Introduction
Idiopathic pulmonary fibrosis (IPF) is a progressive
and usually fatal lung disease characterized by fibroblast
proliferation, and extracellular matrix remodeling (1,2). It is a
common disease in the elderly population, particularly those who
are between 50–70 years old. IPF may be associated with additional
comorbidities, which have an impact on the quality of life and
survival of patients in addition to the progressive exertional
dyspnea. IPF has a small number of treatment options. Therefore, it
is hypothesized that since there are no effective therapies, timely
detection and reduction of the complications are important in order
to improve the quality of life of patients. It has been reported
that IPF is an independent risk factor of lung cancer (1,2), with
non-small cell lung carcinoma (NSCLC) being the main pathological
type. However, the underlying mechanism remains poorly
understood.
Epigenetic alterations are involved in the
pathogenesis of IPF and lung cancer. Long non-coding RNA (lncRNA)
is a class of RNA with the length of >200 bases. With the
development of gene sequencing technology and bioinformatics
technology, increasing evidence has demonstrated that changes in
lncRNA expression levels are associated with numerous diseases
including lung disease and neurological disease (3,4). The p53
gene has been revealed to have the highest number of genetic
correlations with human tumor types and is an important tumor
suppressor gene (5). The p53-mediated
cell-signaling pathway serves an important role in the regulation
of normal cellular activities.
Studies have demonstrated that lncRNA alterations
and the p53-signaling pathway are involved in the process of IPF,
and lung cancer formation. Therefore, it was hypothesized that
there are certain changes in lncRNA related to the p53 gene,
further associating IPF with lung cancer. Thus, the present study
aimed to investigate the differential expression of lncRNAs through
high-throughput sequencing and bioinformatics analysis.
Materials and methods
Study population
A total of 24 patients with IPF, according to
diagnostic criteria established by the American Thoracic Society
(6), and 24 healthy controls were
involved in the study (all males; aged 67±3.2 vs. 64±2.8 years,
respectively; P>0.05). Based on the uniformity of background
including age and gender, four patients with IPF and four healthy
controls were selected for RNA extraction. RNA from peripheral
blood was extracted using high-throughput sequencing and
bioinformatics analysis was performed for the expression of lncRNA.
The remaining 20 patients with IPF and 20 healthy controls were
further studied; RNA extracted from peripheral blood was used to
verify the lncRNA and mRNA. The 3 ml of blood sample was stored in
−70°C for further study. The present study was approved by the
Shanxi Medical University Ethics Committee (Taiyuan, China).
Written informed consent was obtained from each patient and healthy
individuals.
RNA extraction
Total RNA was isolated using TRIzol (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. RNA concentration and quality were assessed using a
NanoDrop ND-1000 Spectrophotometer (NanoDrop Technologies; Thermo
Fisher Scientific, Inc., Waltham, MA, USA). The ratio A260/A280 was
between 1.8–2.1.
RNA sequencing
Ribosomal (r)RNA was removed the total RNA (1 µg)
samples using the Ribo-Zero Gold kit (Illumina, Inc., San Diego,
CA, USA) according to the manufacturer's protocol. The
rRNA-depleted samples were used for library construction using the
NEBNext® Ultra™ RNA Library Prep kit according to the
manufacturer's protocol (New England Biolabs, Inc., Ipswich, MA,
USA). Libraries were sequenced with the Illumina HiSeq Sequencer
according to the manufacturer's protocol (Illumina, San Diego, CA,
USA). Reads were trimmed and cleaned of Illumina adaptors, and low
quality sequences using Cutadapt software (version 1.9.2;
https://github.com/marcelm/cutadapt).
Clean reads were mapped to the Human genome [University of
California Santa Cruz (UCSC) hg19; http://genome.ucsc.edu/cgi-bin/hgGateway] using
TopHat2 software (version 2.1.2; http://ccb.jhu.edu/software/tophat/index.shtml) and
unmapped reads were discarded. Cuffdiff software (version 2.1.2;
part of cufflinks; https://github.com/cole-trapnell-lab/cufflinks) was
used to perform expression analysis and differential expression
analysis. LncRNAs were considered to be differentially expressed
based on Fragments/kb of transcript/million mapped reads (FPKM)
>0.5 and fold change >2.0.
Gene ontology (GO) analysis and
pathway analysis
The GO enrichment analysis was performed for
functional analysis of LncRNA-associated genes using R package
‘topGO’ from Bioconductor (http://www.bioconductor.org/packages/release/bioc/html/topGO.html).
The significant pathways for predicting target genes were
identified according to the Kyoto Encyclopedia of Genes and Genomes
(KEGG) database (http://www.genome.jp/kegg/). The Fisher's exact test
was used to select the significant Gene ontology and pathways, and
the threshold of significance was defined by P<0.05. RNA
Sequencing reads may be mapped into the reference genome. Any gene
of interest may be directly visualized in the Integrative Genomics
Viewer (http://www.broadinstitute.org/igv/).
cDNA synthesis and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was used to make cDNA using PowerScript
RT-PCR kit (Cloud-Seq Inc., Shanghai, China) according to the
manufacturer's protocol with random primers supplied with the kit.
The expression levels of lncRNAs and mRNAs were determined using
SYBR Green I-based qPCR. The qPCR thermocycling conditions were
maintained as follows: 95°C for 10 min; followed by 40 cycles of
95°C for 10 sec and 60°C for 60 sec. Data were analyzed using the
comparative ∆Cq method (7) with
β-actin as an endogenous reference gene. Primers were designed
using Primer (version 5.0; https://wheat.pw.usda.gov/demos/BatchPrimer3/). The
primers used were as follows: Cyclin dependent kinase inhibitor
(CDKN)2B-antisense RNA 1 (AS1) forward, AACCGGGGAGATCTATTTGG and
reverse, GGTGTGGTGTCTCACACCTG; CDKN2A forward, GGCTGTTCCTGGTCATGAT
and reverse, TGTCCAGGAAGCCCTCC.
Statistical analysis
Results are expressed as the mean ± standard
deviation. Differences between groups were analyzed by one-way
analysis of variance (ANOVA). Bonferroni post hoc test was used to
identify which comparison is significantly different after ANOVA
analysis. Statistical significance was determined using SPSS
software (version 17.0; SPSS, Inc., Chicago, IL, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
lncRNA expression
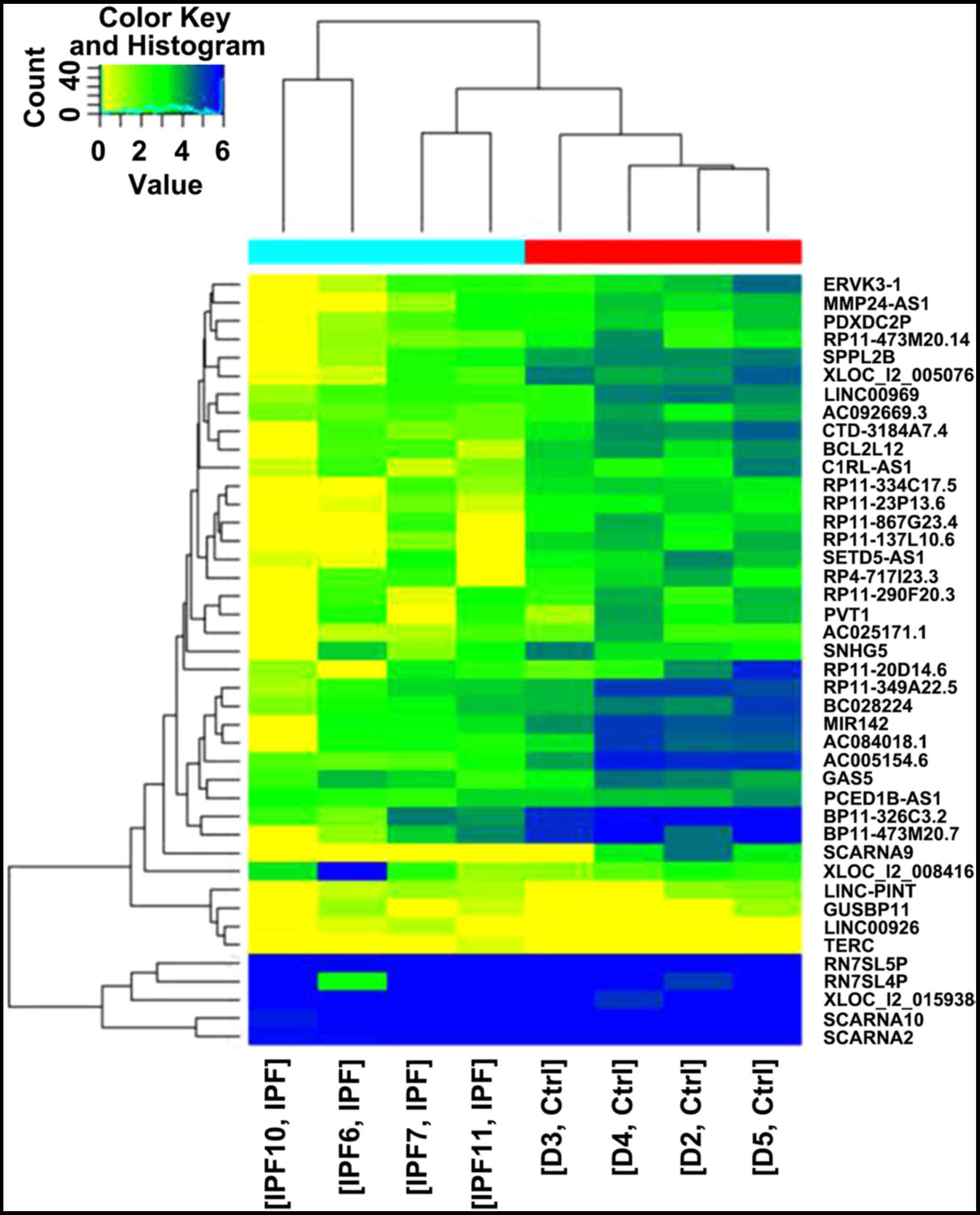
A total of 1,816 differentially expressed lncRNAs
were identified via screening, including 440 upregulated and 1,376
downregulated lncRNAs (Fig. 1). In
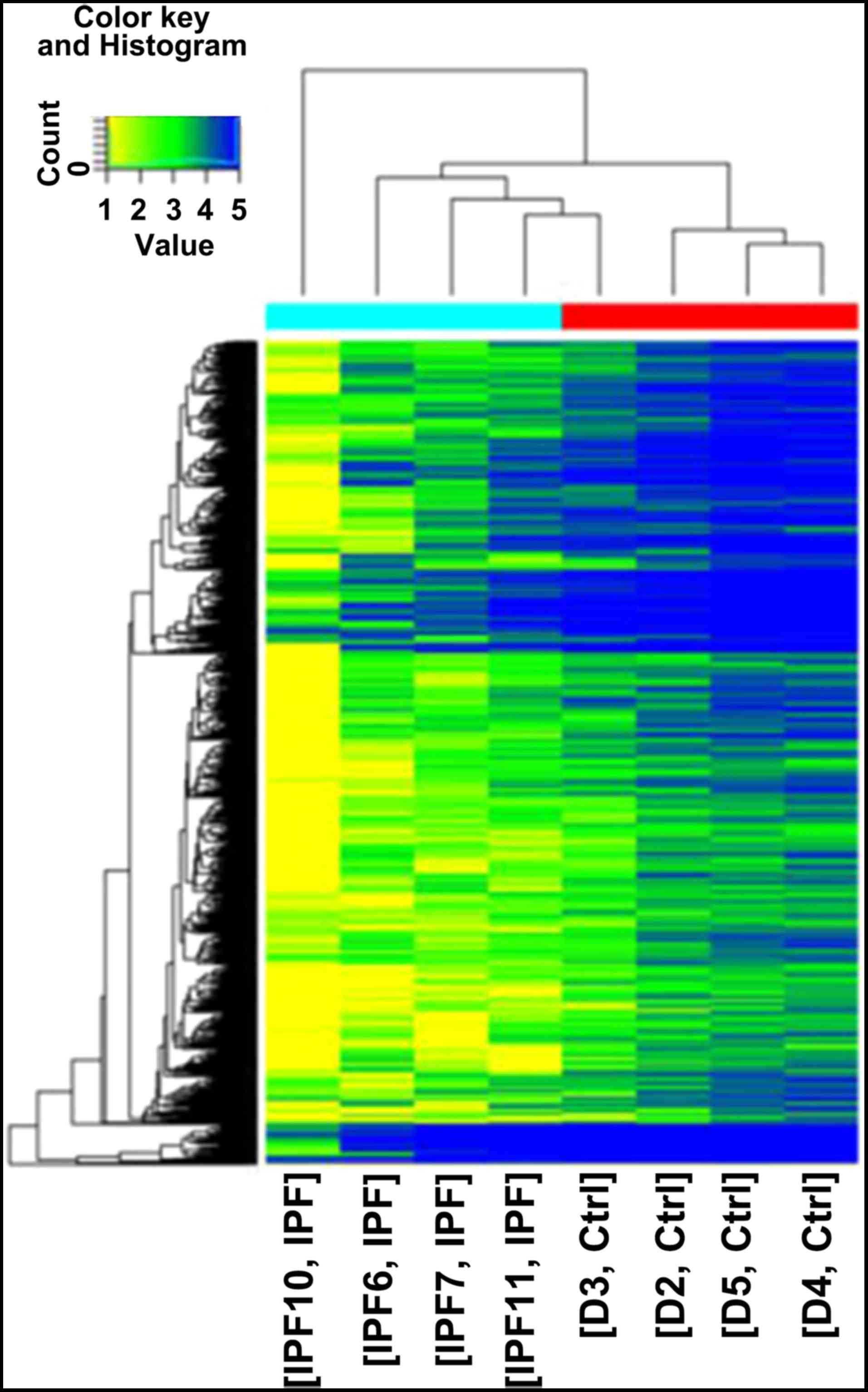
addition, 1,124 differentially expressed mRNAs were identified
(Fig. 2). Notably, downregulated
lncRNAs were more common compared with upregulated lncRNAs. Among
the lncRNAs, CDKN2B-AS1 (chr9:21802541-22121096) was identified to
be the most significantly downregulated lncRNA.
Pathway analysis
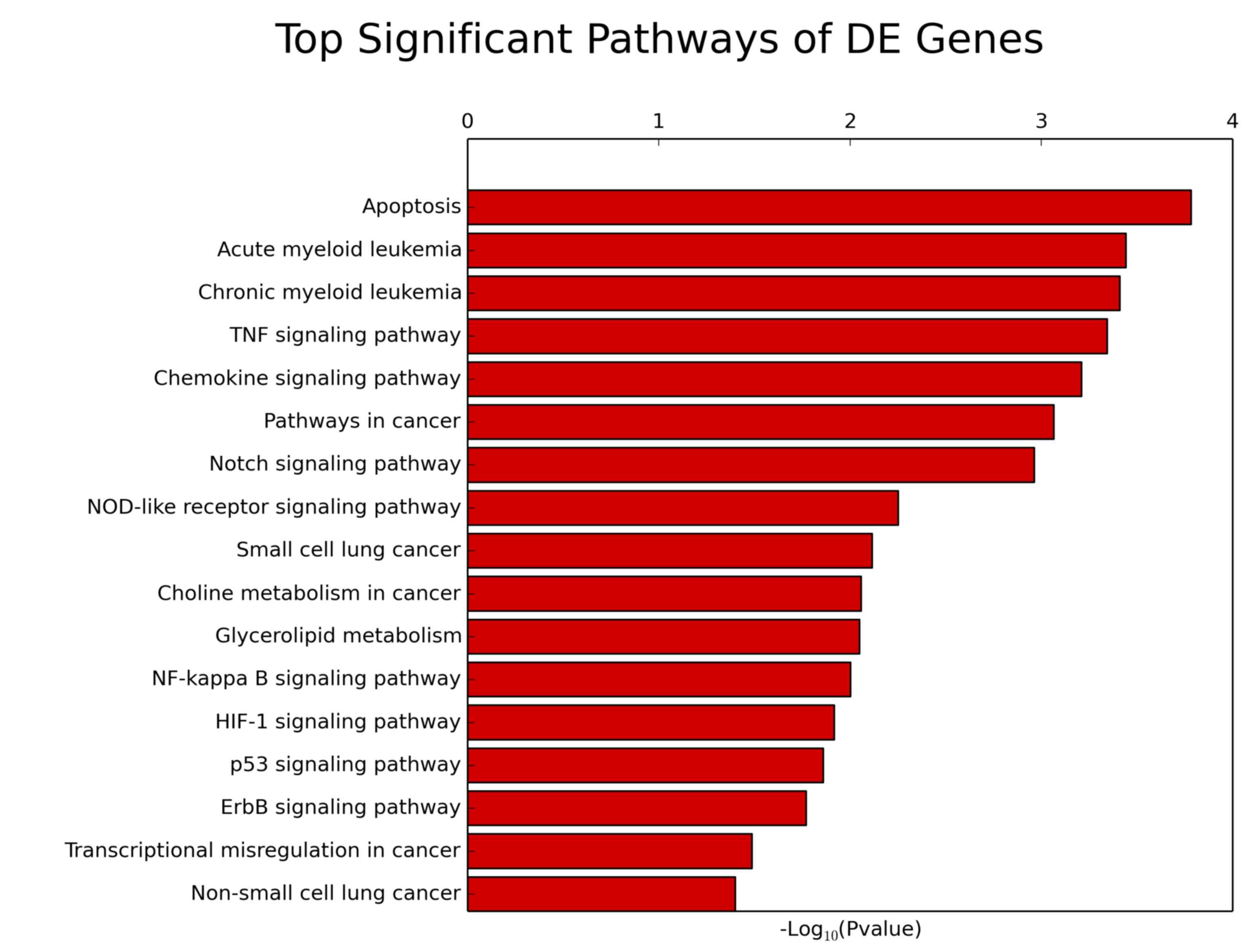
The significant pathways for predicting the target
gene were identified according to the KEGG database using Fishers
exact test. The p53-signaling pathway was significantly associated
with lung cancer (Fisher's exact test; P<0.013) and target
gene-related pathways (Fisher's exact test; P<0.0039; Fig. 3; Table
I).
 | Table I.Target gene-associated pathways. |
Table I.
Target gene-associated pathways.
| Pathway ID | Definition | Fisher P-value | Selection
counts | Size |
Enrichment-score | Genes |
|---|
| hsa04115 | p53-signaling
pathway | 0.013 | 24 | 6890 | 1.86 |
APAF1//BAI1//BAX//BBC3//CASP8//CASP9//CCND3//CCNG2//CD82//CDK2//CDKN1A//CDKN2A//CHEK1//CYCS//DDB2//IGFBP3//RCHY1//RFWD2//RRM2B//SHISA5//TNFRSF10B//TP53//TSC2//ZMAT3 |
| hsa04330 | Notch-signaling
pathway | 0.001 | 21 | 6890 | 2.96 |
APH1A//CREBBP//CTBP2//DTX1//DTX3//DVL1//DVL2//DVL3//HDAC1//HDAC2//JAG2//KAT2A//LFNG//MFNG//NCOR2//NCSTN//NOTCH1//NOTCH2//NUMB//PSENEN//RFNG |
| hsa04621 | NOD-like
receptor-signaling pathway | 0.005 | 22 | 6890 | 2.52 |
CARD6//CARD8//CARD9//CASP5//CASP8//CHUK//CXCL1//IKBKB//MAPK13//MAPK14//MAPK3//MEFV//NFKB1//NLRP1//NLRP3//NOD1//NOD2//PSTPIP1//PYCARD//RELA//SUGT1//TNF |
| hsa04668 | TNF signaling
pathway | <0.001 | 41 | 6890 | 3.34 |
AKT1//AKT2//BCL3//CASP10//CASP8//CEBPB//CHUK//CREB1//CREB3L2//CSF1//CXCL1//CXCL5//FADD//FOS//ICAM1//IKBKB//IL15//JUNB//MAP2K6//MAP2K7//MAP3K5//MAPK13//MAPK14//
MAPK3//MMP14//MMP9//NFKB1//
NOD2//PGAM5//PIK3CD//PIK3R5//PTGS2//RELA//RIPK1//RIPK3//RPS6KA5//TNF//TNFRSF1B//TRADD//TRAF1//TRAF3 |
| hsa05222 | Small cell lung
cancer | 0.008 | 30 | 6890 | 2.11 |
AKT1//AKT2//APAF1//BCL2//CASP9//CDK2//CDKN1B//CDKN2B//CHUK//COL4A3//CYCS//E2F2//FHIT//IKBKB//ITGA3//ITGA6//ITGAV//LAMA5//LAMB2//LAMC3//MYC//NFKB1//PIK3CD//PIK3R5//PTGS2//RELA//TP53//TRAF1//TRAF3//TRAF4 |
| hsa05223 | Non-small cell lung
cancer | 0.004 | 19 | 6890 | 1.399 |
AKT1//AKT2//ARAF//CASP9//CDKN2A//E2F2//FHIT//GRB2//HRAS//MAPK3//PDPK1//PIK3CD//PIK3R5//PLCG1//PRKCA//PRKCB//RASSF1//TGFA//TP53 |
Target lncRNA
CDKN2A is an important component of the
p53-signaling pathway. Using bioinformatics analysis, it was
revealed that the adjacent mRNA gene of CDKN2A was CDKN2B-AS1.
Through the aforementioned selected differentially expressed lncRNA
and mRNA, the key lncRNA is CDKN2B-AS1 (chr9:21802541-22121096)
with change of 3.78-folds. Differentially expressed lncRNA and mRNA
were screened out and it was found that CDKN2B-AS1
(chr9:21802541-22121096) decreased significantly in IPF patients.
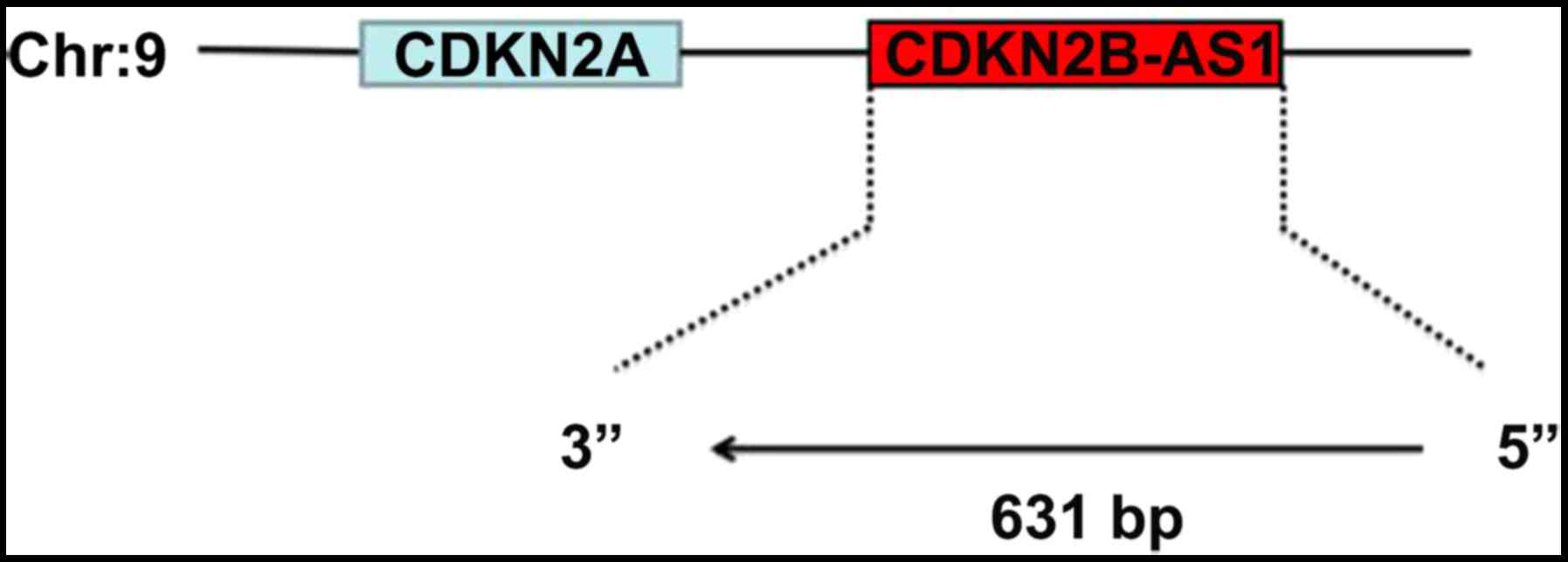
The gene ID of CDKN2B-AS1 is ENSG00000240498. According to the UCSC
database, the CDKN2B-AS1 gene length is 631 bp. CDKN2B-AS1 was
identified in the ‘Homo_sapiens_HG19.sorted.gtf’ database.
From the Ensembl database (http://www.ensembl.org/Homo_sapiens/Info/Index)
and CNKI gene database, the transcript of CDKN2A was demonstrated
to contain an alternate open reading frame (ARF) that encodes a
protein, which is structurally unassociated with the products of
the other variants. This ARF product functions as a stabilizer of
the tumor suppressor protein p53 as they can interact with each
other (8). This gene is known as an
important tumor suppressor gene and is associated with IPF. The
positional association between the two genes is antisense (Fig. 4), and the adjacent gene of the
CDKN2B-AS1 is CDKN2A. It further suggests that these two genes
CDKN2B-AS1 and CDKN2A are simultaneously transcribed. This lncRNA
is located on chromosome 9 at the approximate location
chr9:21802541-22121096 and the adjacent gene is CDKN2A in Fig. 4.
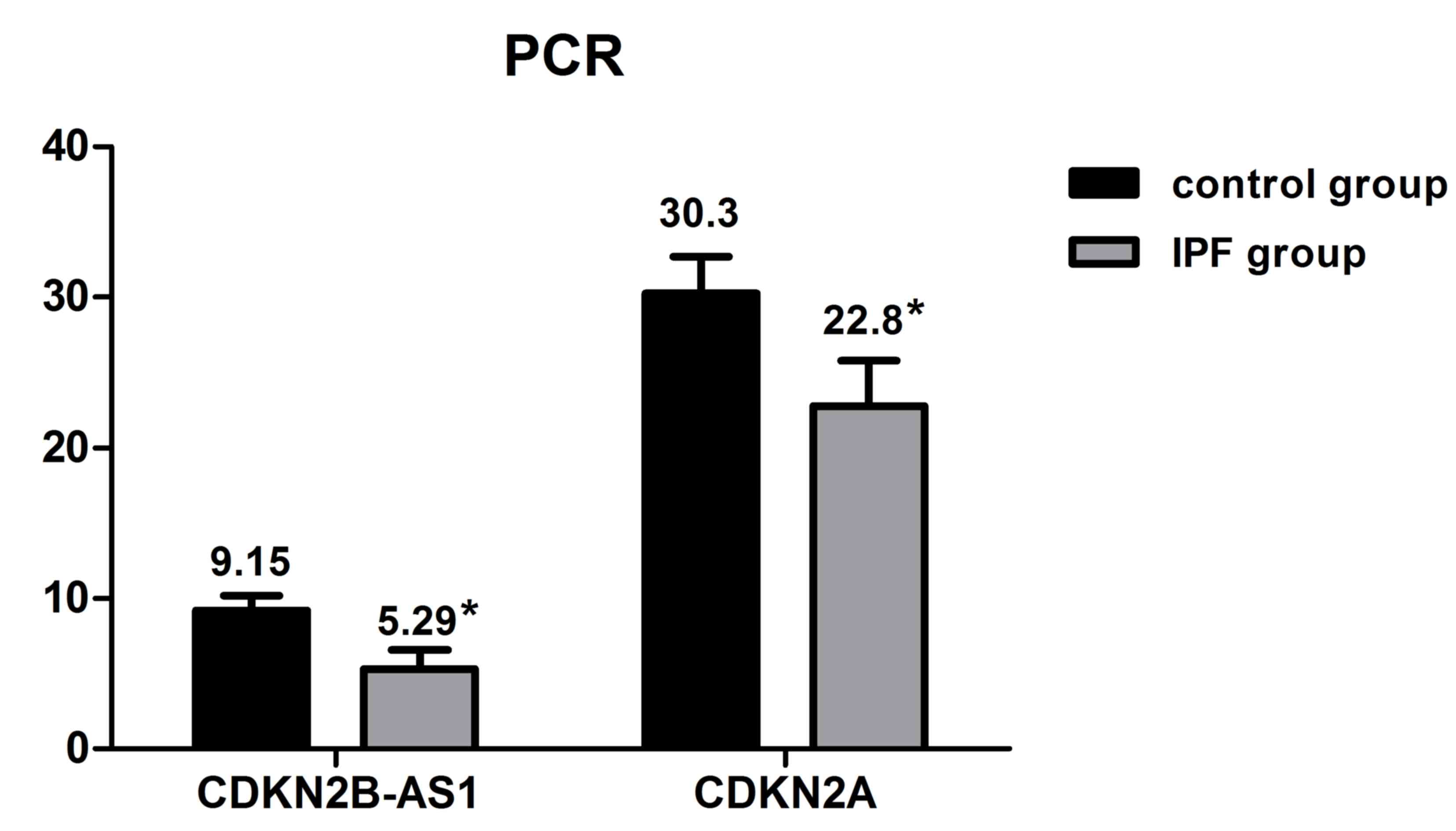
From the aforementioned results, the CDKN2B-AS1 and
CDKN2A expression levels were determined in the remaining IPF and
control group cases (n=20 each) using RT-qPCR technology. The
results revealed that CDKN2B-AS1 and CDKN2A expression levels were
significantly decreased in the IPF group compared with the control
group (*P<0.05; Fig. 5).
Discussion
IPF is a chronic, progressive and fatal diffuse
interstitial lung disease. It has re-emerged as a focus of
scientific study, due to its increasing incidence, the progressive
dyspnea and lack of effective treatments (9). The diagnosis of IPF often requires a
multidisciplinary approach, involving pulmonologists, radiologists,
and pathologists experienced in the field of interstitial lung
diseases (6,10). As early as 1957, the incidence of lung
cancer in patients with IPF was identified to be higher, compared
with healthy control patients. (11),
is has been confirmed that the incidence of lung cancer is
increased in patients with IPF compared to the general population
(12–16). A previous study identified that IPF is
an independent risk factor of lung cancer (14). Recently, the view that lung cancer
occurs as a late complication of IPF rather than being incidental
has been supported (17). Studies
consistently demonstrated that elderly male patients with IPF with
a history of smoking are more likely to develop lung cancer
(18–20).
Typical HRCT findings from lung cancer with IPF are
well-defined nodules with lobulation in the peripheral subpleural
areas inside or adjacent to the fibrosis (21). Kim et al (22) reported that the highest proportion of
IPF with lung cancer cases is adenocarcinoma, but Lee et al
(23) reported that the highest
proportion was squamous cell carcinoma. These two differences may
be associated with the different sample populations that were
chosen; however, patients with IPF are prone to having non-small
cell lung cancer (24). It has been
reported that the incidence of IPF associated with lung cancer is
inconsistent, which may be due to the different diagnostic criteria
used. Certain reports have noted the incidence of lung cancer as
4.8–48% and without pulmonary fibrosis as 2.0–6.4% (1), thus it has been considered that IPF is a
precancerous condition. IPF has been associated with lung cancer;
however, the underlying mechanism remains to be elucidated. There
are a number of genes and signaling pathways involved in this
process, including the p53-signaling pathway.
Of all humans cancers, ~50% lack a wild-type p53
allele and thus fail to produce a normal version of the p53 protein
(5). The presence of multiple
mutations in the p53 gene may explain the high incidence of IPF
complicated by lung carcinoma (25).
Kawasaki et al (26) have
reported that tumor suppressor p53 is altered in squamous
metaplasia, and dysplastic bronchial and alveolar epithelia in
patients with IPF. Exposure to carcinogens, tobacco and aging may
cause the inactivation of tumor suppressor genes, and lead to lung
cancer in patients with IPF (1).
Reduced levels of p53 have been identified in cancer of the colon,
lung, esophagus, breast, liver, brain, reticuloendothelial tissues
and hemopoietic tissues. The incidence of positive anti-p53
antibody in IPF, irrespective of the existence of lung cancer, was
as high as that in lung cancer (27).
These findings suggest that the p53-signaling pathway is associated
with lung cancer (28–30) and with IPF (31). Thus, we hypothesized that p53 mutation
downregulation may be associated with a high incidence of lung
cancer in patients with IPF.
Previously, lncRNA molecules were generally
considered as byproducts formed during the transcription of the
genome, and were defined as transcriptome ‘background noise’
without biological functions. It has now been reported that lncRNAs
are involved in numerous important biological processes, including
gene imprinting, cell proliferation and differentiation, immune
responses, and chromosome structure. LncRNA may serve a role in a
variety of mechanisms, including the following: Rupturing of small
RNA (shortRNAs); specific binding to chromosomes in Hox gene loci;
regulating epigenetic activity by transacting; RNA may also form
DNA-DNA-RNA triple helix structures to inhibit promoter activity;
coding mRNA antisense transcripts to regulate gene activity
(32). Previously, research confirmed
that lncRNA is associated with numerous chronic pulmonary disease
types, which serves important roles in the biological processes of
lung cancer and IPF (33).
Through microarray analysis of bleomycin-induced
pulmonary fibrosis in a mouse model, the differential expression of
lncRNA was confirmed (34).
Furthermore, lncRNA imbalance is a characteristic of numerous types
of cancer, which may be involved in promoting tumor progression,
invasion and metastasis (35–37). lncRNA has been demonstrated to be
involved in cell proliferation, apoptosis, epithelial-mesenchymal
transition and other biological processes, which regulate
tumorigenesis, and metastasis (38–40).
lncRNAs may either facilitate or inhibit the progression of lung
cancer and the various pathways involved (41–43).
In the present study, differentially expressed
lncRNAs were screened in IPF, lncRNA CDKN2B-AS1 was screened and a
fold-change of 3.78 FPKM was observed (P<0.05). The gene ID for
CDKN2B-AS1 was ENSG00000240498. According to the UCSC database, the
CDKN2B-AS1 gene length was 631 bp. However, there is currently
little research on CDKN2B-AS1. Certain reports have noted that
CDKN2B-AS1 is associated with hypertension and myocardial injury
(44). In genome wide association
studies of individual cancer types, genetic polymorphisms in the
CDKN2A/2B-AS1/2B/methylthioadenosine phosphorylase gene cluster
were associated with melanoma (45).
There are several mechanisms of lncRNA, it is able
to inhibit the expression of certain genes and may enhance the
expression of its neighboring gene (46,47). A
previous study demonstrated that lncRNAs serve an important role in
the process of transcription, particularly that of neighboring
genes (33). It has been reported
that adjacent pairs of genes often exhibit correlated expression
patterns throughout the cell cycle (48–50).
lncRNAs may regulate the transcription of adjacent genes, thus
affecting their biological roles. According to the bioinformatics
analysis in the present study, it was revealed that the adjacent
gene mRNA of CDKN2B-AS1 was CDKN2A. CDKN2A is the cyclin-dependent
kinase inhibitor, which is an important tumor suppressor gene. It
is involved in the regulation of cell proliferation and apoptosis,
encoding proteins p16INK4a and p14ARF serve a function via
retinoblastoma protein and p53 protein respectively (51). Altered expression levels of the CDKN2A
gene have been reported in numerous tumor types such as tumors of
lung, breast, brain, bone, skin, bladder, kidney, ovary, and
lymphocyte (52), thus, there it is a
focus of oncogenetic studies. CDKN2A is considered as an exogenous
marker, which is able to be detected at an early stage of sputum
and bronchoalveolar lavage fluid in patients with lung cancer
(53). Busch et al (8) identified that induction of ARF is an
early response in lung tumorigenesis that mounts a barrier against
tumor growth and malignant progression. According to the
GeneWays7.0 database, Li et al (54) reported that CDKN2A is associated with
non-small lung cancer. In addition, Cisneros et al (55) demonstrated that many IPF fibroblasts
exhibit decreased expression of the proapoptotic p14ARF
attributable to promoter hypermethylation (55). The present study revealed that CDKN2A
expression was decreased significantly in patients with IPF, which
was consistent with previous report (55). Furthermore, CDKN2A expression was
concentrated on the p53-signaling pathway according to the
high-throughput sequencing results. It is involved in the
regulation of gene p53, there may be a key factor in IPF patients
with lung cancer.
In conclusion, the current study demonstrated that
CDKN2B-AS1 expression is decreased significantly in patients with
IPF, while its adjacent gene CDKN2A expression is reduced
simultaneously. Thus, it may promote the occurrence of lung cancer
by regulating p53-signaling pathways.
References
|
1
|
Ozawa Y, Suda T, Naito T, Enomoto N,
Hashimoto D, Fujisawa T, Nakamura Y, Inui N, Nakamura H and Chida
K: Cumulative incidence of and predictive factors for lung cancer
in IPF. Respirology. 14:723–728. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Artinian V and Kvale PA: Cancer and
interstitial lung disease. Curr Opin Pulm Med. 10:425–434. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Carninci P, Kasukawa T, Katayama S, Gough
J, Frith MC, Maeda N, Oyama R, Ravasi T, Lenhard B, Wells C, et al:
The transcriptional landscape of the mammalian genome. Science.
309:1559–1563. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mehler MF and Mattick JS: Noncoding RNAs
and RNA editing in brain development, functional diversification,
and neurological disease. Physiol Rev. 87:799–823. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kaelin WG Jr: The emerging p53 gene
family. J Natl Cancer Inst. 91:594–598. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
American Thoracic Society. Idiopathic
pulmonary fibrosis, . Diagnosis and treatment. International
consensus statement. American Thoracic Society (ATS) and the
European Respiratory Society (ERS). Am J Respir Crit Care Med.
161:646–664. 2000.PubMed/NCBI
|
|
7
|
Silver N, Best S, Jiang J and Thein SL:
Selection of housekeeping genes for gene expression studies in
human reticulocytes using real-time PCR. BMC Mol Biol. 7:332006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Busch SE, Moser RD, Gurley KE,
Kelly-Spratt KS, Liggitt HD and Kemp CJ: ARF inhibits the growth
and malignant progression of non-small-cell lung carcinoma.
Oncogene. 33:2665–2673. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Collard HR and Pantilat SZ: Dyspnea in
interstitial lung disease. Curr Opin Support Palliat Care.
2:100–104. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
King TE Jr, Pardo A and Selman M:
Idiopathic pulmonary fibrosis. Lancet. 378:1949–1961. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Spain DM: The association of terminal
bronchiolar carcinoma with chronic interstitial inflammation and
fibrosis of the lungs. Am Rev Tuberc. 76:559–566. 1957.PubMed/NCBI
|
|
12
|
Song DH, Choi IH, Ha SY, Han KM, Lee JJ,
Hong ME, Jeon K, Chung MP, Kim J and Han J: Usual interstitial
pneumonia with lung cancer: Clinicopathological analysis of 43
cases. Korean J Pathol. 48:10–16. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Le Jeune I, Gribbin J, West J, Smith C,
Cullinan P and Hubbard R: The incidence of cancer in patients with
idiopathic pulmonary fibrosis and sarcoidosis in the UK. Respir
Med. 101:2534–2540. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Matsushita H, Tanaka S, Saiki Y, Hara M,
Nakata K, Tanimura S and Banba J: Lung cancer associated with usual
interstitial pneumonia. Pathol Int. 45:925–932. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Turner-Warwick M, Lebowitz M, Burrows B
and Johnson A: Cryptogenic fibrosing alveolitis and lung cancer.
Thorax. 35:496–499. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Stack BH, Grant IW, Irvine WJ and Moffat
MA: Idiopathic diffuse interstitial lung disease. A review of 42
cases. Am Rev Respir Dis. 92:939–948. 1965.PubMed/NCBI
|
|
17
|
Tomassetti S, Gurioli C, Ryu JH, Decker
PA, Ravaglia C, Tantalocco P, Buccioli M, Piciucchi S, Sverzellati
N, Dubini A, et al: The impact of lung cancer on survival of
idiopathic pulmonary fibrosis. Chest. 147:157–164. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Aubry MC, Myers JL, Douglas WW, Tazelaar
HD, Washington Stephens TL, Hartman TE, Deschamps C and Pankratz
VS: Primary pulmonary carcinoma in patients with idiopathic
pulmonary fibrosis. Mayo Clin Proc. 77:pp. 763–770. 2002;
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nagai A, Chiyotani A, Nakadate T and Konno
K: Lung cancer in patients with idiopathic pulmonary fibrosis.
Tohoku J Exp Med. 167:231–237. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Park J, Kim DS, Shim TS, Lim CM, Koh Y,
Lee SD, Kim WS, Kim WD, Lee JS and Song KS: Lung cancer in patients
with idiopathic pulmonary fibrosis. Eur Respir J. 17:1216–1219.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kishi K, Homma S, Kurosaki A, Motoi N and
Yoshimura K: High-resolution computed tomography findings of lung
cancer associated with idiopathic pulmonary fibrosis. J Comput
Assist Tomogr. 30:95–99. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim Y, Kwon Y, Oh I, Kim K, Kim S, Ryu J,
Yum H, Yong S, Lee K, Lee C, et al: National survey of lung cancer
in Korea, 2005. J Lung Cancer. 6:67–73. 2007. View Article : Google Scholar
|
|
23
|
Lee C, Kang KH, Koh Y, Chang J, Chung HS,
Park SK, Yoo K and Song JS: Characteristics of lung cancer in
Korea, 1997. Lung Cancer. 30:15–22. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hironaka M and Fukayama M: Pulmonary
fibrosis and lung carcinoma: A comparative study of metaplastic
epithelia in honeycombed areas of usual interstitial pneumonia with
or without lung carcinoma. Pathol Int. 49:1060–1066. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Takahashi T, Munakata M, Ohtsuka Y,
Nisihara H, Nasuhara Y, Kamachi-Satoh A, Dosaka-Akita H, Homma Y
and Kawakami Y: Expression and alteration of ras and p53 proteins
in patients with lung carcinoma accompanied by idiopathic pulmonary
fibrosis. Cancer. 95:624–633. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kawasaki H, Ogura T, Yokose T, Nagai K,
Nishiwaki Y and Esumi H: p53 gene alteration in atypical epithelial
lesions and carcinoma in patients with idiopathic pulmonary
fibrosis. Hum Pathol. 32:1043–1049. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Oshikawa K and Sugiyama Y: Serum anti-p53
autoantibodies from patients with idiopathic pulmonary fibrosis
associated with lung cancer. Respir Med. 94:1085–1091. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhong G, Chen X, Fang X, Wang D, Xie M and
Chen Q: Fra-1 is upregulated in lung cancer tissues and inhibits
the apoptosis of lung cancer cells by the P53 signaling pathway.
Oncol Rep. 35:447–453. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu ZH, Wang MH, Ren HJ, Qu W, Sun LM,
Zhang QF, Qiu XS and Wang EH: Interleukin 7 signaling prevents
apoptosis by regulating bcl-2 and bax via the p53 pathway in human
non-small cell lung cancer cells. Int J Clin Exp Pathol. 7:870–881.
2014.PubMed/NCBI
|
|
30
|
Li S, Li X, Zhao H, Gao M, Wang F and Li
W: Overexpression of microRNA-125a-3p effectively inhibits the cell
growth and invasion of lung cancer cells by regulating the mouse
double minute 2 homolog/p53 signaling pathway. Mol Med Rep.
12:5482–5486. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chuang CY, Liu HC, Wu LC, Chen CY, Chang
JT and Hsu SL: Gallic acid induces apoptosis of lung fibroblasts
via a reactive oxygen species-dependent ataxia telangiectasia
mutated-p53 activation pathway. J Agric Food Chem. 58:2943–2951.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fu X, Ravindranath L, Tran N, Petrovics G
and Srivastava S: Regulation of apoptosis by a prostate-specific
and prostate cancer-associated noncoding gene, PCGEM1. DNA Cell
Biol. 25:135–141. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gupta RA, Shah N, Wang KC, Kim J, Horlings
HM, Wong DJ, Tsai MC, Hung T, Argani P, Rinn JL, et al: Long
non-coding RNA HOTAIR reprograms chromatin state to promote cancer
metastasis. Nature. 464:1071–1076. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cao G, Zhang J, Wang M, Song X, Liu W, Mao
C and Lv C: Differential expression of long non-coding RNAs in
bleomycin-induced lung fibrosis. Int J Mol Med. 32:355–364. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ricciuti B, Mencaroni C, Paglialunga L,
Paciullo F, Crinò L, Chiari R and Metro G: Long noncoding RNAs: New
insights into non-small cell lung cancer biology, diagnosis and
therapy. Med Oncol. 33:182016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang HM, Lu JH, Chen WY and Gu AQ:
Upregulated lncRNA-UCA1 contributes to progression of lung cancer
and is closely related to clinical diagnosis as a predictive
biomarker in plasma. Int J Clin Exp Med. 8:11824–11830.
2015.PubMed/NCBI
|
|
37
|
Khandelwal A, Bacolla A, Vasquez KM and
Jain A: Long non-coding RNA: A new paradigm for lung cancer. Mol
Carcinog. 54:1235–1251. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lopez-Pajares V: Long non-coding RNA
regulation of gene expression during differentiation. Pflugers
Arch. 468:971–981. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ebisuya M, Yamamoto T, Nakajima M and
Nishida E: Ripples from neighbouring transcription. Nat Cell Biol.
10:1106–1113. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
40
|
Huang C, Yang Y and Liu L: Interaction of
long noncoding RNAs and microRNAs in the pathogenesis of idiopathic
pulmonary fibrosis. Physiol Genomics. 47:463–469. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mercer TR, Dinger ME, Sunkin SM, Mehler MF
and Mattick JS: Specific expression of long noncoding RNAs in the
mouse brain. Proc Natl Acad Sci USA. 105:pp. 716–721. 2008;
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Rinn JL, Kertesz M, Wang JK, Squazzo SL,
Xu X, Brugmann SA, Goodnough LH, Helms JA, Farnham PJ, Segal E and
Chang Y: Functional demarcation of active and silent chromatin
domains in human HOX loci by noncoding RNAs. Cell. 129:1311–1323.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Willingham AT, Orth AP, Batalov S, Peters
EC, Wen BG, Aza-Blanc P, Hogenesch JB and Schultz PG: A strategy
for probing the function of noncoding RNAs finds a repressor of
NFAT. Science. 309:1570–1573. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Bayoglu B, Yuksel H, Cakmak HA, Dirican A
and Cengiz M: Polymorphisms in the long non-coding RNA CDKN2B-AS1
may contribute to higher systolic blood pressure levels in
hypertensive patients. Clin Biochem. 49:821–827. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Timofeeva MN, Hung RJ, Rafnar T,
Christiani DC, Field JK, Bickeböller H, Risch A, McKay JD, Wang Y,
Dai J, et al: Influence of common genetic variation on lung cancer
risk: Meta-analysis of 14 900 cases and 29 485 controls. Hum Mol
Genet. 21:4980–4995. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ørom UA, Derrien T, Beringer M, Gumireddy
K, Gardini A, Bussotti G, Lai F, Zytnicki M, Notredame C, Huang Q,
et al: Long noncoding RNAs with enhancer-like function in human
cells. Cell. 143:46–58. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Mattick JS: Linc-ing long noncoding RNAs
and enhancer function. Dev Cell. 19:485–486. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Cho RJ, Campbell MJ, Winzeler EA,
Steinmetz L, Conway A, Wodicka L, Wolfsberg TG, Gabrielian AE,
Landsman D, Lockhart DJ and Davis RW: A genome-wide transcriptional
analysis of the mitotic cell cycle. Mol Cell. 2:65–73. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kruglyak S and Tang H: Regulation of
adjacent yeast genes. Trends Genet. 16:109–111. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Cohen BA, Mitra RD, Hughes JD and Church
GM: A computational analysis of whole-genome expression data
reveals chromosomal domains of gene expression. Nat Genet.
26:183–186. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
51
|
Quelle DE, Zindy F, Ashmun RA and Sherr
CJ: Alternative reading frames of the INK4a tumor suppressor gene
encode two unrelated proteins capable of inducing cell cycle
arrest. Cell. 83:993–1000. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kamb A, Gruis NA, Weaver-Feldhaus J, Liu
Q, Harshman K, Tavtigian SV, Stockert E, Day RS III, Johnson BE and
Skolnick MH: A cell cycle regulator potentially involved in genesis
of many tumor types. Science. 264:436–440. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Huang SK, Scruggs AM, McEachin RC, White
ES and Peters-Golden M: Lung fibroblasts from patients with
idiopathic pulmonary fibrosis exhibit genome-wide differences in
DNA methylation compared to fibroblasts from nonfibrotic lung. PLoS
One. 9:e1070552014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Li J, Bi L, Sun Y, Lu Z, Lin Y, Bai O and
Shao H: Text mining and network analysis of molecular interaction
in non-small cell lung cancer by using natural language processing.
Mol Biol Rep. 41:8071–8079. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Cisneros J, Hagood J, Checa M,
Ortiz-Quintero B, Negreros M, Herrera I, Ramos C, Pardo A and
Selman M: Hypermethylation-mediated silencing of p14(ARF) in
fibroblasts from idiopathic pulmonary fibrosis. Am J Physiol Lung
Cell Mol Physiol. 303:L295–L303. 2012. View Article : Google Scholar : PubMed/NCBI
|