Introduction
Hydroxycamptothecin (HCPT) has few side effects in
the treatment of various cancers and has been widely used
clinically (1–3). HCPT can inhibit proliferation and induce
apoptosis in some types of cancer treatment, including prostate,
colon and ovarian cancer (4–6). However, the underlying molecular
mechanism by which HCPT affects the development of lung cancer has
not yet been elucidated.
In the 21st Century, lung cancer has accounted for a
marked proportion of morbidity and mortality worldwide according to
the American Cancer Society (7).
Small-cell lung carcinoma (SCLC) and non-SCLC (NSCLC) are the
primary types of lung cancer, 85–90% of lung cancer is NSCLC
(8). Among those patients with
advanced NSCLC and those undergoing first-line platinum-based
double-agent chemotherapy, the remission rate is between 30 and
40%. In addition, the median survival time is reported to be
between 31 and 40 weeks, and the 1-year survival rate is between 30
and 40% (9). Therefore, there is an
urgency to understand the key issues regarding alternative
therapeutic approaches for treating NSCLC.
Autophagy serves a pivotal function in the
physiological and pathological processes. It eliminates misfolded
aggregated proteins to maintain cellular homeostasis (10,11).
Nucleation and elongation of the isolation membrane are the two
major processes in the autophagosome formation. At first, the
formation of the initial film nucleation stage requires a kinase
complex including Beclin-1, a B-cell lymphoma 2 (Bcl-2) homology
domain 3-only protein, which is frequently used as a marker for
monitoring autophagy. Subsequently, the cytosolic protein light
chain 3 (LC3)I is conjugated to phosphatidylethanolamine, forms
LC3II and participates in membrane elongation (12–15). In
addition, autophagy pathways have also been reported to participate
in anticancer drug-induced cell death, such as 5-fluorouracil and
rapamycin (16,17). Notably, it has been demonstrated that
the appropriate modification of autophagy is able to accelerate the
process of apoptosis and enhance the curative effect of
chemotherapy (18–20). However, the effects of autophagy on
the ability of HCPT to inhibit the proliferation of lung cancer
cells remain unknown.
Materials and methods
Chemicals and antibodies
3-Methyladenine (3-MA) and rapamycin were purchased
from Sigma; Merck KGaA (Darmstadt, Germany). A Cyto-ID autophagy
detection kit was purchased from Enzo Life Sciences, Inc.
(Farmingdale, NY, USA; cat. no. ENZ-51031-K200). HCPT was purchased
from Bailingwei Technology Co., Ltd. (Beijing, China) and an MTT
cell viability assay kit was purchased from Zhejiang Tianshun
Biotechnology Co., Ltd. (Zhejiang, China; http://tianshunbiotech.com/index_en.asp). An Annexin
V-propidium iodide (PI) apoptosis kit was purchased from Yeasen
Biotechnology Co., Ltd. (Shanghai, China). Rabbit polyclonal
anti-Beclin-1 (cat. no. 4122), rabbit polyclonal
anti-phosphorylated mammalian target of rapamycin (p-mTOR) (cat.
no. 5536), rabbit polyclonal anti-Bcl-2-associated X protein (Bax)
(cat. no. 2772), rabbit polyclonal anti-Bcl-2 (cat. no. 2876),
rabbit polyclonal anti-GAPDH (cat. no. 5174), goat anti-rabbit
immunoglobulin secondary antibody (cat. no. 14708), Tubulin
antibody (cat. no. 2146) were purchased from Cell Signaling
Technology, Inc. (Danvers, MA, USA); Rabbit polyclonal anti-LC3
(cat. no. L7543) were purchased from Sigma; Merck KGaA (Darmstadt,
Germany).
Cell culture and treatments
The A549 NSCLC cells were obtained from the Chinese
Academy of Sciences (Beijing, China) and maintained in RPMI-1640
medium (Shanghai Haoran Biological Technology Co., Ltd., Shanghai,
China) supplemented with 10% fetal bovine serum (FBS; Shanghai
Haoran Biological Technology Co., Ltd.) at 37°C in a humidified
atmosphere containing 5% CO2. When cells reached 70–80%
confluence, (0–400 µM) HCPT was added to the medium for 24 h.
Cell viability assay
In brief, A549 cells were plated in a 96-well plate
at 5×104 cells/well and were treated with (0–400 µM)
HCPT. After 24 h, 10 µl 5 mg/ml MTT solution was added to each well
prior to incubation at 37°C for an additional 4 h. Following
careful removal of the medium, 150 µl MTT solvent (DMSO) was added
to each well. Cells were protected from light and mixed on an
orbital shaker (80 rpm) for 15 min. The absorbance values were read
at 590 nm, with a reference filter of 620 nm. Each experiment was
performed in triplicate.
Apoptosis assay
A549 cells were grown to 70–80% confluence and HCPT
group A549 cells were treated with (0–400 µM) HCPT for 24 h, (5 mM)
3-MA group A549 cells were treated with (5 mM) 3-MA for 1 h and
(50–400 µM) HCPT+(5 mM) 3-MA group A549 cells were treated with (5
mM) 3-MA for 1 h then were treated with (50–400 µM) HCPT for 24 h.
Analysis of apoptosis in cells stained with fluorescein
isothiocyanate (FITC) Annexin V and propidium iodide (PI) was
performed using a flow cytometer and Cell Quest Pro software
version 5.1 (BD Biosciences, San Jose, CA, USA). Each experiment
was performed in triplicate.
Determination of autophagosome
formation using Cyto-ID staining
The cell nucleus was stained blue by Hoechst 33342
(5 µg/ml) in the dark for 30 min at 37°C and Cyto-ID Green dye
(diluted in 5% FBS at 1:1,000) was employed to selectively label
autolytic enzyme bodies and indicate autophagy at 37°C in the dark
for 30 min, which are expressed as green dots as described in a
previous study (21). A Cyto-ID
autophagy detection kit was employed to analyze autophagy. In
brief, A549 cells were washed with 1X assay buffer containing 5%
FBS and cells were incubated in Cyto-ID solution at 37°C in the
dark for 30 min. Finally, all samples were visualized by
laser-scanning confocal microscopy (FV1000; Olympus Corporation,
Tokyo, Japan), (confocal microscope image ×40 magnification).
Western blot analysis
Whole cell lysate was prepared with lysis using
Triton X-100/glycerol buffer, containing 50 mM Tris-HCl (pH 7.4), 4
mM EDTA, 2 mM EGTA, and 1 mM dithiothreitol, supplemented with 1%
Triton X-100, 1% SDS, and protease inhibitors, and then separated
on a SDS-PAGE gel and transferred to PVDF membrane. Western blot
analysis was performed using appropriate primary antibodies and
horseradish peroxidase-conjugated suitable secondary antibodies,
followed by detection with enhanced chemiluminescence (Pierce
Chemical). A total of 30 mg protein lysate (the protein
determination method adopt BCA) was separated by SDS-PAGE (8–13%)
and then transferred onto a polyvinylidene difluoride membrane
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). Membranes were
incubated overnight at 4°C with primary antibodies against LC3
(dilution, 1:1,000), Beclin-1 (dilution, 1:1,000), p-mTOR
(dilution, 1:1,000), Bax (dilution, 1:1,000), Bcl-2 (dilution,
1:1,000), GAPDH (dilution, 1:1,000) and Tubulin (dilution,
1:1,000), prior to incubating membranes with goat anti-rabbit
immunoglobulin secondary antibody (dilution, 1:1,000) for 2 h at
room temperature. The bands were visualized by enhanced
chemiluminescence (Genshare, Shaanxi, China). Image J software
version 1.4.3.67 (National Institutes of Health, Bethesda, MD, USA)
was employed to quantify protein expression levels. GAPDH or
Tubulin (Tu) were used as loading controls.
Statistical analysis
Data were analyzed using SPSS software (version
21.0; IBM Corp., Armonk, NY, USA). Data are expressed as the mean ±
standard deviation. One-way analysis of variance followed by a
Least Significance Differences (LSD) post-hoc testing was used to
examine differences between groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
HCPT inhibits cell proliferation and
induces apoptosis in A549 cells
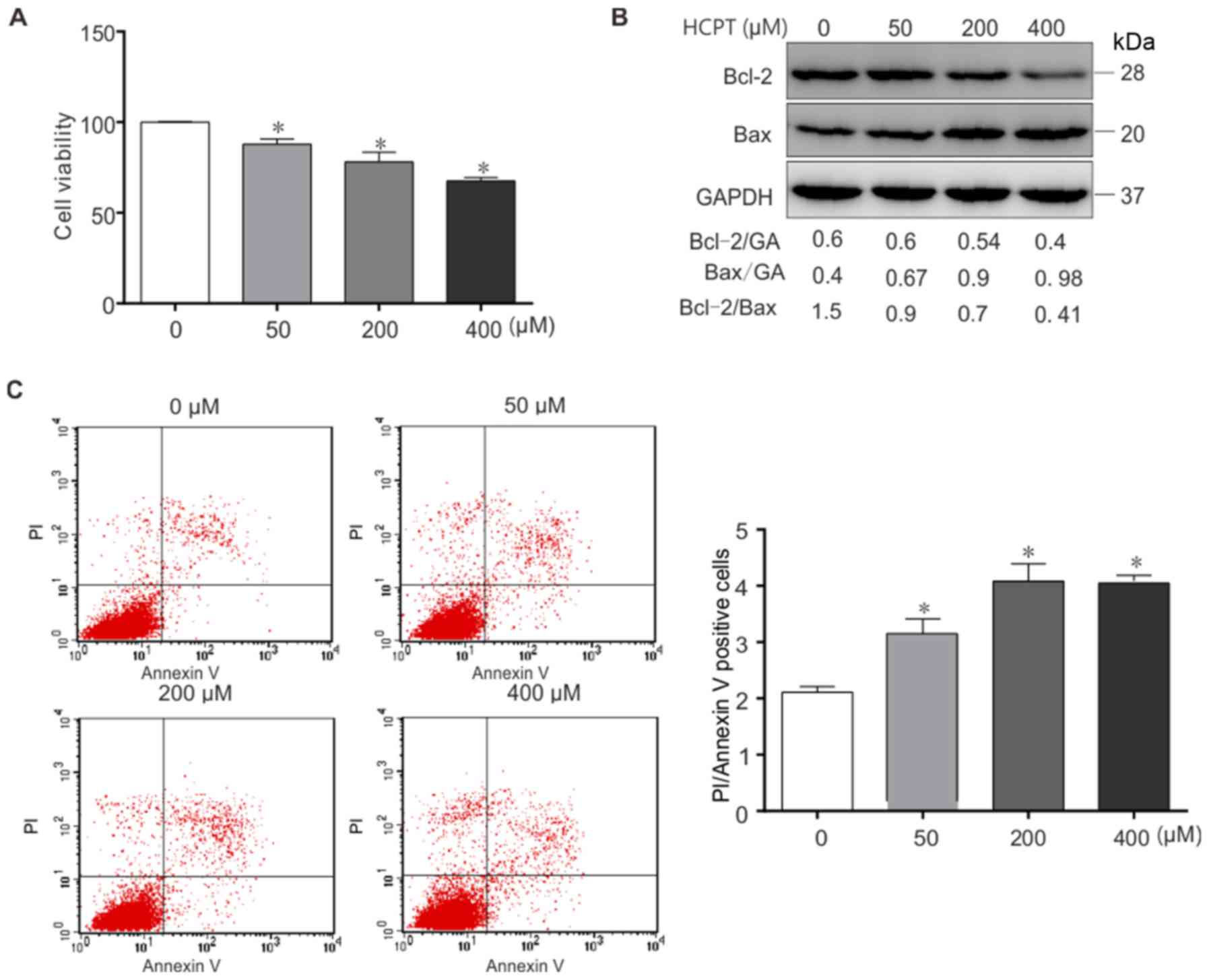
As presented in Fig.
1A, treatment with (0–400 µM) HCPT resulted in a dose-dependent
decrease in A549 cell viability. In order to determine whether HCPT
induced apoptosis of A549 cells, the expression of the
apoptosis-specific proteins Bax and Bcl-2 was determined in (0–400
µM) HCPT-treated A549 cells by western blot analysis (Fig. 1B). The results indicated that HCPT
downregulates Bcl-2 expression and increases Bax expression in
vitro. Furthermore, the Bcl-2/Bax ratio was decreased in
response to HCPT (Fig. 1B). Similar
results were acquired using flow cytometric analysis (Fig. 1C), Annexin+PI+
represent apoptosis. Taken together, these results suggest that
HCPT induces apoptotic cell death in A549 cells.
HCPT induces autophagy in A549
cells
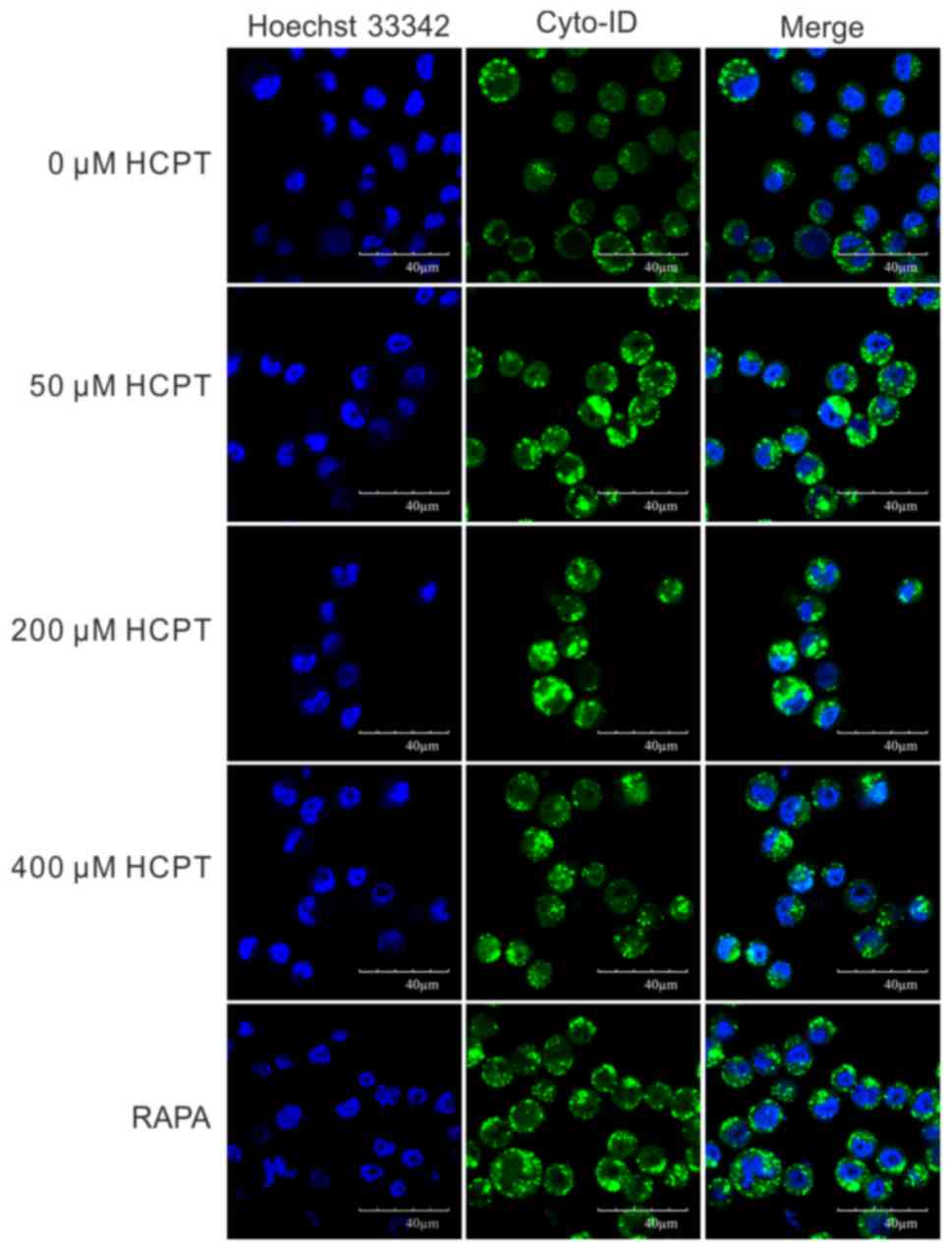
In order to determine whether HCPT was able to
induce autophagy in A549 cells, a Cyto-ID autophagy detection kit
was employed. As presented in Fig. 2,
the number of Cyto-ID-positive cells gradually increased with
increasing concentrations of HCPT treatment in A549 cells. In order
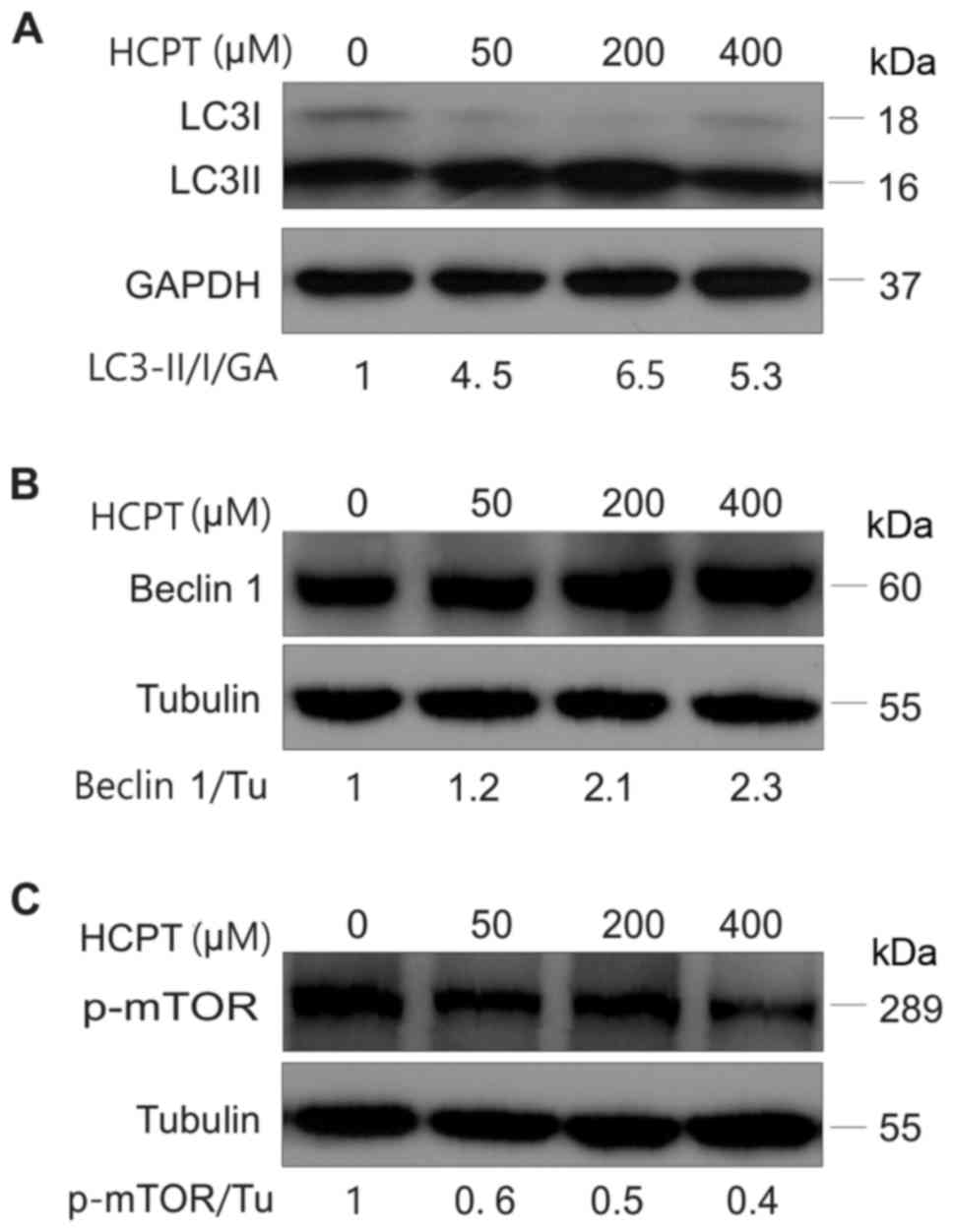
to further verify this result, the expression of
autophagy-associated proteins, including LC3, Beclin-1 and p-mTOR,
was detected in A549 cells treated with HCPT (RPMI-1640 medium with
0–400 µM HCPT) by western blot analysis. The results indicated that
HCPT increased the conversion of LC3I into LC3II (Fig. 3A) and increased Beclin-1 protein
expression (Fig. 3B), but decreased
the expression of p-mTOR (Fig. 3C),
suggesting that HCPT induces autophagy in A549 cells.
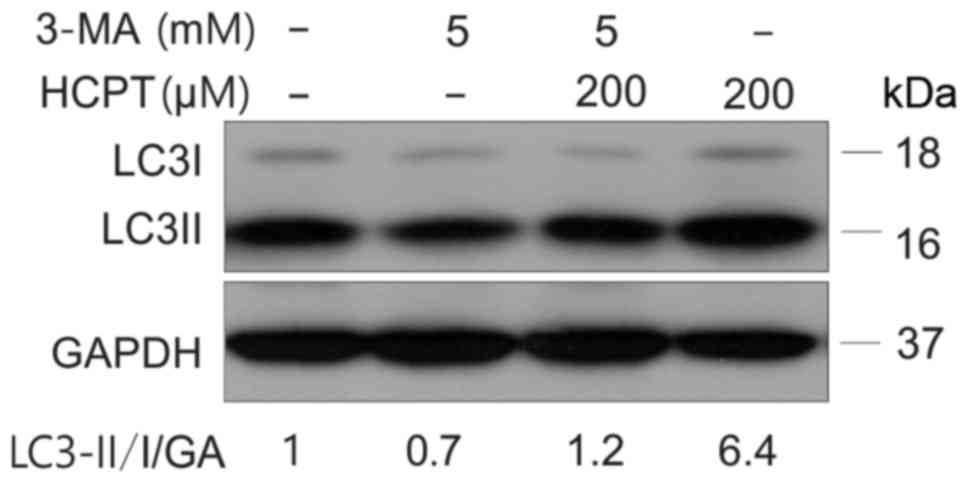
Effect of autophagy inhibitors
Autophagy is able to be inhibited by activation of
the phosphoinositide 3-kinase signaling pathway (22). In order to further prove that HCPT is
able to induce an increase in autophagy, cells were treated with an
autophagy inhibitor (3-MA) prior to treatment with HCPT. A549 cells
were treated with (5 mM) 3-MA, prior to (200 µM) HCPT treatment for
1 h (Fig. 4). The results from this
experiment demonstrated that the combination of 3-MA and HCPT
significantly decreased cell viability (Fig. 5A) and the Bcl-2/Bax ratio decreased
(Fig. 5B) and increased apoptosis
(Fig. 5C)
(Annexin+PI+ represent apoptosis) in response
to 3-MA and 200 µM HCPT treatment in A549 cells. These results
suggested that inhibition of autophagy decreased cell viability and
increased apoptosis induced by HCPT in A549 cells.
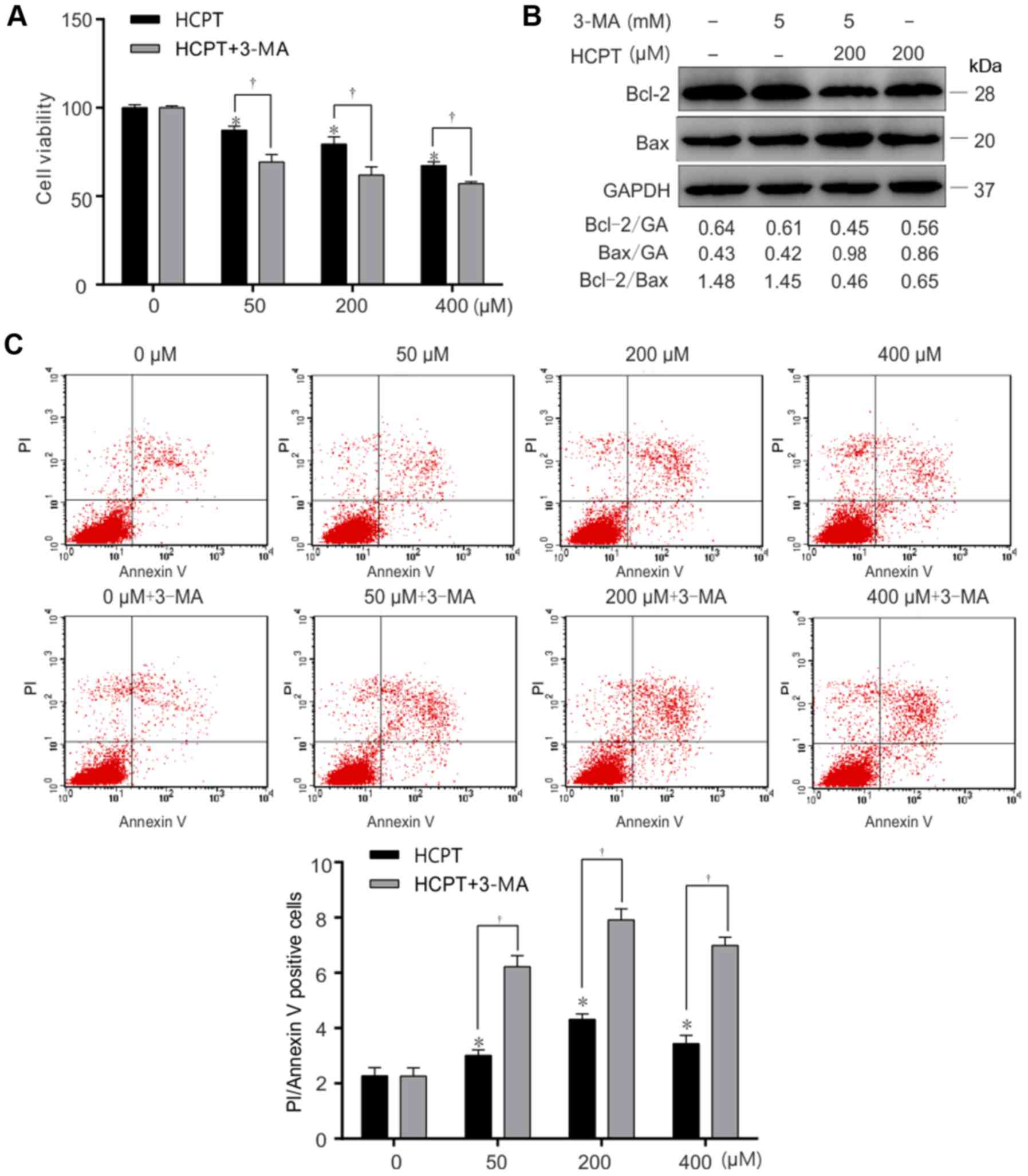 | Figure 5.Inhibition of autophagy accelerates
HCPT-induced apoptotic cell death in A549 cells. A549 cells were
exposed to (0, 50, 200 or 400 µM) HCPT with or without 3-MA (5 mM),
and harvested at 24 h. (A) Relative cell viability of A549 cells
treated with (0, 50, 200 or 400 µM) HCPT, (5 mM) 3-MA or with (0,
50, 200 or 400 µM) HCPT+ (5 mM) 3-MA, determined using an MTT
assay. (B) Apoptosis determined in A549 cells which were treated
with (0, 50, 200 or 400 µM) HCPT, (5 mM) 3-MA or with (0, 50, 200
or 400 µM) HCPT+ (5 mM) 3-MA, using Annexin V/PI staining. (C)
Western blot analysis of the Bcl-2/Bax ratio in A549 cells which
were treated with (0 or 200 µM) HCPT, (5 mM) 3-MA or (0 or 200 µM)
HCPT+ (5 mM) 3-MA. GAPDH (GA) was used as a loading control.
Results are expressed as the mean ± standard deviation. *P<0.05
vs. (0 µM) HCPT (control); †P<0.05 vs. (0, 50, 200 or
400 µM) HCPT. HCPT, hydroxycamptothecin; Bax, Bcl-2-associated X
protein; Bcl-2, B-cell lymphoma 2; 3-MA, 3-methyladenine. |
Discussion
Uncontrolled proliferation and deregulated apoptosis
are important features of malignant tumor cells, and there are a
number of anticancer drugs that target tumor cell proliferation and
induce apoptosis (23–25). The Bcl-2 family proteins serve an
important function in regulating apoptosis. For example, Bcl-2 is
the main inhibitor of apoptosis and Bax is the main promoter of
apoptosis in the Bcl-2 family. Furthermore, Bcl-2 and Bax regulate
the release of apoptotic activators, including cytochrome c, to
affect the state of cells by controlling the permeability of
mitochondrial membrane (26). The Bax
dimer opens the channel on the membrane in order to increase its
permeability, Bcl-2 and Bax form a heteropolymer which reduces
permeability (27), when Bax forms a
homologous dimer, it induces apoptosis. The Bax-Bcl-2 allodimer
inhibits cell apoptosis (28).
Furthermore, Bcl-2 and Bax regulate tumor cell apoptosis (29). The Bcl-2/Bax ratio is associated with
tumor occurrence and development (30,31). In
the present study, Bax and Bcl-2 protein levels were assessed by
western blot analysis. The results indicated that HCPT decreased
Bcl-2 protein levels and increased Bax protein levels. Furthermore,
the Bcl-2/Bax ratio was decreased in response to HCPT treatment.
Flow cytometric analysis also confirmed these results, suggesting
that HCPT induces apoptotic cell death in A549 cells. In addition,
dose-dependent concentrations of (0–400 µM) HCPT decreased cell
viability of A549 cells as determined using an MTT assay. Taken
together, the results of the present study suggest that HCPT
inhibits cancer development by preventing tumor cell proliferation
and inducing apoptosis.
The results of the present study indicate that HCPT
induces autophagy in A549 cells and that by targeting autophagy
using 3-MA, an autophagy inhibitor, the cells become more sensitive
to HCPT treatment. Autophagy is responsible for maintaining the
steady state of cells by degrading misfolded proteins and
eliminating damaged organelles (32).
However, evidence suggests that autophagy can lead to cell death
via a process distinct from apoptosis (33), termed autophagic cell death. A number
of anticancer drugs contribute to the antitumor process by inducing
autophagy and apoptosis at the same time (34–36). It is
important to note that a number of factors may induce autophagy,
including hypoxia, DNA damage and damaged organelles (36,37).
A growing body of evidence suggest a possible
function of autophagy in controlling pathogens (38). In response to chemotherapy,
autophagy-deficient tumors fail to elicit an anticancer immune
(39). Therefore, future research
should consider the cell context-specific functions of
autophagy.
The results of the present study indicated that
exposing A549 cells to HCPT significantly increases autophagy.
Treatment with an autophagy inhibitor (3-MA) led to a statistically
significant decrease in viability and increased apoptosis in A549
cells in response to HCPT treatment. Therefore, inhibiting
autophagy may decrease the viability and apoptosis of A549 cells
treated with HCPT. Combining autophagy inhibitors with HCPT may
enhance the efficacy of HCPT for treating lung cancer. The results
of a recent study suggested that HCPT confers antitumor efficacy on
HeLa cells via activating autophagy and mediating apoptosis in
cervical cancer (40), which is also
consistent with the results of the present study. Nevertheless, it
is worth mentioning that the ratio of LC3II/I in the 200 µM HCPT
group was increased compared with that of the 400 µM HCPT group.
Similarly, the rate of change in the growth inhibition and
apoptosis in the 200 µM HCPT+3-MA group, relative to the 200 µM
HCPT group, was increased compared with that of the respective 400
µM HCPT groups. These data suggest that the ability of HCPT to
enhance autophagy in A549 cells is HCPT dose-dependent and that
autophagy inhibition combined with 200 µM HCPT treatment may be
optimal for the treatment of lung cancer.
Autophagy has a dual function in tumorigenesis
(41). Cancer is a complex disease,
and autophagy serves different roles in patients depending on the
type of cancer. Therefore, modulation of autophagy as a therapeutic
strategy has a different sensitivity between the various types of
cancer (42). Chemotherapy also
serves an important role in current cancer treatment; furthermore,
it interacts with autophagy (43).
Autophagy is involved in a wide variety of physiological and
pathological processes and is closely associated cancer (44,45). Under
normal circumstances, autophagy clears misfolded proteins and
organelles, preventing stress reaction and cancer incidence
(46). However, although autophagy is
primarily a protective process, it can also promote cancer
viability by degrading abnormal proteins and organelles in cancer
cells (44,46). Induction of autophagy may affect
cancer drug curative effects (47,48). The
results of the present study suggest that the inhibition of
autophagy induces a statistically significant decrease in viability
and apoptosis in A549 cells in response to HCPT treatment. Taken
together, the results of the present study lead to a deeper
understanding of the molecular mechanisms underlying HCPT-induced
autophagy in lung cancer.
In summary, the results of the present study support
the hypothesis that autophagy is a survival mechanism for A549
cells in response to HCPT treatment. The results have marked
implications because HCPT resistance and autophagy are associated
with human cancer and resistance to treatment (1,3,49). Therefore, inhibiting autophagy in
tumors may be a means to increase the efficacy of anticancer
treatment.
Acknowledgements
The authors thank all members of the Medical
Research Center, North China University of Science and Technology.
The abstract was presented at the ATS 2017 Conference 19–24 May
2017 in Washington, DC, USA, and published as abstract no. A3124 in
Am J Respir Crit Care Med 195 (Suppl 1): 2017.
Funding
The present study was supported by the North China
University of Science and Technology Research (grant no.
201610081026), the Key Projects of Science and Technology Research
in Hebei Province (grant no. ZD2017063), the North China University
of Science and Technology Research (grant no. X2016026) and the
Tangshan International Technological Cooperation Projects (grant
no. 14160201B).
Availability of data and materials
All materials described in the manuscript, including
all relevant raw data, will be freely available to any scientist
wishing to use them for non-commercial purposes, without breaching
participant confidentiality.
Author's contributions
HW and WT designed the study. YW wrote the
manuscript and performed the western blotting. CL conducted the
cell culture and performed the cell viability assay. YZ prepared
the cell samples for the autophagy assay. HH performed the
laser-scanning confocal microscopy. XH prepared the cell samples
for the apoptosis assay and performed the imaging. GZ and HL helped
to conduct the cell culture and write the manuscript, and all
authors read and approved the final manuscript.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing of
interests.
References
|
1
|
Urasaki Y, Takebayashi Y and Pommier Y:
Activity of a novel camptothecin analogue, homocamptothecin, in
camptothecin-resistant cell lines with topoisomerase I alterations.
Cancer Res. 60:6577–6580. 2000.PubMed/NCBI
|
|
2
|
Zhang R, Li Y, Cai Q, Liu T, Sun H and
Chambless B: Preclinical pharmacology of the natural product
anticancer agent 10-hydroxycamptothecin, an inhibitor of
topoisomerase I. Cancer Chemother Pharmacol. 41:257–267. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang G, Ding L, Renegar R, Wang X, Lu Q,
Huo S and Chen YH: Hydroxycamptothecin-loaded Fe3O4 nanoparticles
induce human lung cancer cell apoptosis through caspase-8 pathway
activation and disrupt tight junctions. Cancer Sci. 102:1216–1222.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nie F, Cao J, Tong J, Zhu M, Gao Y and Ran
Z: Role of Raf-kinase inhibitor protein in colorectal cancer and
its regulation by hydroxycamptothecine. J Biomed Sci. 22:562015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu Z, Zhu G, Getzenberg RH and Veltri RW:
The upregulation of PI3K/Akt and MAP kinase pathways is associated
with resistance of microtubule-targeting drugs in prostate cancer.
J Cell Biochem. 116:1341–1349. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bian Z, Yu Y, Quan C, Guan R, Jin Y, Wu J,
Xu L, Chen F, Bai J, Sun W and Fu S: RPL13A as a reference gene for
normalizing mRNA transcription of ovarian cancer cells with
paclitaxel and 10-hydroxycamptothecin treatments. Mol Med Rep.
11:3188–3194. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fu T, Wang L, Jin XN, Sui HJ, Liu Z and
Jin Y: Hyperoside induces both autophagy and apoptosis in non-small
cell lung cancer cells in vitro. Acta Pharmacol Sin. 37:505–518.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mizushima N, Yoshimori T and Levine B:
Methods in mammalian autophagy research. Cell. 140:313–326. 2016.
View Article : Google Scholar
|
|
11
|
Baehrecke EH: Autophagy: Dual roles in
life and death? Nat Rev Mol Cell Biol. 6:505–510. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang H and Baehrecke EH: Eaten alive:
Novel insights into autophagy from multicellular model systems.
Trends Cell Biol. 25:376–387. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Weckman A, Rotondo F, Di Ieva A, Syro LV,
Butz H, Cusimano MD and Kovacs K: Autophagy in endocrine tumors.
Endocr Relat Cancer. 22:R205–R218. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Djavaheri-Mergny M, Maiuri M and Kroemer
G: Cross talk between apoptosis and autophagy by caspase-mediated
cleavage of Beclin 1. Oncogene. 29:1717–1719. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kang R, Zeh HJ, Lotze MT and Tang D: The
Beclin 1 network regulates autophagy and apoptosis. Cell Death
Differ. 18:571–580. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pan X, Zhang X, Sun H, Zhang J, Yan M and
Zhang H: Autophagy inhibition promotes 5-fluorouraci-induced
apoptosis by stimulating ROS formation in human non-small cell lung
cancer A549 cells. PLoS One. 8:e566792013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ravikumar B, Berger Z, Vacher C, O'Kane CJ
and Rubinsztein DC: Rapamycin pre-treatment protects against
apoptosis. Hum Mol Genet. 15:1209–1216. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cui Q, Tashiro S, Onodera S, Minami M and
Ikejima T: Autophagy preceded apoptosis in oridonin-treated human
breast cancer MCF-7 cells. Biol Pharm Bull. 30:859–864. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Iwamaru A, Kondo Y, Iwado E, Aoki H,
Fujiwara K, Yokoyama T, Mills GB and Kondo S: Silencing mammalian
target of rapamycin signaling by small interfering RNA enhances
rapamycin-induced autophagy in malignant glioma cells. Oncogene.
26:1840–1851. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cheng Y, Chen G, Hu M, Huang J, Li B, Zhou
L and Hong L: Has-miR-30a regulates autophagic activity in cervical
cancer upon hydroxycamptothecinexposure. Biomed Pharmacother.
75:67–74. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Desai SD, Reed RE, Babu S and Lorio EA:
ISG15 deregulates autophagy in genotoxin-treated ataxia
telangiectasia cells. J Biol Chem. 288:2388–2402. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Eskelinen EL, Prescott AR, Cooper J,
Brachmann SM, Wang L, Tang X, Backer JM and Lucocq JM: Inhibition
of autophagy in mitotic animal cells. Traffic. 3:878–893. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vilgelm AE, Pawlikowski JS, Liu Y, Hawkins
OE, Davis TA, Smith J, Weller KP, Horton LW, McClain CM, Ayers GD,
et al: Mdm2 and aurora kinase a inhibitors synergize to block
melanoma growth by driving apoptosis and immune clearance of tumor
cells. Cancer Res. 75:181–193. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhu M, Xu Y, Ge M, Gui Z and Yan F:
Regulation of UHRF1 by microRNA-9 modulates colorectal cancer cell
proliferation and apoptosis. Cancer Sci. 106:833–839. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hamedani FS, Cinar M, Mo Z, Cervania MA,
Amin HM and Alkan S: Crizotinib (PF-2341066) induces apoptosis due
to downregulation of pSTAT3 and BCL-2 family proteins in NPM-ALK(+)
anaplastic large cell lymphoma. Leuk Res. 38:503–508. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Edlich F, Banerjee S, Suzuki M, Cleland
MM, Arnoult D, Wang C, Neutzner A, Tjandra N and Youle RJ: Bcl-x(L)
retrotranslocates Bax from the mitochondria into the cytosol. Cell.
145:104–116. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rossé T, Olivier R, Monney L, Rager M,
Conus S, Fellay I, Jansen B and Borner C: Bcl-2 prolongs cell
survival after Bax-induced release of cytochrome c. Nature.
391:496–499. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lalier L, Cartron PF, Juin P, Nedelkina S,
Manon S, Bechinger B and Vallette FM: Bax activation and
mitochondrial insertion during apoptosis. Apoptosis. 12:887–896.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Guo B, Zhai D, Cabezas E, Welsh K,
Nouraini S, Satterthwait AC and Reed JC: Humanin peptide suppresses
apoptosis by interfering with Bax activation. Nature. 423:456–461.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tallman MS: New strategies for the
treatment of acute myeloid leukemia including antibodies and other
novel agents. Hematology Am Soc Hematol Educ Program. 1–150.
2005.PubMed/NCBI
|
|
31
|
Chen XP, Ren XP, Lan JY, Chen YG and Shen
ZJ: Analysis of HGF, MACC1, C-met and apoptosis-related genes in
cervical carcinoma mice. Mol Biol Rep. 41:1247–1256. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yuan Y, Wang H, Wei Z and Li W: Impaired
autophagy in hilar mossy cells of the dentate gyrus and its
implication in schizophrenia. J Genet Genomics. 42:1–8. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gewirtz DA: The four faces of autophagy:
Implications for cancer therapy. Cancer Res. 74:647–651. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Maiuri MC, Zalckvar E, Kimchi A and
Kroemer G: Self-eating and self-killing: Crosstalk between
autophagy and apoptosis. Nat Rev Mol Cell Biol. 8:741–752. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Maiuri MC, Le Toumelin G, Criollo A, Rain
JC, Gautier F, Juin P, Tasdemir E, Pierron G, Troulinaki K,
Tavernarakis N, et al: Functional and physical interaction between
Bcl-XL and a BH3-like domain in Beclin-1. EMBO J. 26:2527–2539.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yorimitsu T and Klionsky DJ: Autophagy:
Molecular machinery for self-eating. Cell Death Differ. 12 Suppl
2:S1542–S1552. 2005. View Article : Google Scholar
|
|
37
|
Mizushima N, Levine B, Cuervo AM and
Klionsky DJ: Autophagy fights disease through cellular
self-digestion. Nature. 451:1069–1075. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Schreiber RD, Old LJ and Smyth MJ: Cancer
immunoediting: Integrating immunity's roles in cancer suppression
and promotion. Science. 331:1565–1570. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Michaud M, Martins I, Sukkurwala AQ,
Adjemian S, Ma Y, Pellegatti P, Shen S, Kepp O, Scoazec M, Mignot
G, et al: Autophagy-dependent anticancer immune responses induced
by chemotherapeutic agents in mice. Science. 334:1573–1577. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cheng YX, Zhang QF, Pan F, Huang JL, Li
BL, Hu M, Li MQ and Chen Ch: Hydroxycamptothecin shows antitumor
efficacy on HeLa cells via autophagy activation mediated apoptosis
in cervical cancer. Eur J Gynaecol Oncol. 37:238–243.
2016.PubMed/NCBI
|
|
41
|
White E and DiPaola RS: The double-edged
sword of autophagy modulation in cancer. Clin Cancer Res.
15:5308–5316. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lin L and Baehrecke EH: Autophagy, cell
death, and cancer. Mol Cell Oncol. 2:e9859132015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Bailly C: Homocamptothecins: Potent
topoisomerase I inhibitors and promising anticancer drugs. Crit Rev
Oncol Hematol. 45:91–108. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Levine B and Kroemer G: Autophagy in the
pathogenesis of disease. Cell. 132:27–42. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yan J, Yang H, Wang G, Sun L, Zhou Y, Guo
Y, Xi Z and Jiang X: Autophagy augmented by troglitazone is
independent of EGFR transactivation and correlated with
AMP-activated protein kinase signaling. Autophagy. 6:67–73. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Levine B and Klionsky DJ: Development by
self-digestion: Molecular mechanisms and biological functions of
autophagy. Dev Cell. 6:463–477. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Amaravadi RK, Lippincott-Schwartz J, Yin
XM, Weiss WA, Takebe N, Timmer W, DiPaola RS, Lotze MT and White E:
Principles and current strategies for targeting autophagy for
cancer treatment. Clin Cancer Res. 17:654–666. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Sui X, Chen R, Wang Z, Huang Z, Kong N,
Zhang M, Han W, Lou F, Yang J, Zhang Q, et al: Autophagy and
chemotherapy resistance: A promising therapeutic target for cancer
treatment. Cell Death Dis. 4:e8382013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Liu Z, Shi A, Song D, Han B, Zhang Z, Ma
L, Liu D and Fan Z: Resistin confers resistance to
doxorubicin-induced apoptosis in human breast cancer cells through
autophagy induction. Am J Cancer Res. 7:574–583. 2017.PubMed/NCBI
|