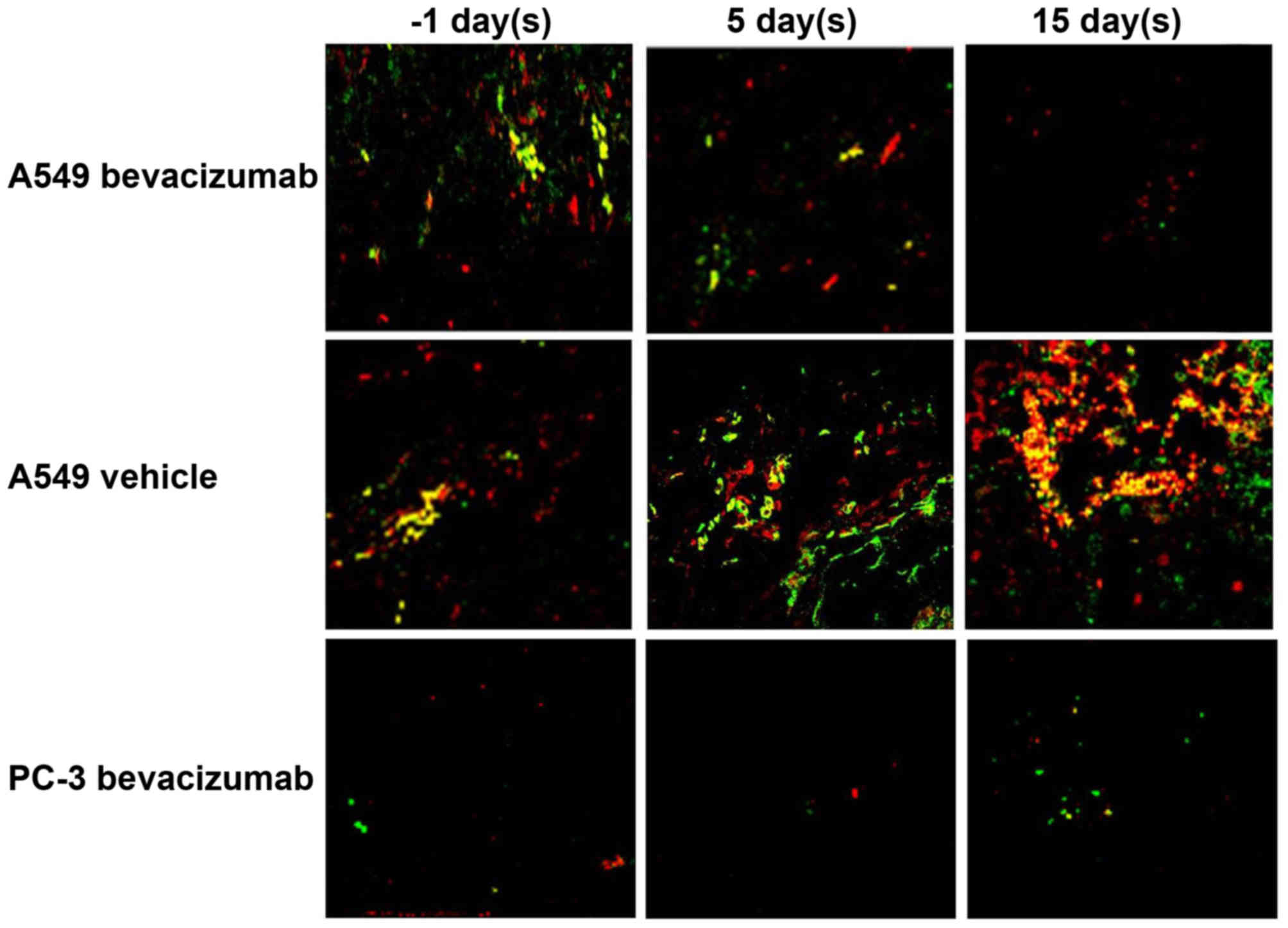
Fig. 5 depicts overlay
images of A549 and PC-3 tumor tissues after immunohistochemical
staining for integrin β3 and CD31 in A549 Bevacizumab,
A549 Vehicle and PC-3 Bevacizumab groups. The PC-3 model exhibited
low integrin β3 levels. At 5 days after the start of
therapy, the integrin β3 level in the
bevacizumab-treated models decreased. No evident alterations in the
levels of CD31 were observed following bevacizumab-treatment. In
the vehicle-treated model, the expression level of integrin
β3 was elevated. At 15 days after the start of therapy,
the integrin β3 and CD31 levels in the
bevacizumab-treated models decreased. In the vehicle-treated model,
tumor volume increased and integrin β3 and CD31 levels decreased,
which may be due to the lack of neovascularization in the deep
tumor tissue.
Discussion
Multiple growth factors, including VEGF and
platelet-derived growth factor (PDGF) elevate integrin
αvβ3 levels in vitro (15). Consequently, suppressing the
expression or signaling of VEGF and PDGF receptors may decrease
integrin αvβ3 levels. The target site of
bevacizumab is VEGF and PDGF receptors. Therefore, integrin
αvβ3 may be deemed to be a relevant biomarker
for the evaluation of the biological effects that occur following
bevacizumab therapy. Previous enhanced magnetic resonance imaging
studies have indicated that, either in preclinical or clinical
studies, a tumor vasculature system experiences alterations
following bevacizumab therapy (16–18). In
the present study, SPECT/CT was used to dynamically observe changes
in 99mTc-3PRGD2 uptake at two imaging time
points, prior to and following dosing with bevacizumab in
xenografts. At 5 days after the start of therapy, a significant
decrease in the tumor uptake in the A549 model was observed. At 15
days after the start of therapy, the tumor volume in the A549 model
mildly decreased. In comparison, the tumor volume in the PC-3 model
and the vehicle-treated model increased. Additionally, the tumor
uptake consistently increased in the vehicle-treated model.
Therefore, 99mTc-3PRGD2 SPECT/CT may be used
in early therapy monitoring in bevacizumab treatment in the A549
model, but not in the PC-3 model.
The present study revealed that the degree of
increase in %ID/g and T/M values were evident in the A549 model.
Previous studies indicated that the treatment effect of bevacizumab
was closely associated with MVD and integrin
αvβ3 levels in different tumor types, which
may explain differences with the present study (18). Compared with the PC-3 model, which had
low MVD, the A549 model, with moderately high MVD, may express
higher levels of integrin αvβ3. Further
studies using models with poor integrin αvβ3
expression are required to investigate whether SPECT/CT could be
used to rule out integrin αvβ3 expression
non-invasively in an experimental and clinical setting.
MVD is the number of vessels per unit area of tumor
tissue; it directly reflects the capability of formation of new
tumor vessels. MVD is positively associated with tumor metastasis
and proliferation, and can be used as an index for evaluating
therapeutic effects of solid tumors (19,20).
Through observing alterations in tumor microvessels via H&E
staining, the present study demonstrated that the MVD of the
bevacizumab treated model significantly decreased. The
immunofluorescence staining results during therapy suggested that
at 5 days after the start of therapy, a decrease of integrin
αvβ3 on the surface of tumor cell was not
evident, whereas the decrease of integrin
αvβ3 within the new epithelial cells was.
These results indicated the anti-angiogenic effect of bevacizumab
and may explain the decrease of 99mTc-3PRGD2
SPECT/CT uptake in tumors. 18F-labeled peptides such as
18F-fluciclatide and 18F-FPPRGD2
have been used to monitor the therapeutic effects of
anti-angiogenic agents, including sunitinib, ZD4190 and functional
paclitaxel (21–23). The results of the present study are in
accordance with these previous studies. Considering the
availability of pharmacokinetics, biodistribution, radiation dose
and 9mTc-3PRGD2, we hypothesize that
99mTc-3PRGD2 SPECT/CT could be more
cost-effective compared with the 18F-labeled analogs.
The T/M ratio of tumor RGD uptake was linearly associated with
integrin αvβ3 and CD31 expression, as
previously reported (13).
Quantitative analysis revealed that
99mTc-3PRGD2 SPECT/CT was prominent in future
clinical applications (24). However,
to understand the association between T/M ratio and the treatment
effects better, further research is required.
In conclusion, in the A549 model,
99mTc-3PRGD2 tumor uptake decreased following
treatment with bevacizumab.
99mTc-3PRGD2SPECT/CT may be used as a
non-invasive tool to evaluate the early biological effects of
anti-angiogenic therapy.
Acknowledgements
This study was supported by the Research Fund of
Science and Technology Department of Jilin Province (grant nos.
20150520154JH and 20160101064JC), the Foundation of National Health
and Family Planning Commission of Jilin Province (grant nos.
2015Q020 and 2016Q038), the Department of Education of Jilin
Province for Thirteen-Five Scientific Technique Research [grant no.
(2016) 460], the Norman Bethune Program of Jilin University (grant
no. 2015437) and Jilin University Funding Project for Young Teacher
Cultivation Plan (grant no. 419080500365).
References
|
1
|
Rayson D, Vantyghem SA and Chambers AF:
Angiogenesis as a target for breast cancer therapy. J Mammary Gland
Biol Neoplasia. 4:415–423. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferrara N and Kerbel RS: Angiogenesis as a
therapeutic target. Nature. 438:967–974. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rodgers M, Soares M, Epstein D, Yang H,
Fox D and Eastwood A: Bevacizumab in combination with a taxane for
the first-line treatment of her2-negative metastatic breast cancer.
Health Technol Assess. 15 Suppl 1:S1–S12. 2011. View Article : Google Scholar
|
|
4
|
Sheng J, Yang YP, Yang BJ, Zhao YY, Ma YX,
Hong SD, Zhang YX, Zhao HY, Huang Y and Zhang L: Efficacy of
addition of antiangiogenic agents to taxanes-containing
chemotherapy in advanced nonsmall-cell lung cancer: A meta-analysis
and systemic review. Medicine (Baltimore). 94:e12822015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee SM, BAAS P and Wakelee H:
Anti-angiogenesis drugs in lung cancer. Respirology. 15:387–392.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lange A, Prenzler A, Frank M, Golpon H,
Welte T and von der Schulenburg JM: A systematic review of the
cost-effectiveness of targeted therapies for metastatic non-small
cell lung cancer (nsclc). BMC Pulm Med. 14:1922014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schreuder SM, Lensing R, Stoker J and
Bipat S: Monitoring treatment response in patients undergoing
chemoradiotherapy for locally advanced uterine cervical cancer by
additional diffusion-weighted imaging: A systematic review. J Magn
Reson Imaging. 42:572–594. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lei L, Wang X and Chen Z: PET/CT imaging
for monitoring recurrence and evaluating response to treatment in
breast cancer. Adv Clin Exp Med. 25:377–382. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ji B, Chen B, Wang T, Song Y, Chen M, Ji
T, Wang X, Gao S and Ma Q: 99mTc-3PRGD2 SPECT
to monitor early response to neoadjuvant chemotherapy in stage II
and III breast cancer. Eur J Nucl Med Mol Imaging. 42:1362–1370.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ma Q, Min K, Wang T, Chen B, Wen Q, Wang
F, Ji T and Gao S: (99m)Tc-3PRGD 2 SPECT/CT predicts the outcome of
advanced nonsquamous non-small cell lung cancer receiving
chemoradiotherapy plus bevacizumab. Ann Nucl Med. 29:519–527. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jia B, Liu Z, Zhu Z, Shi J, Jin X, Zhao H,
Li F, Liu S and Wang F: Blood clearance kinetics, biodistribution,
and radiation dosimetry of a kit-formulated integrin αvβ3-selective
radiotracer 99mTc-3PRGD2 in non-human primates. Mol Imaging Biol.
13:730–736. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Niu G and Chen X: Why integrin as a
primary target for imaging and therapy. Theranostics. 1:30–47.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang L, Shi J, Kim YS, Zhai S, Jia B, Zhao
H, Liu Z, Wang F, Chen X and Liu S: Improving tumor-targeting
capability and pharmacokinetics of (99m)Tc-labeled cyclic RGD
dimers with PEG(4) linkers. Mol Pharm. 6:231–245. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou Y, Kim YS, Chakraborty S, Shi J, Gao
H and Liu S: 99mTc-labeled cyclic RGD peptides for noninvasive
monitoring of tumor integrin αvβ3 expression.
Mol Imaging. 10:386–397. 2011.PubMed/NCBI
|
|
15
|
Distler JH, Hirth A, Kurowska-Stolarska M,
Gay RE, Gay S and Distler O: Angiogenic and angiostatic factors in
the molecular control of angiogenesis. Q J Nucl Med. 47:149–161.
2003.PubMed/NCBI
|
|
16
|
Wong CI, Koh TS, Soo R, Hartono S, Thng
CH, McKeegan E, Yong WP, Chen CS, Lee SC, Wong J, et al: Phase I
and biomarker study of ABT-869, a multiple receptor tyrosine kinase
inhibitor, in patients with refractory solid malignancies. J Clin
Oncol. 27:4718–4726. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jiang F, Albert DH, Luo Y, Tapang P, Zhang
K, Davidsen SK, Fox GB, Lesniewski R and McKeegan EM: ABT-869, a
multitargeted receptor tyrosine kinase inhibitor, reduces tumor
microvascularity and improves vascular wall integrity in
preclinical tumor models. J Pharmacol Exp Ther. 338:134–142. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tannir NM, Wong YN, Kollmannsberger CK,
Ernstoff MS, Perry DJ, Appleman LJ, Posadas EM, Cho D, Choueiri TK,
Coates A, et al: Phase 2 trial of linifanib (ABT-869) in patients
with advanced renal cell cancer after sunitinib failure. Eur J
Cancer. 47:2706–2714. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gullino PM: Angiogenesis and neoplasia. N
Engl J Med. 305:884–885. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Koukourakis MI, Giatromanolaki A, Sivridis
E and Fezoulidis I: Cancer vascularization: Implications in
radiotherapy? Int J Radiat Oncol Biol Phys. 48:545–553. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Morrison MS, Ricketts SA, Barnett J,
Cuthbertson A, Tessier J and Wedge SR: Use of a novel Arg-Gly-Asp
radioligand, 18F-AH111585, to determine changes in tumor
vascularity after antitumor therapy. J Nucl Med. 50:116–122. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Battle MR, Goggi JL, Allen L, Barnett J
and Morrison MS: Monitoring tumor response to antiangiogenic
sunitinib therapy with 18F-fluciclatide, an 18F-labeled
αVbeta3-integrin and αVbeta5-integrin imaging agent. J Nucl Med.
52:424–430. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sun X, Yan Y, Liu S, Cao Q, Yang M,
Neamati N, Shen B, Niu G and Chen X: 18F-FPPRGD2 and 18F-FDG PET of
response to Abraxane therapy. J Nucl Med. 52:140–146. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhou Y, Kim YS, Lu X and Liu S: Evaluation
of 99mTc-labeled cyclic RGD dimers: Impact of cyclic RGD peptides
and 99mTc chelates on biological properties. Bioconjug Chem.
23:586–595. 2012. View Article : Google Scholar : PubMed/NCBI
|