Introduction
Head and neck squamous cell carcinomas (HNSCC)
represent 4% of all cancer cases worldwide (1). They primary source (41% of cases) is the
oral cavity, but they are also often found in the pharynx and
larynx, (22 and 24% of cases, respectively) (2). The majority of patients with HNSCC are
clinically identified prior to metastasis, and therefore are
potentially able to be cured by an aggressive therapy regimen,
comprising surgery, chemotherapy and/or radiotherapy (3). Nevertheless, despite recent improvements
to utilized surgical techniques, chemotherapy and radiation
delivery, and supportive care, which have improved the quality of
life of patients, the HNSCC disease recurrence rate remains
unacceptably high, occurring in up to 50% of patients within the
first 2 years of diagnosis (4,5). Thus,
there is an urgent requirement for the development of novel drugs
that can be safely integrated into current treatment regimens to
improve the tolerability and the efficacy of current treatments.
Currently, patients with HNSCC are treated with several anticancer
drugs, including paclitaxel, 5-fluorouracil (5-FU), cisplatin, and
docetaxel.
Adenosine is a purine nucleoside that is composed of
an adenine molecule covalently attached to a ribose sugar molecule,
and is found in abundance intra- and extracellularly (6). Adenosine is known to induce growth
suppression and apoptosis in multiple types of cancer cells via at
least two independent pathways. For example, adenosine induces
apoptosis by activating specific extracellular A1,
A2a, A2b, and A3 receptors in a
number of types of cancer, including gastric cancer (7), breast cancer (8), colon cancer (9), leukemia (10) and melanoma (11). Similarly, the intracellular
transportation of adenosine induces the inhibition of cell growth
and apoptosis via non-receptor-mediated pathways. The apoptotic
effects induced by numerous anticancer drugs occur via diverse
signaling pathways, and are regulated by complex
mitochondrial-extrinsic and -intrinsic pathways. Two crucial
apoptotic pathways are distinguished according to the presence or
absence of caspase involvement. The first of these is the
mitochondrial-intrinsic pathway, in which activation of B-cell
lymphoma-2 (Bcl-2)-family proteins leads to mitochondrial
permeabilization and the release of cytochrome c from the
mitochondria to the cytoplasm, and thus to the activation of the
initiator (caspase-9) and downstream effector (caspase-3) caspases
(12). Activated caspase-3 then
cleaves various substrates, including cytokeratins,
poly(ADP-ribose) polymerase (PARP), and nuclear mitotic apparatus
protein, ultimately mediating the morphological and biochemical
changes that are characteristic of apoptotic cells (13). Alternatively, Bcl-2 activation is
regulated by the post-translational phosphorylation of
phosphoinositide-3 kinase (PI3K)/RAC serine/threonine-protein
kinase (Akt) signaling (14,15). In fact, PI3K/Akt signaling is
frequently dysregulated and/or constitutively activated in multiple
cancer types, including breast, bladder, prostate, thyroid, ovarian
and non-small cell lung cancer (16,17). Akt
is capable of phosphorylating substrates involved in a number of
processes, including cell proliferation (p27, cyclin D1), survival
(Bcl-2 family, caspases), and growth [mechanistic target of
rapamycin (mTOR)]; thus, drugs that target Akt either directly, or
via elements of the Akt pathway, are promising targets for cancer
therapy (18).
In the present study, the FaDu human pharyngeal
squamous carcinoma cell line was used to investigate whether
adenosine induced cell death via the adenosine receptors, and to
establish the mechanism underlying this process, in the context of
HNSCC.
Materials and methods
Materials
Adenosine and ATL-444 (a selective antagonist for
the A1 and A2a adenosine receptors) were
purchased from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany). The
primary antibodies anti-PI3K, anti-phospho PI3K, anti-Akt,
anti-phospho Akt (ser473), anti-mTOR, anti-phospho mTOR (ser2448),
anti-phospho S6 kinase β1 (S6K1), anti-phospho eukaryotic
translation initiation factor 4E-binding protein 1 (4EBP1),
anti-phospho eukaryotic translation initiation factor 4 γ1 (eIF4G),
anti-caspase-9, anti-caspase-3, anti-PARP, anti-Bcl-associated X
(Bax), anti-Bcl-2, and anti-cytochrome c were purchased from Cell
Signaling Technology, Inc. (Danvers, MA, USA). The primary
antibodies anti-adenosine A1 receptor, anti-adenosine
A2a receptor, anti-adenosine A2b receptor,
anti-adenosine A3 receptor were purchased from Abcam
(Cambridge, MA, USA). The primary anti-β-actin (cat. no. sc-47778)
antibody was purchased from Santa Cruz Biotechnology, Inc. (Dallas,
TX, USA).
Cell culture
Normal human oral keratinocytes (NHOKs) were
purchased from ScienCell Research Laboratories, Inc. (San Diego,
CA, USA). NHOKs were maintained in Keratinocyte Growth Medium
supplemented using a supplementary growth factor bullet kit
(Clonetics Corp., San Diego, CA, USA). The FaDu human pharynx
squamous carcinoma cell line was maintained in Dulbecco's modified
Eagle's medium supplemented with 10% heat-inactivated fetal bovine
serum, 100 U/ml penicillin, and 100 µg/ml streptomycin (all from
Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA), at 37°C
in a humidified atmosphere with 5% CO2.
MTT assay
NHOKs and FaDu cells were seeded (5×105
cells/well) in 12-well plates and maintained in DMEΜ at 37°C with
5% CO2. Once cells reached 60–80% confluency, they were
treated with adenosine (0, 1.5, or 3 mM), and co-treated with
adenosine (3 mM) and/or ATL-444 (50 µM). After the indicated times,
20 µl of MTT (5 mg/ml in PBS) was added to each well, and the cells
were incubated for another 4 h at 37°C. The supernatants were then
carefully aspirated, and 100 µl of dimethyl sulfoxide was added to
each well. The plates were shaken for an additional 15 min, and
then absorbance values were detected using a microplate reader at
592 nm.
RNA isolation and reverse
transcription-polymerase chain reaction (RT-PCR) analysis
Total RNA was isolated from cells using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocol, and reverse-transcribed using the
Reverse Transcription system (Promega Corporation, Madison, WI,
USA). Specifically, the sequential heating steps necessary for
reverse transcription comprised 10 min at 25°C, 60 min at 42°C, and
5 min at 95°C. GAPDH was used as an internal control. The following
primers were used for RT-PCR: A1 receptor forward,
5′-ACCTAATCCGCAAGCAGCTCAA-3′ and reverse,
5′-ATCCTCGTCAATGGGAGGTGCA-3′; A2a receptor forward,
5′-TCCCATGCTAGGTTGGAACAACTG-3′ and reverse,
5′-TGATGGCCAGTGACTTGGCAGC-3′; A2b receptor forward,
5′-AGTCGACAGATACCTGGCCATCTG-3′ and reverse,
5′-CATGGCCAGTGACTTGGCTGCA-3′; A3 receptor forward,
5′-TCATCTGCGTGGTCAAGCTGAACC-3′ and reverse,
5′-CATGTAGTCCATTCTCATGACGGA-3′;. GAPDH forward,
5′-GGACTGTGGTCATGAGCCCTTCCA-3′ and reverse,
5′-ACTCACGGCAAATTCAACGGCAC-3′. PCR products were visualized by gel
electrophoresis with a 1.5% agarose gel.
DNA fragmentation assay
Cells were washed twice with PBS, and pelleted via
centrifugation (400 × g, 3 min) at 4°C. Cell pellets were lysed by
incubation (30 min, on ice) in lysis buffer (10 µM Tris-Cl, pH 7.5,
10 µM EDTA, and 0.5% Triton X-100). Cells were incubated for 1 h at
37°C with RNase A (sufficient volume to achieve a final
concentration of 0.5 µg/ml in the solution), and then for 8 h at
50°C with proteinase K (sufficient volume to achieve a final
concentration of 0.2 µg/ml in the solution). DNA was extracted
using phenol: Chloroform:isoamyl alcohol (25:24:1), precipitated
via incubation (for 24 h, −70°C) with an equal volume of
isopropanol, and centrifuged (15,000 × g, 15 min, 4°C). The
precipitated DNA was the air-dried, resuspended in 30 µl of TE
buffer [10 mM Tris-HCl (pH 8.0), 0.1 mM EDTA] and quantified
according to its absorbance at 260 nm in a UV spectrophotometer. A
total of 10 µg DNA was separated on a 1.5% agarose gel (with added
ethidium bromide) by electrophoresis at 50 V for 50 min. DNA
fragments were visualized using a UV trans-illuminator.
Assay for nuclear apoptosis (Hoechst
staining)
To determine DNA chromatin morphological features,
the cells were treated with Hoechst 33342 stain, as described by
Díaz-Ruiz et al (19).
Subsequent to washing twice in PBS, the cells were fixed with
formaldehyde (4%, ice-cold), re-washed with PBS and Hoechst 33342
(2 µg/ml) and incubated for 30 min at 37°C. Subsequent to
re-washing with PBS, cell nuclei were observed in five random
fields using a fluorescence microscope (Olympus Corporation, Tokyo,
Japan, magnification, ×100).
Flow cytometric analysis with Annexin
V-fluorescein isothiocyanate (FITC) and propidium iodine (PI)
staining
The rate of apoptosis was evaluated using a Vybrant
apoptosis assay kit (Molecular Probes; Thermo Fisher Scientific,
Inc.) in accordance with the manufacturer's protocol. Briefly, the
cells were plated (2–4×105 cells/dish) in six-well
plates, incubated overnight, and treated for 24 h with adenosine (0
and 3 mM). They were then harvested, washed in PBS, and combined
with a binding buffer containing Alexa Fluor 488 Annexin V-FITC and
PI. Following incubation for 15 min at 37°C, the cells were
analyzed via flow cytometry using the Cell Lab Quanta™
SC flow cytometer and associated Cell Lab Quanta SC MPL analysis
software version 1.0 (Beckman Coulter, Inc., Brea, CA, USA).
Western blot analysis
Cells were lysed (30 min, on ice) in protein
extraction lysis buffer (Intron Biotechnology, Inc., Seongnam,
Korea), and centrifuged (12,000 × g, 15 min, 4°C). The resulting
supernatant was transferred to a fresh tube, and the concentration
of extracted protein was quantified via the BCA protein assay
(Pierce; Thermo Fisher Scientific, Inc.), using bovine serum
albumin (BSA; Pierce; Thermo Fisher Scientific, Inc.) as a
standard. Approximately 10 µg of protein from each lysate was
solubilized in Laemmli sample buffer, separated by 3–8 or 4–20%
SDS-PAGE. Separated proteins were transferred to a polyvinylidene
difluoride nanofiber membrane (Amomedi, Gwangju, Korea). The
membranes were blocked for 1 h at room temperature with 5% BSA, and
incubated overnight at 4°C with primary antibodies comprising
anti-PI3K, anti-phospho PI3K, anti-Akt, anti-phospho Akt (ser473),
anti-caspase-9, anti-caspase-3, anti-PARP, anti-Bax, anti-Bcl-2,
anti-cytochrome c, and anti-β-actin (all from Cell Signaling
Technology, Danvers, MA, USA). They were then washed three times
with TBS-T (0.1% Tween-20, 50 µM Tris-HCl pH 7.5, and 150 µM NaCl),
and incubated for 1 h at room temperature with secondary
antibodies, prior to being rewashed a further three times with
TBS-T. Protein signals were visualized using the WestSave Up ECL
kit (AB Frontier Co., Ltd., Seoul, Korea), and detected using the
Microchemi 4.2 device (DNR Bioimaging Systems, Jerusalem,
Israel).
Statistical analysis
Experiments were performed in triplicate and
expressed as the mean ± standard deviation (SD). The differences in
protein expression between untreated cells and treated cells were
analyzed by a one-way analysis of variance followed by Dunnett's
t-test w, using GraphPad Prism Software version 6.0 (GraphPad
Software Inc., La Jolla, CA, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Adenosine suppresses cell growth via
the A1 and A2a adenosine receptors in FaDu
cells
To evaluate the effects of adenosine on the
viability of FaDu cells, MTT assays was performed in which FaDu and
oral keratinocytes cells were treated with 1–3 mM of adenosine. As
presented in Fig. 1A, the growth of
NHOKs was unaffected by treatment with adenosine concentrations
<1.5 mM, but was inhibited by 8.6% in response to treatment with
3 mM adenosine, although this change was not statistically
significant. By contrast, FaDu cell growth was inhibited in
response to treatment with 1.5 mM adenosine, and furthermore, this
effect increased dose-dependently until it reached a maximum level
at 3 mM adenosine. RT-PCR was used to confirm the presence of
adenosine receptors in FaDu cells. The results of PCR revealed that
the FaDu cells expressed A1 and A2a adenosine
receptor mRNA expression, with the A2a adenosine
receptor mRNA being most strongly expressed of the two adenosine
receptors. However, the A3 adenosine receptor was not
detected (Fig. 1B). Furthermore, the
results of western blot analysis revealed that the FaDu cells
expressed A1 and A2a adenosine receptor
proteins, with the expression level of A2a receptor
protein being the more strongly expressed of the two genes. The
A3 adenosine receptor protein was not detected (Fig. 1C). To evaluate the effects of the
adenosine receptors on the growth of adenosine-treated FaDu cells,
MTT assays were performed with ATL-444, an A1 and
A2a adenosine receptor antagonist. The results of these
assays confirmed that inhibition of the growth of adenosine-treated
FaDu cells was reversed upon ATL-444 treatment (Fig. 1D). Taken together, these findings
indicate that adenosine suppresses the growth of FaDu cells via two
types of adenosine receptors.
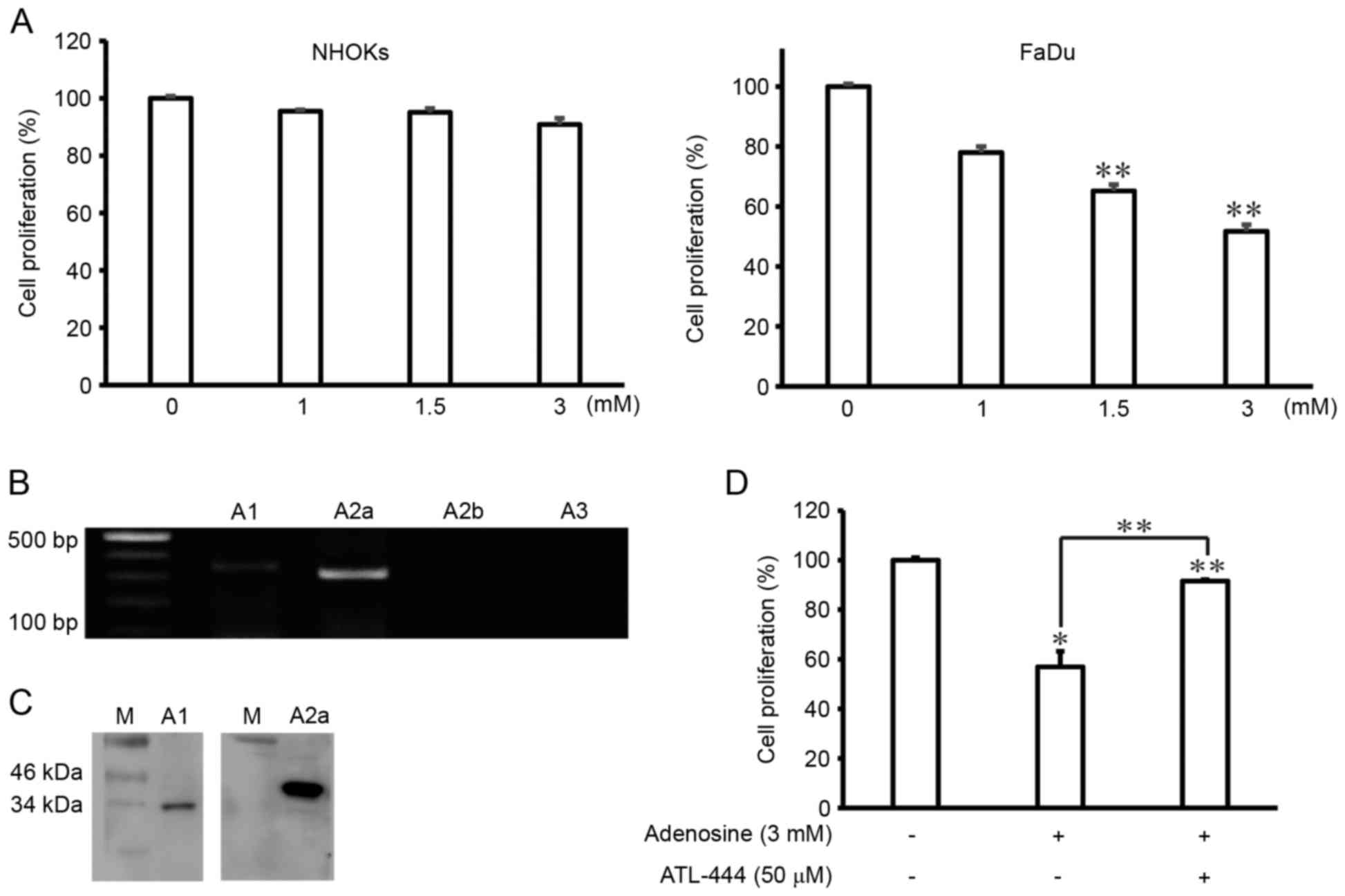 | Figure 1.Adenosine induces cell death in FaDu
cells via the A1, and A2a adenosine
receptors. (A) NHOKs and FaDu human hypopharynx squamous carcinoma
cells were treated with different doses of adenosine (0, 1, 1.5, or
3 mM) for 24 h. The resultant cell viability was analyzed using an
MTT assay. The results of three experiments (n=3) are summarized.
(B) Polymerase chain reaction products for A1,
A2a, A2b, and A3 generated using a
cDNA template isolated from total RNA of FaDu cells. (C) A1 and A2a
adenosine receptors protein expression confirmed by western blot
analysis from FaDu cells. (D) FaDu cells were co-treated with
adenosine (3 mM) and ATL-444 (50 µM, an adenosine-receptor
antagonist) for 24 h. The resulting cell viability was analyzed
using an MTT assay. Data represent percentages of cell viability in
comparison to control cells that were arbitrarily set to 100% and
are reported as the mean ± SD of three independent experiments.
*P<0.05 and **P<0.01 vs. untreated group, one-way ANOVA,
Dunnett's t-test. |
Adenosine induces apoptosis in FaDu
cells
A DNA fragmentation assay was used to ascertain
whether the mechanism underlying the adenosine-induced cytotoxic
effect on FaDu cells was associated with apoptosis. DNA ladder
formation was observed in cells treated with 3 mM adenosine for 24
h (Fig. 2A), indicative of the
induction of apoptosis. This observed apoptotic cell death was
subsequently analyzed and quantified using Hoechst 33342 staining
and fluorescence microscopy, respectively. The results of these
analyses revealed that whereas the majority of control cells
contained intact genomic DNA, adenosine-treated FaDu cells instead
exhibited condensed chromatin (Fig.
2B). To study the potential mechanisms by which adenosine
induced apoptosis in the FaDu cells, the effect of adenosine on the
proportion of apoptotic cells was evaluated by flow cytometry using
Annexin V-FITC and PI staining. FaDu cells were treated with 3 mM
adenosine for 24 h, and the cell population was then analyzed. As
shown in Fig. 2C, the percentage of
early and late apoptotic/necrotic adenosine-treated cells increased
to 7.3 and 29.1%, respectively.
Adenosine activates cleavage of
caspase-3, caspase-9 and PARP via the mitochondrial intrinsic
pathway in FaDu cells
To elucidate the mechanisms underlying the effects
of adenosine on the early or late induction of apoptosis, western
blot analysis was performed to assess the levels of Bax, Bcl-2, and
cytochrome c in adenosine-treated cells, since each of these
molecules are key to the intrinsic apoptotic pathway. Adenosine
treatment of FaDu cells was found to increase and suppress the
expression of Bax and Bcl-2, respectively, and to induce the
release of cytochrome c from the mitochondria into the cytosol
(Fig. 3A). Furthermore, whether
adenosine activated caspases was assessed, since these enzymes are
critical to the apoptotic signaling cascade. Indeed, adenosine
treatment was shown to induce the activation of the cleaved,
catalytically active forms of caspase-3 and −9 in FaDu cells, and
furthermore, also promoted the cleavage of PARP, a substrate of
activated caspases that mediates apoptotic signaling (Fig. 3B).
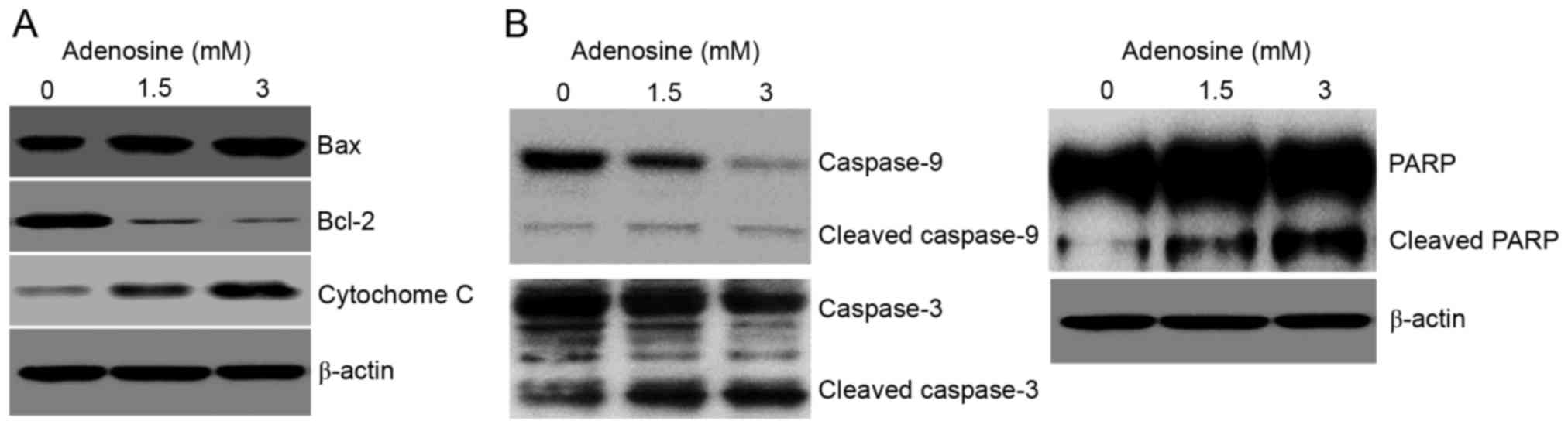 | Figure 3.Bax, Bcl-2, and cytochrome c mediate
adenosine-induced apoptosis via the mitochondrial intrinsic
pathway. FaDu cells were treated with 3 mM adenosine for 24 h,
prior to collection of whole cell lysates. Samples were separated
using (8–15%) SDS-PAGE, and resolved by incubation with primary
antibodies against (A) Bax, Bcl-2, cytochrome c, and (B) caspase-9,
caspase-3, and PARP. β-actin was used as an internal control for
the western blot analysis. Data are representative of three
experiments that produced similar results. Sample bands (n=3) were
densitometrically evaluated. Bcl-2, B-cell lymphoma 2; Bax,
Bcl-associated X; PARP, poly(ADP-ribose) polymerase. |
Adenosine suppresses PI3K/Akt/mTOR
phosphorylation in FaDu cells
The PI3K/Akt signaling pathway regulates cell
proliferation and apoptotic signaling in a variety of cancer cells
(16,17). Several biological effects of PI3K are
mediated via the activation of Akt, a downstream target of PI3K
that regulates cell proliferation via the phosphorylation of its
own downstream targets, including mTOR. In the present study,
adenosine treatment (3 mM) of FaDu cells decreased the
phosphorylation levels of PI3K, Akt and mTOR (Fig. 4A). Given this result, western blot
analysis was performed to evaluate the phosphorylation levels of
the downstream mTOR signaling pathway components S6K1, 4E-BP1, and
eIF4G in response to adenosine treatment. The results of this
analysis demonstrated that adenosine-treated FaDu cells exhibited
significantly reduced S6K1, 4E-BP1 and eIF4G phosphorylation levels
compared with non-treated cells (Fig.
4B).
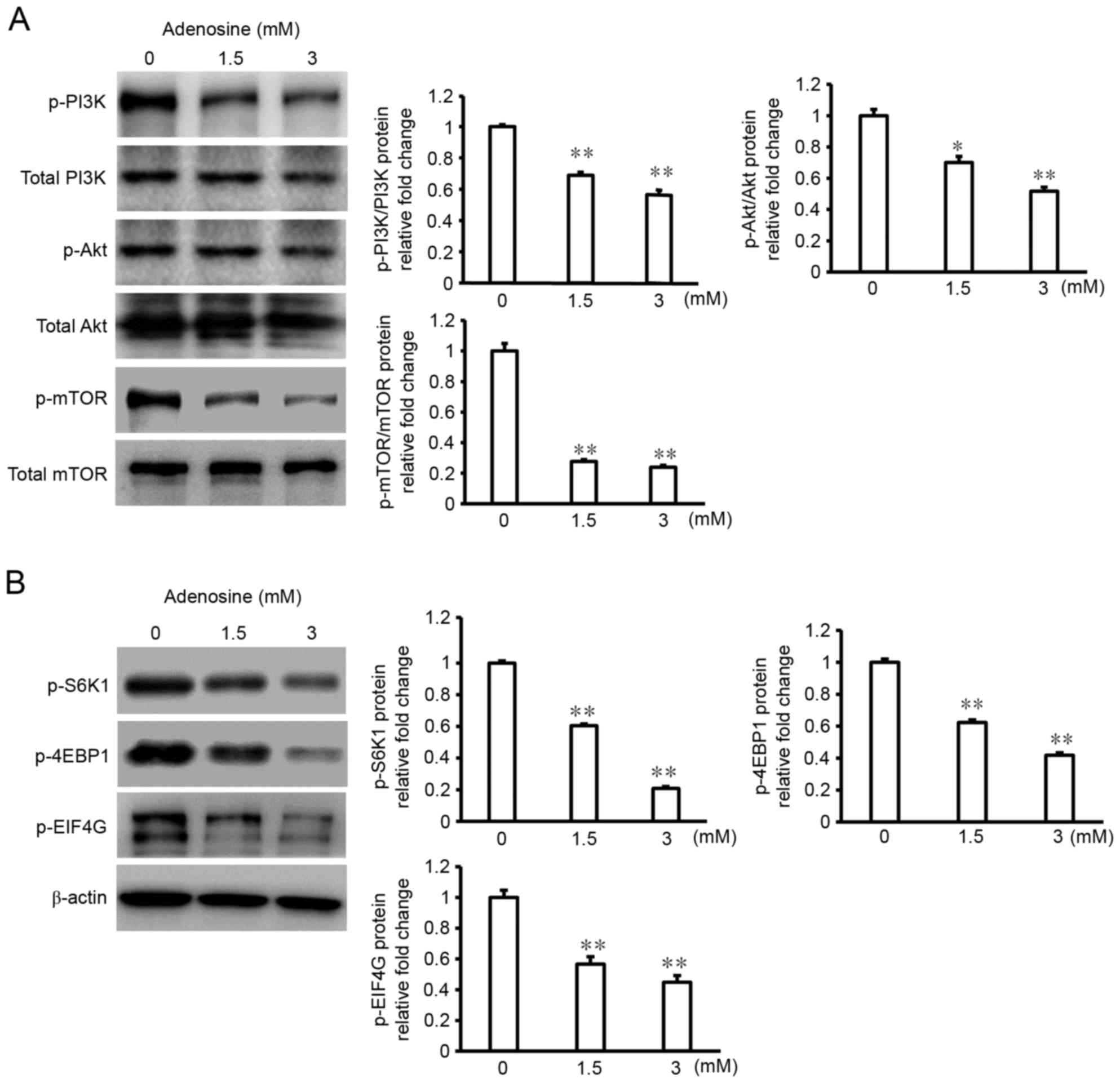 | Figure 4.Adenosine treatment of FaDu cells
suppresses the PI3K/Akt/mTOR signaling pathway. FaDu cells were
treated with 3 mM adenosine for 24 h prior to collection of
whole-cell lysates. Samples were separated using (8–15%) SDS-PAGE,
and resolved by incubation with primary antibodies against (A)
phospho-PI3K, total PI3K, phospho-Akt, total Akt, phospho-mTOR,
total mTOR, and (B) phospho-S6K1, phospho-4EBP1, and phospho-EIF4
G. β-actin was used as an internal control for the western blot
analysis. Data are representative of three experiments that
produced similar results. Sample bands (n=3) were densitometrically
evaluated. *P<0.05 and **P<0.01. PI3K, phosphoinositide
3-kinase; Akt, RAC serine/threonine-protein kinase; mTOR,
mechanistic target of rapamycin; 4EBP1, eukaryotic translation
initiation factor 4E-binding protein 1; EIF4 G, eukaryotic
translation initiation factor 4 γ1. |
Discussion
Adenosine is critical to various physiological
processes and pharmacological actions, including apoptosis, cell
proliferation, and differentiation (20). In particular, a number of previously
published studies have reported that adenosine inhibits cell growth
via diverse biological pathways in a number of cancer cells
(21); however, the effect and
underlying mechanism of adenosine in head and neck cancer cell
lines remains unknown.
Thus, the present study examined the effect of
adenosine treatment on cell death in the FaDu human pharyngeal
squamous carcinoma cell line. Treatment with 3.0 mM of adenosine
was confirmed to significantly decrease the viability of FaDu
cells. Next, whether the adenosine A1, A2a,
A2b, and/or A3 receptors were expressed in
the FaDu cells was investigated, since these receptors have been
previously demonstrated to be highly expressed in a number of
different cancer cells (22), and to
critically mediate the growth and death of cancerous and normal
cells. The expression of the A1 and A2a
receptors were confirmed to be expressed in FaDu cells. Thus,
whether adenosine induces cell death via these two receptors in
FaDu cells was next investigated, by attempting to rescue
adenosine-induced cell-growth inhibition with ATL-444, a known
A1 and A2a receptor antagonist. The results
of this analysis indicated that the observed adenosine-induced
inhibition of FaDu cell viability was receptor-dependent.
Furthermore, adenosine-treated FaDu cells were observed to exhibit
condensed nuclei and apoptotic morphology. Similarly, adenosine
treatment increased the proportion of early apoptotic and late
apoptotic/secondary necrotic (Annexin V-positive and PI-negative)
cells via the conducted flow cytometric analysis. Given that these
results were obtained by observing inter-nucleosomal DNA
fragmentation and early or late apoptotic cell populations, we
hypothesize that the adenosine-induced FaDu cell growth inhibition
is predominantly due to apoptosis.
A number of studies have reported that adenosine
induces apoptosis via the activation of caspase-3 and caspase-9,
and the subsequent stimulation of mitochondrial reactive oxygen
species production in BEL-7404 human liver cancer cells (23,24).
Adenosine has also been shown to downregulate expression of the
anti-apoptotic factor Bcl-xl, and upregulate the expression of the
pro-apoptotic factor BH3 interacting-domain death agonist via the
A2a adenosine receptor, thereby disrupting mitochondrial
membrane potentials to facilitate the efflux of cytochrome c from
the mitochondria into the cytosol to activate caspase-9 and −3 in
human liver cancer HepG2 cells (25).
Similarly, adenosine has been reported to induce apoptosis in human
colon cancer CW2 and Caco-2 cells by activating the cleavage of
caspase-9, −3, and PARP via the A1 and A2a
receptors (26,27). Adenosine treatment in the present
study increased the protein expression of the pro-apoptotic factor
Bax whereas that of the anti-apoptotic factor Bcl-2 was markedly
decreased, with the release of cytochrome c into the cytosol was
stimulated in FaDu cells. Together, these results confirm that the
anticancer effect exerted by adenosine in FaDu cells induces cell
death associated via the altered expression of Bax, Bcl-2 and
cytochrome c.
Currently, therapies targeting the epidermal growth
factor receptor, insulin-like growth factor receptor and
PI3K/Akt/mTOR have attracted attention as promising novel
treatments for HNSCC patients (28).
Of these, inhibition of the PI3K/Akt/mTOR signaling pathway is
predicted to be an extremely effective HNSCC anticancer therapy,
since increased PI3K/Akt overexpression is observed in up to 60% of
all HNSCC cases (29,30). Akt is a critical downstream target of
PI3K and is known to exert numerous biological effects (31), including inhibition of apoptosis via
the phosphorylation and inactivation of several targets active in
apoptotic pathways (32).
Furthermore, mTOR is a downstream target of the Akt and adenosine
monophosphate activated protein kinase signaling pathways, as a
crucial regulator of cell growth and metabolism in HNSCC (32), and as a component of two similar
complexes, mTOR complex 1 and 2 (33). The former of these complexes promotes
mRNA translation and protein synthesis via the phosphorylation of
S6K1 and 4E-BP1, whereas the latter organizes cytoskeleton
function, and regulates Akt phosphorylation (34). To the best of our knowledge, no study
has investigated whether adenosine induces apoptosis in oral cancer
cells via changes in PI3K/Akt/mTOR signaling. In the present study,
treatment of FaDu cells with 3 mM adenosine resulted in decreased
PI3K, Akt and mTOR phosphorylation levels. Furthermore, S6K1,
4E-BP1 and eIF4G phosphorylation levels were investigated, and
shown to be significantly reduced in adenosine-treated FaDu cells
compared with untreated cells following western blot analyses.
Thus, the present study provides novel evidence that adenosine
treatment in FaDu cells decreases PI3K, Akt and mTOR
phosphorylation levels.
In conclusion, the results of the present study
demonstrate that adenosine induced cell death through the
mitochondrial intrinsic apoptotic pathway via PI3K/Akt/mTOR
signaling pathways in human pharyngeal squamous carcinoma cells.
The presented data suggest that adenosine may be a useful
chemotherapeutic agent for the treatment of pharyngeal squamous
carcinoma.
Acknowledgements
Not applicable.
Funding
The present study was supported by research funding
from Chosun University (2014).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
CSK and SMM conceptualized this experiments. MSC and
SMM performed the investigation in the present study. SAL and BRP
analyzed the expression of variable proteins by GraphPad Prism
Software version of the program 6.0 in this work. JSK analyzed flow
cytometry using the Cell Lab Quanta TM SC flow cytometer and
associated Cell Lab Quanta SC MPL analysis software version 1.0 in
the present study. DKK and YHK interpreted all data. CSK gave final
approval of the published article.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Vigneswaran N and Williams MD:
Epidemiological trends in head and neck cancer and aids in
diagnosis. Oral Maxillofac Surg Clin North Am. 26:123–141. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rothenberg SM and Ellisen LW: The
molecular pathogenesis of head and neck squamous cell carcinoma. J
Clin Invest. 122:1951–1957. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Whang SN, Filippova M and Duerksen-Hughes
P: Recent progress in therapeutic treatments and screening
strategies for the prevention and treatment of HPV-associated head
and neck cancer. Viruses. 7:5040–5065. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chaturvedi AK, Engels EA, Pfeiffer RM,
Hernandez BY, Xiao W, Kim E, Jiang B, Goodman MT, Sibug-Saber M,
Cozen W, et al: Human papillomavirus and rising oropharyngeal
cancer incidence in the United States. J Clin Oncol. 29:4294–4301.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Argiris A, Karamouzis MV, Raben D and
Ferris RL: Head and neck cancer. Lancet. 371:1695–1709. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao Z, Kapoian T, Shepard M and Lianos
EA: Adenosine-induced apoptosis in glomerular mesangial cells.
Kidney Int. 61:1276–1285. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Saitoh M, Nagai K, Nakagawa K, Yamamura T,
Yamamoto S and Nishizaki T: Adenosine induces apoptosis in the
human gastric cancer cells via an intrinsic pathway relevant to
activation of AMP-activated protein kinase. Biochem Pharmacol.
67:2005–2011. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Panjehpour M and Karami-Tehrani F:
Adenosine modulates cell growth in the human breast cancer cells
via adenosine receptors. Oncol Res. 16:575–585. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gessi S, Merighi S, Varani K, Cattabriga
E, Benini A, Mirandola P, Leung E, Mac Lennan S, Feo C, Baraldi S
and Borea PA: Adenosine receptors in colon carcinoma tissues and
colon tumoral cell lines: Focus on the A(3) adenosine subtype. J
Cell Physiol. 211:826–836. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tanaka Y, Yoshihara K, Tsuyuki M and
Kamiya T: Apoptosis induced by adenosine in human leukemia HL-60
cells. Exp Cell Res. 213:242–252. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Merighi S, Mirandola P, Milani D, Varani
K, Gessi S, Klotz KN, Leung E, Baraldi PG and Borea PA: Adenosine
receptors as mediators of both cell proliferation and cell death of
cultured human melanoma cells. J Invest Dermatol. 119:923–933.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Luo X, Budihardjo I, Zou H, Slaughter C
and Wang X: Bid, a Bcl2 interacting protein, mediates cytochrome c
release from mitochondria in response to activation of cell surface
death receptors. Cell. 94:481–490. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Slee EA, Adrain C and Martin SJ:
Executioner caspase-3, −6 and −7 perform distinct, non-redundant
roles during the demolition phase of apoptosis. J Biol Chem.
276:7320–7326. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Johnstone RW, Ruefli AA and Lowe SW:
Apoptosis: A link between cancer genetics and chemotherapy. Cell.
108:153–164. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Stokoe D, Stephens LR, Copeland T, Gaffney
PR, Reese CB, Painter GF, Holmes AB, McCormick F and Hawkins PT:
Dual role of phosphatidylinositol-3,4,5-trisphosphate in the
activation of protein kinase B. Science. 277:567–570. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bartholomeusz C and Gonzalez-Angulo AM:
Targeting the PI3K signaling pathway in cancer therapy. Exp Opin
Ther Targets. 16:121–130. 2012. View Article : Google Scholar
|
|
17
|
Kandoth C, McLellan MD, Vandin F, Ye K,
Niu B, Lu C, Lu C, Xie M, Zhang Q, McMichael JF, et al: Mutational
landscape and significance across 12 major cancer types. Nature.
502:333–339. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Moral M and Paramio JM: Akt pathway as a
target for therapeutic intervention in HNSCC. Histol Histopathol.
23:1269–1278. 2008.PubMed/NCBI
|
|
19
|
Díaz-Ruiz C, Montaner B and Pérez-Tomás R:
Prodigiosin induces cell death and morphological changes indicative
of apoptosis in gastric cancer cell line HGT-1. Histol Histopathol.
16:415–421. 2001.PubMed/NCBI
|
|
20
|
Ohana G, Bar-Yehuda S, Barer F and Fishman
P: Differential effect of adenosine on tumor and normal cell
growth: Focus on the A3 adenosine receptor. J Cell Physiol.
186:19–23. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gessi S, Merighi S, Sacchetto V, Simioni C
and Borea PA: Adenosine receptors and cancer. Biochim Biophys Acta.
1808:1400–1412. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fishman P, Bar-Yehuda S, Synowitz M,
Powell JD, Klotz KN, Gessi S and Borea PA: Adenosine receptors and
cancer. Handb Exp Pharmacol. 193:399–441, 209. View Article : Google Scholar
|
|
23
|
Thakur S, Du J, Hourani S, Ledent C and Li
JM: Inactivation of adenosine A2A receptor attenuates basal and
angiotensin II-induced ROS production by Nox2 in endothelial Cells.
J Biol Chem. 285:40104–40113. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ma Y, Zhang J, Zhang Q, Chen P, Yu S, Liu
H, Liu F, Song C, Yang D and Liu J: Adenosine induces apoptosis in
human liver cancer cells through ROS production and mitochondrial
dysfunction. Biochem Biophys Res Commun. 448:8–14. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shirali S, Aghaei M, Shabani M, Fathi M,
Sohrabi M and Moeinifard M: Adenosine induces cell cycle arrest and
apoptosis via cyclinD1/Cdk4 and Bcl-2/Bax pathways in human ovarian
cancer cell line OVCAR-3. Tumour Biol. 34:1085–1095. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yasuda Y, Saito M, Yamamura T, Yaguchi T
and Nishizaki T: Extracellular adenosine induces apoptosis in
Caco-2 human colonic cancer cells by activating caspase-9/-3 via
A2a adenosine receptors. J Gastroenterol. 44:56–65. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Saito M, Yaguchi T, Yasuda Y, Nakano T and
Nishizaki T: Adenosine suppresses CW2 human colonic cancer growth
by inducing apoptosis via A1 adenosine receptors. Cancer let.
290:211–215. 2010. View Article : Google Scholar
|
|
28
|
Gao W, Li JZ, Chan JY, Ho WK and Wong TS:
mTOR pathway and mTOR inhibitors in head and neck cancer. ISRN
Otolaryngol. 2012:9530892012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kundu SK and Nestor M: Targeted therapy in
head and neck cancer. Tumour Biol. 33:707–721. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Machiels JP and Schmitz S: New advances in
targeted therapies for squamous cell carcinoma of the head and
neck. Anticancer Drugs. 22:626–633. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Amornphimoltham P, Sriuranpong V, Patel V,
Benavides F, Conti CJ, Sauk J, Sausville EA, Molinolo AA and
Gutkind JS: Persistent activation of the Akt pathway in head and
neck squamous cell carcinoma: A potential target for UCN-01. Clin
Cancer Res. 10:4029–4037. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Merighi S, Mirandola P, Varani K, Gessi S,
Leung E, Baraldi PG, Tabrizi MA and Borea PA: A glance at adenosine
receptors: Novel target for antitumor therapy. Pharmacol Ther.
100:31–48. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Vanhaesebroeck B and Waterfield MD:
Signaling by distinct classes of phosphoinositide 3-kiness. Exp
Cell Res. 253:239–254. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Altomare DA and Khaled AR: Homeostasis and
the importance for a balance between AKT/mTOR activity and
intracellular signaling. Curr Med Chem. 19:3748–3762. 2012.
View Article : Google Scholar : PubMed/NCBI
|


















