Introduction
Diffuse large B-cell lymphoma (DLBCL) is a common
histological form of non-Hodgkin's lymphoma (NHL), accounting for
30–40% of all adult NHLs (1,2). A diagnosis is made according to the
morphology and immunophenotype of B cells (3). First-line management of DLBCL is a
combination of chemotherapy drugs, rituximab, cyclophosphamide,
adriamycin, vincristine and prednisone (R-CHOP) (4). The International Prognostic Index (IPI)
(5) and age-adjusted International
Prognostic Index (aaIPI) (6) serve
important functions in daily practice to determine the treatment
strategies and prognosis of individual cases. Neither the IPI nor
the aaIPI identifies a risk group with <50% chance of survival
(6). Thus, novel prognostic models
are highly sought after. Inflammatory cells and cytokines located
in tumors are more likely to contribute to cancer growth, spread,
progression and immunosuppression than they are to mount an
effective host antitumor activity (7–10). The
inflammatory response can be represented by the level of serum
neutrophils, lymphocytes, platelets, C-reactive protein and albumin
(7) Previously, several combinations
of these factors, including the neutrophil-lymphocyte ratio (NLR),
lymphocyte-monocyte ratio (LMR) and platelet-lymphocyte ratio (PLR)
have been reported to be useful prognostic factors in various
malignant tumors (8,11–14).
However, to date, no reports have investigated whether platelet
count (PLT) and PLR are prognostic factors for DLBCL. The objective
of this study was to investigate the prognostic ability of PLT and
PLR in patients with DLBCL, and to obtain a novel prognostic
scoring system for these metrics in order to predict the prognosis
of the patients.
Patients and methods
Patients and clinicopathological
variables
The clinical characteristics of 309 patients
(including 186 males and 123 females) diagnosed with DLBCL between
March 2009 and February 2015 at Tianjin Medical University Cancer
Institute and Hospital (Tianjin, China) were retrospectively
analyzed. All patients who were treated with R-CHOP-21 [rituximab
(375 mg/m2) on day 1; cyclophosphamide (750
mg/m2), doxorubicin (50 mg/m2) and
vincristine (1.4 mg/m2; maximal dose, 2 mg) on day 2;
and prednisone (100 mg/day) on days 2 to 6] were diagnosed with
DLBCL via pathological analysis. The dose and number of
chemotherapy cycles were decided by physicians. The mean age of all
patients was 58 years (range, 16–90 years), The median follow-up
for the study was 47 months (range, 1–89 months). All patients
included in the study were diagnosed with untreated DLBCL. Patients
who had a previous history of malignancy, immunosuppression or
previous treatment were excluded from the study. The available
clinical parameters included age, gender, germinal center
B-cell-like or non-germinal center B-cell-like disease, systemic B
symptoms, Ann Arbor stage (15), IPI
or aaIPI, hemoglobin (HGB), absolute lymphocyte count (ALC),
absolute monocyte count (AMC), absolute neutrophil count (NEUT),
PLT, serum albumin, serum lactate dehydrogenase level (LDH: Normal
range 114–285 U/l), β2-microglobulin (β2m: Normal range
0.97–2.64 mg/l). The cell of origin was analyzed by
immunohistochemistry. ALC, AMC, NEUT and PLT were obtained from
pre-treatment CBC counts. According to IPI or aaIPI value, the
patients were divided into four groups: i) Low-risk patients with
IPI <2 scores or aaIPI=0; ii) low-intermediate risk patients
with IPI=2 or aaIPI=1; iii) intermediate-high risk patients with
IPI=3 or aaIPI=2; iv) high risk patients with IPI=4,5 or aaIPI=3.
This study was approved by the ethics committee of Tianjin Medical
University Cancer Institute and Hospital and was conducted in
accordance with the Declaration of Helsinki.
Time from diagnosis of DLBCL to mortality from any
cause was designated OS time. The time from diagnosis to lymphoma
relapse, progression or mortality due to any cause was designated
PFS time. The aim of the present study was to assess the effect of
PLT, NLR, LMR and PLR on OS and PFS.
Statistical analysis
Statistical analysis was performed using SPSS
software (version 17.0; SPSS, Inc., Chicago, IL, USA). Receiver
operating characteristic (ROC) curves was used to determine the
optimal threshold values of ALC, PLT, NLR, LMR and PLR. Patient
characteristics were compared between PLR <170 vs. PLR ≥170
using the Pearson χ2 test. The survival curves were
determined using the Kaplan-Meier method and the log-rank test.
Multivariate analysis, which used Cox's proportional hazards model,
was performed for the variables identified as statistically
significant in univariate analysis to exclude confounding factors.
A novel prognostic scoring system was then created to combine these
factors, which were identified as statistically significant.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Clinical characteristics of the
patients with DLBCL
This study included 309 patients with newly
diagnosed DLBCL. The mean age was 58 years (range, 16–90 years). A
total of 186 patients were male, and 181/309 (58.6%) patients
remained alive at the time of writing. Among the 128 mortalities,
123 individuals succumbed to disease recurrence, 4 individuals
succumbed to infectious shock and 1 individual succumbed to
respiratory failure. Baseline clinical and laboratory parameters
are presented in Table I.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Baseline
characteristics | Patients, n
(%) |
|---|
| Age |
|
|
<60 | 178 (57.6) |
|
≥60 | 131 (42.4) |
| Sex |
|
|
Male | 186 (60.2) |
|
Female | 123 (39.8) |
| B symptoms |
|
|
Absent | 209 (67.6) |
|
Present | 100 (32.4) |
| Ann Arbor
stage |
|
|
I–II | 177 (57.3) |
|
III–IV | 132 (42.7) |
| IPI or aaIPI risk
group |
|
|
Low-risk | 112 (36.2) |
|
Low-intermediate risk | 95
(30.8) |
|
High-intermediate risk | 56
(18.1) |
| High
risk | 46
(14.9) |
| Subtype |
|
|
GCB | 125 (40.5) |
|
n-GCB | 184 (59.5) |
| Albumin, g/l |
|
|
≥35 | 259 (83.8) |
|
<35 | 50
(16.2) |
| HGB, g/l |
|
|
≥110 | 232 (75.1) |
|
<110 | 77
(24.9) |
| ALC,
×109/l |
|
|
≥1.45 | 172 (55.7) |
|
<1.45 | 137 (44.3) |
| PLT,
×109/l |
|
|
≥250 | 186 (60.2) |
|
<250 | 123 (39.8) |
| NLR |
|
|
≥2.9 | 125 (40.5) |
|
<2.9 | 184 (59.5) |
| LMR |
|
|
≥2.65 | 193 (62.5) |
|
<2.65 | 116 (37.5) |
| PLR |
|
|
≥170 | 165 (53.4) |
|
<170 | 144 (46.6) |
| LDH (114–285
U/l) |
|
|
>Normal | 128 (41.4) |
|
≤Normal | 181 (58.6) |
| β2m (0.97–2.64
mg/l) |
|
|
>Normal | 131 (42.4) |
|
<Normal | 178 (57.6) |
Selection of the best threshold values
of ALC, PLT and PLR for DCBCL patients
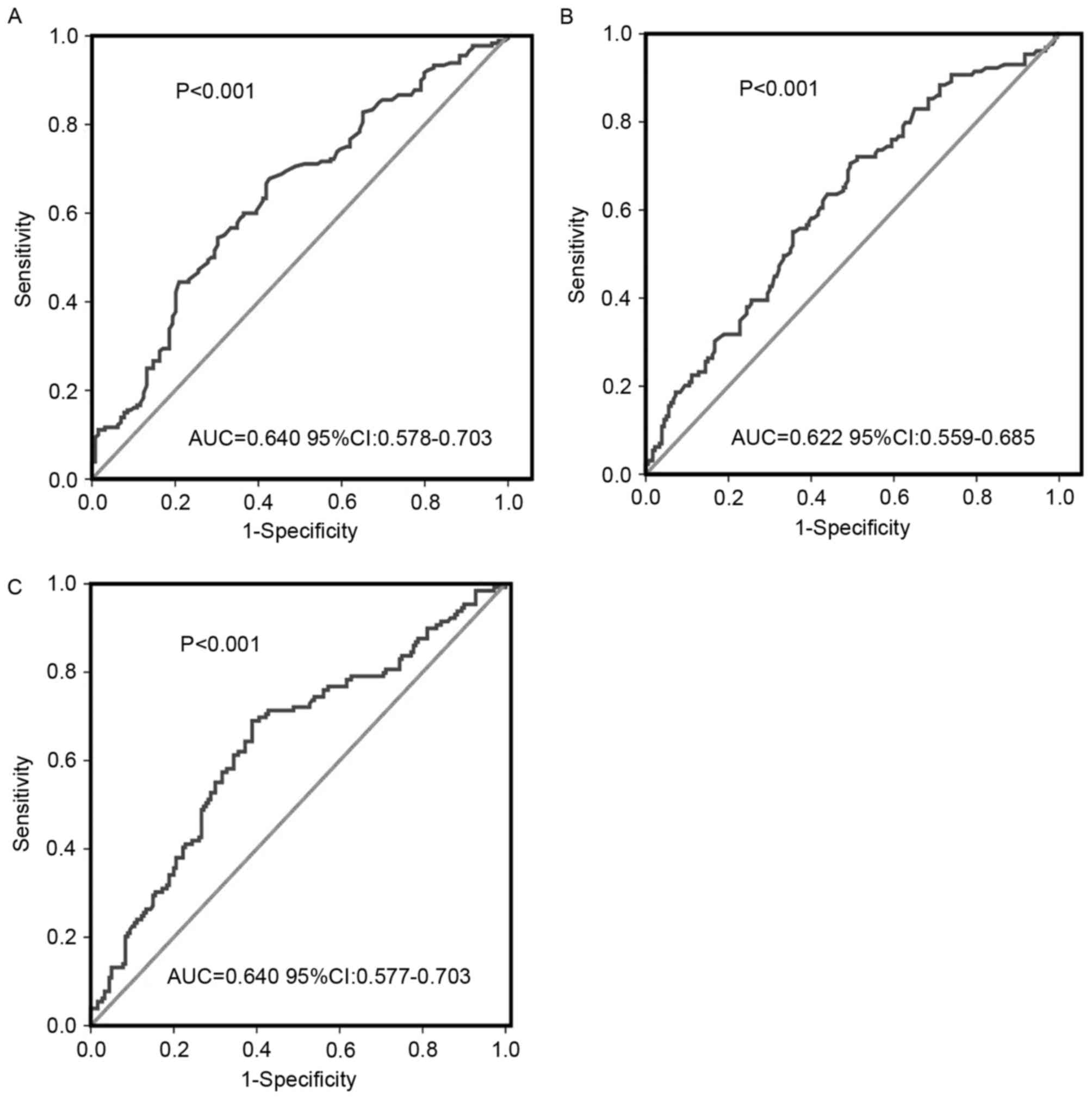
ROC curve analyses determined the optimal threshold
values for ALC, PLT and PLR. The optimal ALC threshold value was
1.45×109/l, with an area under the curve (AUC) value of
0.640 [95% confidence interval (CI), 0.578–0.703; P<0.001;
Fig. 1A]. ROC curve analysis
identified 250×109/l as the threshold value of PLT for
predicting survival with an AUC of 0.622 (95% CI, 0.559–0.685;
P<0.001; Fig. 1B). The threshold
value of PLR was 170, with an AUC of 0.640 (95% CI, 0.577–0.703;
P<0.001; Fig. 1C).
Association of PLR with the clinical
characteristics in patients with DLBCL
ROC curve analyses determined the that the sum of
the specificity and the sensitivity was the largest when PLR was
170, with an AUC of 0.640 (95% CI, 0.577–0.703; P<0.001).
Therefore, a PLR of 170 was chosen as the threshold for division in
to groups. Patients were divided into two groups: PLR <170 group
or PLT ≥170 group. A total of 144 patients (46.6%) had a PLR
<170 and 165 patients (53.4%) had a PLR ≥170. Compared with the
patients with a PLR <170, the patients with PLR ≥170 were
significantly associated with the presence of B syndromes,
increased Ann-Arbor stages (15),
high-intermediate risk or high risk (IPI ≥3, aaIPI ≥2), decreased
albumin levels, decreased HGB levels and increased LDH. However,
there was no significant association between PLR and age, gender,
subtype or β2m (Table II).
 | Table II.Clinical comparison between patients
with PLR<170 and PLR≥170. |
Table II.
Clinical comparison between patients
with PLR<170 and PLR≥170.
| Baseline
characteristics |
PLR<170a, n |
PLR≥170b,
n | P-value |
|---|
| Age, years |
|
| >0.999 |
|
<60 | 83 | 95 |
|
|
≥60 | 61 | 70 |
|
| Sex |
|
| 0.728 |
|
Male | 85 | 101 |
|
|
Female | 59 | 64 |
|
| B symptoms |
|
| <0.001 |
|
Absent | 115 | 94 |
|
|
Present | 29 | 71 |
|
| Ann Arbor
stage |
|
| <0.001 |
|
I–II | 101 | 76 |
|
|
III–IV | 43 | 89 |
|
| IPI or aaIPI risk
group |
|
| <0.001 |
|
Low-risk | 75 | 37 |
|
|
Low-intermediate risk | 43 | 52 |
|
|
High-intermediate risk | 14 | 43 |
|
|
High-risk | 12 | 33 |
|
| Subtype |
|
| >0.999 |
|
GCB | 59 | 67 |
|
|
n-GCB | 85 | 98 |
|
| Albumin, g/l |
|
| <0.001 |
|
≥35 | 137 | 122 |
|
|
<35 | 7 | 43 |
|
| HGB, g/l |
|
| <0.001 |
|
≥110 | 132 | 100 |
|
|
<110 | 12 | 65 |
|
| LDH (114–285
U/l) |
|
| <0.001 |
|
>Normal | 39 | 89 |
|
|
≤Normal | 105 | 76 |
|
| β2m (0.97–2.64
mg/l) |
|
| 0.728 |
|
>Normal | 42 | 89 |
|
|
<Normal | 102 | 76 |
|
Prognostic significance of PLT and
PLR
Univariate analysis revealed that an age ≥60 years
(P<0.001), the presence of B symptoms (P=0.001), stage III–IV
disease (P<0.001), high-intermediate risk or high risk
(P<0.001), decreased albumin levels (P<0.001), decreased HGB
levels (P<0.001), ALC (P<0.001), NLR (P=0.019), LMR
(P<0.001), increased LDH levels (P<0.001) and β2m
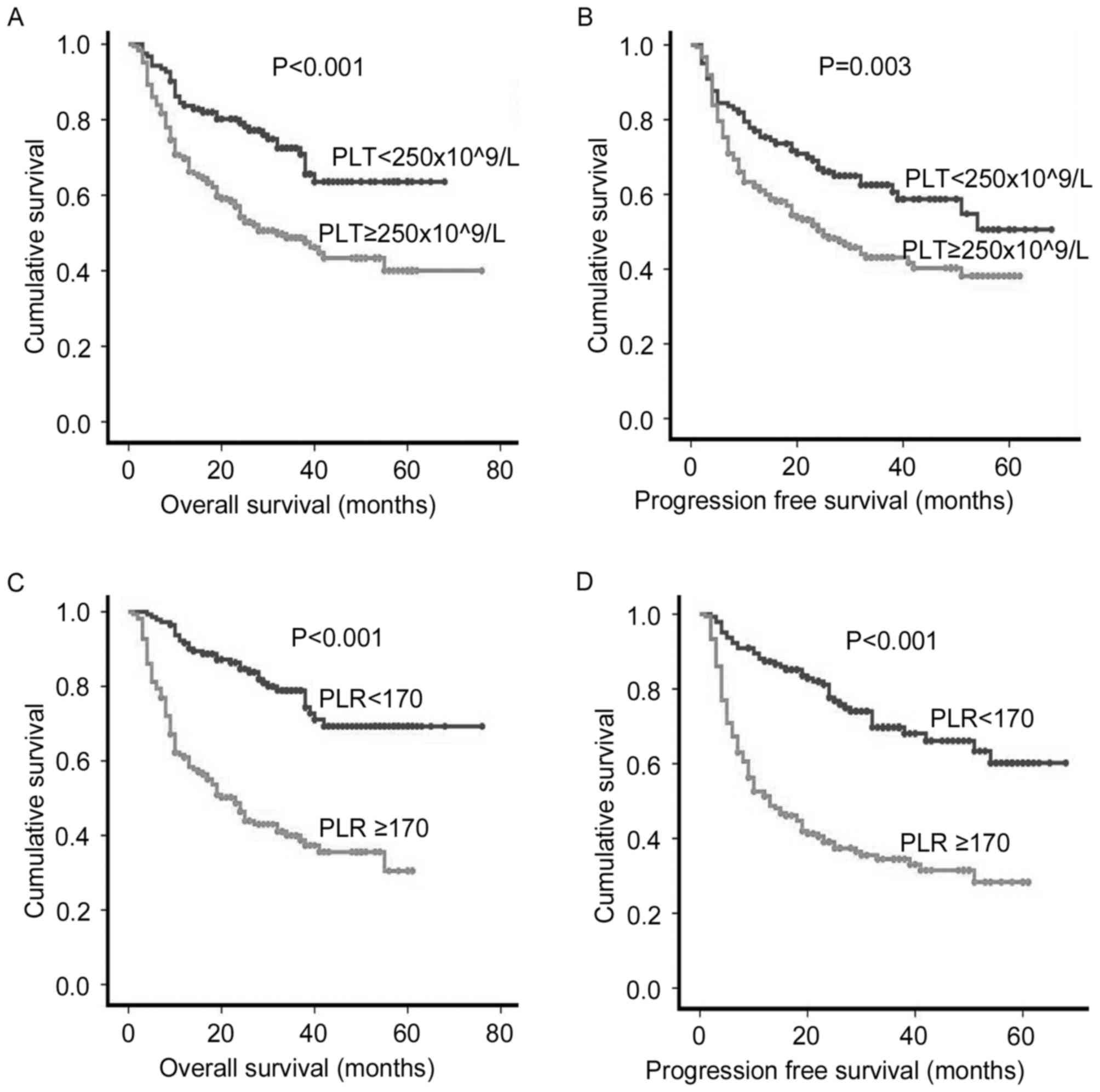
(P<0.001) were poor prognostic factors (Table III). Patients with a PLT
≥250×109/l experienced a significantly decreased OS rate
(5-year OS rate, 50.0 vs. 70.7%; P<0.001 Fig. 2A) and PFS (5-year PFS rate, 45.7 vs.
61.5%; P=0.003 Fig. 2B) than those
with PLT<250×109/l. The OS and PFS rate in patients
with a PLR ≥170 were significantly decreased compared with those
with a PLR <170 at diagnosis (5-year OS rate, 41.8 vs. 77.1%,
P<0.001 Fig. 2C; 5-year PFS rate,
35.8 vs. 70.6%, P<0.001, Fig. 2D).
Furthermore, via multivariate analysis, age, IPI or aaIPI rick
groups, β2m and PLR were identified to be independent prognostic
factors for OS, whereas age, Ann-Arbor stage and PLR may
independently predict poor PFS (Table
IV).
 | Table III.Analysis of prognostic factors for OS
and PFS (univariate analysis) in 309 patients with DLBCL. |
Table III.
Analysis of prognostic factors for OS
and PFS (univariate analysis) in 309 patients with DLBCL.
| Baseline
characteristics | OS | P-value | PFS | P-value |
|---|
| Age, years |
| <0.001 |
| <0.001 |
|
<60 | 68.5 |
| 61.8 |
|
|
≥60 | 44.3 |
| 38.5 |
|
| Sex |
| 0.836 |
| 0.715 |
|
Male | 58.6 |
| 51.6 |
|
|
Female | 57.7 |
| 52.5 |
|
| B symptoms |
| 0.002 |
| 0.001 |
|
Absent | 63.2 |
| 57.2 |
|
|
Present | 48.4 |
| 41.0 |
|
| Ann Arbor
stage |
| <0.001 |
| <0.001 |
|
I–II | 72.3 |
| 65.5 |
|
|
III–IV | 39.4 |
| 33.6 |
|
| IPI or aaIPI risk
groups |
| <0.001 |
| <0.001 |
|
Low-risk | 77.7 |
| 72.3 |
|
|
Low-intermediate risk | 62.1 |
| 51.6 |
|
|
High-intermediate risk | 36.8 |
| 29.8 |
|
| High
risk | 28.9 |
| 29.5 |
|
| Subtype |
| 0.552 |
| 0.155 |
|
GCB | 62.1 |
| 58.9 |
|
|
n-GCB | 55.5 |
| 47.0 |
|
| Albumin, g/l |
| <0.001 |
| <0.001 |
| 35 | 62.9 |
| 57.0 |
|
|
<35 | 34.0 |
| 26.1 |
|
| HGB, g/l |
| <0.001 |
| <0.001 |
|
≥110 | 63.8 |
| 57.6 |
|
|
<110 | 41.6 |
| 35.1 |
|
| ALC
(×109/l) |
| <0.001 |
| <0.001 |
|
≥1.45 | 69.8 |
| 64.3 |
|
|
<1.45 | 43.8 |
| 36.5 |
|
| PLT
(×109/l) |
| <0.001 |
| 0.003 |
|
≥250 | 50.0 |
| 45.7 |
|
|
<250 | 70.7 |
| 61.5 |
|
| NLR |
| 0.019 |
| 0.003 |
|
≥2.9 | 51.2 |
| 43.2 |
|
|
<2.9 | 63.0 |
| 57.9 |
|
| LMR |
| <0.001 |
| <0.001 |
|
≥2.65 | 66.3 |
| 60.4 |
|
|
<2.65 | 44.8 |
| 37.9 |
|
| PLR |
| <0.001 |
| <0.001 |
|
≥170 | 41.8 |
| 35.8 |
|
|
<170 | 77.1 |
| 70.6 |
|
| LDH (114–285
U/l) |
| <0.001 |
| <0.001 |
|
>Normal | 43.2 |
| 63.5 |
|
|
≤Normal | 69.1 |
| 35.4 |
|
| β2m (0.97–2.64
mg/l) |
| <0.001 |
| <0.001 |
|
>Normal | 39.7 |
| 33.6 |
|
|
≤Normal | 71.9 |
| 65.5 |
|
 | Table IV.Multivariate analysis of prognostic
factors for survival in testing set. |
Table IV.
Multivariate analysis of prognostic
factors for survival in testing set.
|
| Overall
survival | Progression-free
survival |
|---|
|
|
|
|
|---|
|
Characteristics | P-value | HR (95% CI) | P-value | HR (95% CI) |
|---|
| Age | <0.001 | 2.410
(1.678–3.460) | <0.001 | 2.022
(1.488–2.823) |
| B symptoms | 0.348 | 1.209
(0.814–1.797) | 0.185 | 1.277
(0.889–1.834) |
| Ann Arbor
stage | 0.123 |
1.441(0.906–2.294) | 0.038 | 1.575
(1.026–2.418) |
| IPI or aaIPI risk
groups | 0.009 | 1.402
(1.088–1.806) | 0.161 | 1.183
(0.935–1.495) |
| Albumin | 0.413 | 1.231
(0.748–2.026) | 0.478 | 1.183
(0.743–1.885) |
| HGB | 0.354 | 0.807
(0.513–1.269) | 0.627 | 0.900
(0.589–1.375) |
| PLT | 0.221 | 1.321
(0.846–2.064) | 0.852 | 0.961
(0.636–1.454) |
| ALC | 0.080 | 1.472
(0.955–2.270) | 0.067 | 1.456
(0.937–2.178) |
| NLR | 0.314 | 0.815
(0.548–1.213) | 0.764 | 0.945
(0.652–1.369) |
| LMR | 0.139 | 0.758
(0.525–1.095) | 0.083 | 0.738
(0.523–1.040) |
| PLR | <0.001 | 0.327
(0.205–0.521) | <0.001 | 0.418
(0.274–0.640) |
| LDH | 0.240 | 1.294
(0.842–1.989) | 0.036 | 1.536
(1.028–2.295) |
| β2m | 0.043 | 1.499
(1.012–2.219) | 0.133 | 1.327
(0.917–1.920) |
Prognostic significance of the PLR and
β2m combined with IPI or aaIPI
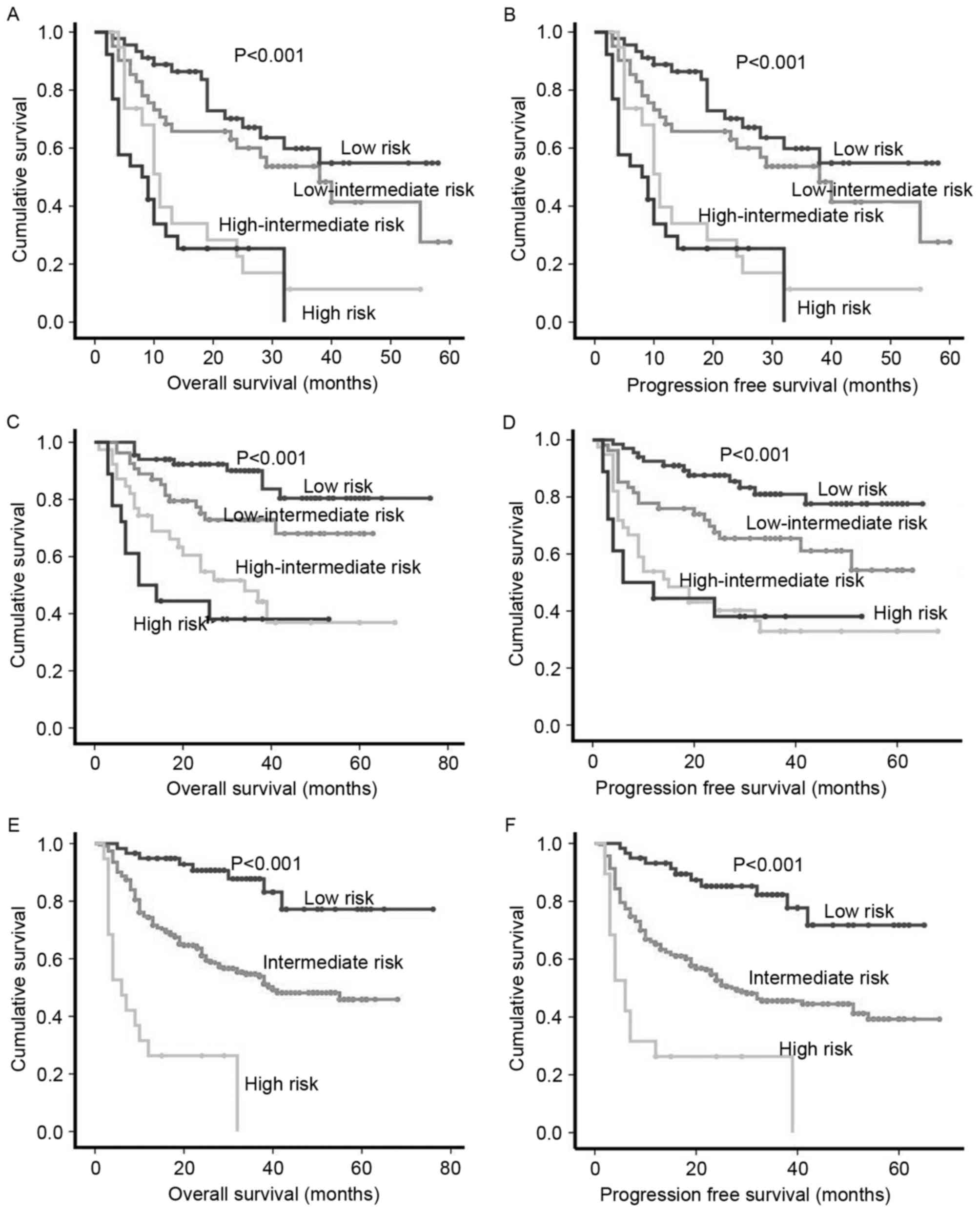
As the diagnosis and treatment of DLBCL has
improved, the ability of IPI or aaIPI to differentiate between risk
groups, particularly high-risk groups, has declined (Fig. 3A-D). Thus, according to the results of
the Cox regression analysis, a novel score was created combining
the PLR and β2m with IPI or aaIPI, 309 patients were split into
three groups: i) Low-risk patients with a PLR <170, IPI <2
scores or aaIPI=0 and normal β2m; ii) high-risk patients with a PLR
≥170, IPI ≥4 or aaIPI=3 and high level of β2m; and iii)
intermediate-risk patients. This novel score predicted a 5-year OS
rate of 86.4, 54.1 and 21.1% in the low-, intermediate- and
high-risk groups, respectively (P<0.001; Fig. 3E). The estimated 5-year PFS rate with
this stratification was: 81.4% for the low-risk group, 47.0% for
the intermediate-risk group and 21.1% for the high-risk group
(P<0.001; Fig. 3F).
Discussion
DLBCL is a highly aggressive NHL with varied
clinical manifestations and variable patient prognosis. IPI is the
widely accepted prognostic factor index for patients with DLBCL
(5). The addition of rituximab to
conventional chemotherapy has led to a marked improvement in
patient survival rates (4). As a
result, the ability of IPI or aaIPI to differentiate between risk
groups, particularly high-risk groups, has declined (5). The revised International Prognostic
Index is unable to identify a risk group with a <50% chance of
survival (6). This means that more
sensitive prognostic factors are required. Inflammation is
recognized as a major hallmark of cancer. As early as 1863, Rudolf
Virchow, who suggested that lymphoreticular infiltration reflected
the origin of cancer at sites of chronic inflammation, identified a
connection between inflammation and cancer (16). Subsequently, numerous studies have
provided evidence that the host inflammatory responses serve a
critical function in various aspects of cancer, including cancer
initiation, promotion, progression and metastasis (7,17–19) Previously, a number of inflammatory
markers have been proposed to have potential for use as predictors
of OS and PFS for solid tumors (8,12,20–23). The
present study identified that NLR, LMR and PLR may be able to
predict the prognosis of patients with DLBCL.
Monocytes and neutrophils, which are important
components in the active defense system, are potent regulators of
macrophages, mast cells and epithelial cells, and serve an
important function in inflammatory events (16). These cell types are able to
differentiate into tumor-associated macrophages in tumor tissue,
which undergo tumor-promotion and M2-like macrophage polarization
and secrete angiogenic factors, including interleukin-8, vascular
endothelial growth factor (VGEF) and fibroblast growth factor, then
induce further tumor angiogenesis and progression (24,25).
Furthermore, monocyte-derived cells may provide nutritional factors
that directly promote the growth and survival of malignant tumors
(17–19).
Lymphocytes are the basic components of the immune
system; they can induce cytotoxic cell death and produce cytokines
in cancer cells (26,27). Lymphocytopenia impairs the antitumor
immune response of the host, which in turn promotes tumor expansion
and leads to a poor patient prognosis.
Tumors require the formation of new blood vessels to
provide nutrients and oxygen for continued lesion growth. Platelets
release VEGF upon their activation, thus promoting angiogenesis
(28). Platelet activation also
protects tumor cells from natural killer cells, and platelets
support spontaneous metastasis (22–23).
Platelet-derived lysophosphatidic acid enhances bone metastatic
growth and progression, and platelet-released factors may serve a
function in tumor progression (29).
Therefore, these results provide evidence that platelet counts may
serve as inflammatory biomarkers and could aid the prediction of
the prognosis of patients.
β2m is a human leukocyte antigen-class I molecule
that is expressed on the membrane of almost all nucleated cells.
Studies have reported that β2m, which regulates p21-activated
kinases, VEGF and fatty acid synthase-mediated growth and survival
signaling pathways, is a growth factor and signaling molecule in
cancer cells (30,31); it serves a critical function in the
proliferation, apoptosis and metastasis of cancer cells (30,32).
Furthermore, β2m is a useful prognostic factor in multiple types of
cancer, including breast (33),
gallbladder (34), prostate and lung
cancer (31), chronic lymphocytic
leukemia (35) and follicular
lymphoma (36). In the present study,
an increased level of β2m was associated with poor patient
prognosis in DLBCL.
In summary, PLT and PLR were associated with poor
prognosis in patients with DLBCL. The NLR and LMR at diagnosis, as
biomarkers combining an estimate of host immune and tumor
microenvironment, were previously hypothesized to be powerful
prognostic factors in patients with newly diagnosed DLBCL (37); the results of the present study
confirmed this. Furthermore, it was identified that PLR was an
independent predictor of survival in patients who were newly
diagnosed with DLBCL. Additionally, it was identified that the
patients who have a poor prognosis may be divided through a novel
prognostic scoring system (PLR and β2m combined with IPI or aaIPI),
which is of great significance for the evaluation of prognosis and
guiding treatment.
Acknowledgements
The authors would like to thank Dr Xiaofang Wang for
encouragement and guidance. The present study was funded by the
National Natural Science Foundation of China (grant no.
81272562).
Glossary
Abbreviations
Abbreviations:
|
DLBCL
|
diffuse large B-cell lymphoma
|
|
IPI
|
international prognostic index
|
|
aaIPI
|
age-adjusted International Prognostic
Index
|
|
ALC
|
absolute lymphocyte count
|
|
PLT
|
platelet count
|
|
NLR
|
neutrophil-lymphocyte ratio
|
|
LMR
|
lymphocyte-monocyte ratio
|
|
PLR
|
platelet-lymphocyte ratio
|
|
LDH
|
lactate dehydrogenase
|
|
β2m
|
β2-microglobulin
|
|
OS
|
overall survival
|
|
PFS
|
progression-free survival
|
References
|
1
|
Yıldırım M, Kaya V, Demirpençe Ö and
Paydaş S: The role of gender in patients with diffuse large B cell
lymphoma treated with rituximab-containing regimens: A
meta-analysis. Arch Med Sci. 11:708–714. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yu X and Li Z: New insights into MicroRNAs
involves in drug resistance in diffuse large B cell lymphoma. Am J
Transl Res. 7:2536–2542. 2015.PubMed/NCBI
|
|
3
|
Liu W, Ha M, Wang X and Yin N: Clinical
significance of GRHL3 expression in diffuse large B cell lymphoma.
Tumour Biol. 37:9657–9661. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Meng F, Zhong D, Zhang L, Shao Y and Ma Q:
Efficacy and safety of rituximab combined with chemotherapy in the
treatment of diffuse large B-cell lymphoma: A meta-analysis. Int J
Clin Exp Med. 8:17515–17522. 2015.PubMed/NCBI
|
|
5
|
Ziepert M, Hasenclever D, Kuhnt E, Glass
B, Schmitz N, Pfreundschuh M and Loeffler M: Standard International
prognostic index remains a valid predictor of outcome for patients
with aggressive CD20+ B-cell lymphoma in the rituximab era. J Clin
Oncol. 28:2373–2380. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sehn LH, Berry B, Chhanabhai M, Fitzgerald
C, Gill K, Hoskins P, Klasa R, Savage KJ, Shenkier T, Sutherland J,
et al: The revised international prognostic index (R-IPI) is a
better predictor of outcome than the standard IPI for patients with
diffuse large B-cell lymphoma treated with R-CHOP. Blood.
109:1857–1861. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Balkwill F and Mantovani A: Inflammation
and cancer: Back to Virchow? Lancet. 357:539–545. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Goh BK, Chok AY, Allen JC Jr, Quek R, Teo
MC, Chow PK, Chung AY, Ong HS and Wong WK: Blood
neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios are
independent prognostic factors for surgically resected
gastrointestinal stromal tumors. Surgery. 159:1146–1156. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tagawa T, Anraku M, Morodomi Y, Takenaka
T, Okamoto T, Takenoyama M, Ichinose Y, Maehara Y, Cho BC, Feld R,
et al: Clinical role of a new prognostic score using
platelet-to-lymphocyte ratio in patients with malignant pleural
mesothelioma undergoing extrapleural pneumonectomy. J Thorac Dis.
7:1898–1906. 2015.PubMed/NCBI
|
|
10
|
Jung SH, Kim JS, Lee WS, Oh SJ, Ahn JS,
Yang DH, Kim YK, Kim HJ and Lee JJ: Prognostic value of the inverse
platelet to lymphocyte ratio (iPLR) in patients with multiple
myeloma who were treated up front with a novel agent-containing
regimen. Ann Hematol. 95:55–61. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Polat M, Senol T, Ozkaya E, Ogurlu Pakay
G, Cikman MS, Konukcu B, Ozten MA and Karateke A: Neutrophil to
lymphocyte and platelet to lymphocyte ratios increase in ovarian
tumors in the presence of frank stromal invasion. Clin Transl
Oncol. 18:457–463. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shirai Y, Shiba H, Sakamoto T, Horiuchi T,
Haruki K, Fujiwara Y, Futagawa Y, Ohashi T and Yanaga K:
Preoperative platelet to lymphocyte ratio predicts outcome of
patients with pancreatic ductal adenocarcinoma after pancreatic
resection. Surgery. 158:360–365. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gunaldi M, Goksu S, Erdem D, Gunduz S,
Okuturlar Y, Tiken E, Kahraman S, Inan YO, Genc TB and Yildirim M:
Prognostic impact of platelet/lymphocyte and neutrophil/lymphocyte
ratios in patients with gastric cancer: A multicenter study. Int J
Clin Exp Med. 8:5937–5942. 2015.PubMed/NCBI
|
|
14
|
Yodying H, Matsuda A, Miyashita M,
Matsumoto S, Sakurazawa N, Yamada M and Uchida E: Prognostic
significance of Neutrophil-to-Lymphocyte ratio and
platelet-to-lymphocyte ratio in oncologic outcomes of esophageal
cancer: A systematic review and meta-analysis. Ann Surg Oncol.
23:646–654. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Musshoff K and Schmidt-Vollmer H:
Proceedings: Prognosis of non-Hodgkin's lymphomas with special
emphasis on the staging classification. Z Krebsforsch Klin Onkol
Cancer Res Clin Oncol. 83:323–341. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shalapour S and Karin M: Immunity,
inflammation, and cancer: An eternal fight between good and evil. J
Clin Invest. 125:3347–3355. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Peng H, Chen L, Tang LL, Zhang Y, Li WF,
Mao YP, Zhang F, Guo R, Liu LZ, Tian L, et al: Primary tumor
inflammation in gross tumor volume as a prognostic factor for
nasopharyngeal carcinoma patients. Oncotarget. 7:14963–14972.
2016.PubMed/NCBI
|
|
18
|
Ma Q, Liu W, Jia R, Jiang F, Duan H, Lin
P, Zhang L, Long H, Zhao H and Ma G: Inflammation-based prognostic
system predicts postoperative survival of esophageal carcinoma
patients with normal preoperative serum carcinoembryonic antigen
and squamous cell carcinoma antigen levels. World J Surg Oncol.
14:1412016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Maeda K, Shibutani M, Otani H, Nagahara H,
Ikeya T, Iseki Y, Tanaka H, Muguruma K and Hirakawa K:
Inflammation-based factors and prognosis in patients with
colorectal cancer. World J Gastrointest Oncol. 7:111–117. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lian L, Xia YY, Zhou C, Shen XM, Li XL,
Han SG, Zheng Y, Mao ZQ, Gong FR, Wu MY, et al: Application of
platelet/lymphocyte and neutrophil/lymphocyte ratios in early
diagnosis and prognostic prediction in patients with resectable
gastric cancer. Cancer Biomark. 15:899–907. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li X, Han Z, Cheng Z, Yu J, Yu X and Liang
P: Clinical significance of preoperative platelet-to-lymphocyte
ratio in recurrent hepatocellular carcinoma after thermal ablation:
A retrospective analysis. Int J Hyperthermia. 31:758–763. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Miglani RK, Bhateja N, Bhat RS and Kumar
KV: Diagnostic role of platelet lymphocyte ratio(PLR) in pancreatic
head masses. Indian J Surg. 75:4–9. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li F, Hu H, Gu S, Chen X and Sun Q:
Platelet to lymphocyte ratio plays an important role in prostate
cancer's diagnosis and prognosis. Int J Clin Exp Med.
8:11746–11751. 2015.PubMed/NCBI
|
|
24
|
Coffelt SB, Chen YY, Muthana M, Welford
AF, Tal AO, Scholz A, Plate KH, Reiss Y, Murdoch C, De Palma M and
Lewis CE: Angiopoietin 2 stimulates TIE2-expressing monocytes to
suppress T cell activation and to promote regulatory T cell
expansion. J Immunol. 186:4183–4190. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Coffelt SB, Tal AO, Scholz A, De Palma M,
Patel S, Urbich C, Biswas SK, Murdoch C, Plate KH, Reiss Y and
Lewis CE: Angiopoietin-2 regulates gene expression in
TIE2-expressing monocytes and augments their inherent proangiogenic
functions. Cancer Res. 70:5270–5280. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huber C, Bobek N, Kuball J, Thaler S,
Hoffarth S, Huber C, Theobald M and Schuler M: Inhibitors of
apoptosis confer resistance to tumour suppression by adoptively
transplanted cytotoxic T-lymphocytes in vitro and in vivo. Cell
Death Differ. 12:317–325. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chhabra A: Mitochondria-centric activation
induced cell death of cytolytic T lymphocytes and its implications
for cancer immunotherapy. Vaccine. 28:4566–4572. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Etulain J, Mena HA, Negrotto S and
Schattner M: Stimulation of PAR-1 or PAR-4 promotes similar pattern
of VEGF and endostatin release and pro-angiogenic responses
mediated by human platelets. Platelets. 26:799–804. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gay LJ and Felding-Habermann B:
Contribution of platelets to tumour metastasis. Nat Rev Cancer.
11:123–134. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Huang WC, Wu D, Xie Z, Zhau HE, Nomura T,
Zayzafoon M, Pohl J, Hsieh CL, Weitzmann MN, Farach-Carson MC and
Chung LW: beta2-microglobulin is a signaling and growth-promoting
factor for human prostate cancer bone metastasis. Cancer Res.
66:9108–9116. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nissen MH, Bjerrum OJ, Plesner T, Wilken M
and Rørth M: Modification of beta-2-microglobulin in sera from
patients with small cell lung cancer: Evidence for involvement of a
serine protease. Clin Exp Immunol. 67:425–432. 1987.PubMed/NCBI
|
|
32
|
Huang WC, Havel JJ, Zhau HE, Qian WP, Lue
HW, Chu CY, Nomura T and Chung LW: Beta2-microglobulin signaling
blockade inhibited androgen receptor axis and caused apoptosis in
human prostate cancer cells. Clin Cancer Res. 14:5341–5347. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li K, Du H, Lian X, Yang S, Chai D, Wang
C, Yang R and Chen X: Characterization of β2-microglobulin
expression in different types of breast cancer. BMC Cancer.
14:7502014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sun J, Yang ZL, Miao X, Zou Q, Li J, Liang
L, Zeng G and Chen S: ATP5b and β2-microglobulin are predictive
markers for the prognosis of patients with gallbladder cancer. J
Mol Histol. 46:57–65. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Thompson PA, O'Brien SM, Xiao L, Wang X,
Burger JA, Jain N, Ferrajoli A, Estrov Z, Keating MJ and Wierda WG:
β2-microglobulin normalization within 6 months of ibrutinib-based
treatment is associated with superior progression-free survival in
patients with chronic lymphocytic leukemia. Cancer. 122:565–573.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cánovas A, Alonso JJ, Barreiro G and
Aguirre C: Prognostic factors in follicular lymphoma: The
importance of beta-2 microglobulin. Tumori. 96:117–121.
2010.PubMed/NCBI
|
|
37
|
Koh YW, Park CS, Yoon DH, Suh C and Huh J:
Should the cut-off values of the lymphocyte to monocyte ratio for
prediction of prognosis in diffuse large B-cell lymphoma be changed
in elderly patients? Eur J Haematol. 93:340–348. 2014. View Article : Google Scholar : PubMed/NCBI
|