Introduction
Lung cancer remains the leading cause of
cancer-associated mortality, and accounted for 19.4% of the total
number of cancer mortalities worldwide in 2012 (1). Non-small cell lung carcinoma (NSCLC)
accounts for ~85% of diagnosed lung cancer cases and is associated
with an overall 5-year survival rate of <20% (2). Despite advances in the diagnosis and
treatment of patients with NSCLC, the majority of patients with
succumb to the disease. Therefore, there is an urgent requirement
to develop more effective therapies for patients with this
neoplasm.
Nuclear factor κB (NF-κB) is a family of inducible
transcription factors that exert a variety of evolutionarily
conserved roles in the immune system (3,4). NF-κB is
able to induce rapid transcription of genes regulating
inflammation, cell survival, proliferation and differentiation
(5,6).
The NF-κB family consists of five related proteins, p50 (NF-κB1)
and p52 (NF-κB2), p65 (RelA), RelB and c-Rel (Rel), which exist in
unstimulated cells as homo- or heterodimers bound to IκB family
proteins in the cytoplasm. Following stimulation by
pro-inflammatory cytokines, including tumor necrosis factor-α
(TNF-α) or lipopolysaccharide (LPS), the phosphorylated IκB kinase
(IKK) degrades the inhibitory protein IκB, and releases NF-κB.
NF-κB (p50/p65 or p52/Rel-B) then translocates into the nucleus and
results in the activation of target genes, including cytokines,
chemokines and antiapoptotic genes (7).
The association of NF-κB with tumorigenesis is well
documented in both colitis-associated and hepatitis-associated
cancer models (8,9). The constitutive activation of NF-κB in
cancer cells was demonstrated to result in anti-apoptosis, cell
growth, angiogenesis and metastasis of tumor cells (10–15). By
contrast, blocking NF-κB activation prevents inflammation-mediated
tumor growth and metastasis (16,17).
Therefore, NF-κB is an innovative target for antitumor therapy.
The circumsporozoite protein (CSP) is the main
surface protein of sporozoite of the malaria parasite. Previously,
it was shown that the transfer of the CSP from the parasitophorous
vacuole to the cytoplasm is essential for the development of the
malaria parasite in the hepatocytes, due to its ability to block
NF-κB activation in hepatocytes (18). Therefore, the effect of CSP on the
growth of the human Lung cell line, A549, was investigated and the
results indicated that CSP, via its NLS motif, was able to suppress
the proliferation and survival of A549 through competition with the
nuclear translocation of NF-κB.
Materials and methods
Recombinant plasmid construction
Total RNA of 5×106 Plasmodium
yoelii 265BY sporozoite (gifted from the Academy of Military
Medical Sciences of China, Beijing, China) was extracted using
TRIzol™ Reagent (Thermo Fisher Scientific, MA, USA) and reverse
transcribed to cDNA using PrimeScript™ RT reagent kit with gDNA
Eraser (Takara Bio, Inc., Otsu, Japan). The full-length CSP coding
sequence was amplified using polymerase chain reaction (PCR) from
the cDNA with the primers PCSP3
(5′-CCCaagcttGGGAAGAAGTGTACCATTTTAG-3; the Hind III
restriction site is underlined) and PCSP1284
(5′-CGggatccCGTTAATTAAAGAATACTAATAC-3′, the BamH I
restriction site is underlined). The amplified fragment, which was
1281 bp in length, was cloned into the pFLAG-CMV8 plasmid,
resulting in the recombinant plasmid pFLAG-CMV8-CSP. The PCR
conditions consisted of an initial predenature at 94°C for 2 min,
denaturation at 98°C for 10 sec, annealing at 50°C for 30 sec
followed by amplification for 40 cycles of 45 sec at 68°C. A second
plasmid, pFLAGCMV8-CSP NLS, was constructed through ligation of the
annealing nuclear location signal (NLS) oligonucleotide to the
Hind III and BamH I restriction sites of pFLAG-CMV8.
To construct the recombinant plasmid pFLAG-CMV8-CSPΔNLS, the CSP
lacking the NLS was obtained by overlapping PCR using a KOD FX kit
(Toyobo Life Science, Osaka, Japan) and then cloned into the
Hind III and BamH I restriction sites of pFLAG-CMV8.
Two fragments of CSP without NLS were amplified from the full CSP
coding sequence (1284 bp). The primer sequences used are as
follows: first fragment (1–1119 bp), forward,
3′-AAGCTTGGGAAGAAGTGTACCATTTTAGTTG-5′, and reverse,
3′-TTCTGGTTGCTTGTTACCAGAACCACAGGTTAC-5′, and second fragment
(1147–1284 bp), forward, 3′-AACAAGCAACCAGAAAATTTGACCTTAGAGG-5′, and
reverse, 3′-GGATCCTTAATTAAAGAATACTAATACTAATAATATTAC-5′.
The PCR conditions for the two fragments consisted
of pre-denaturation at 94°C for 3 min; 35 cycles of denaturation at
94°C for 30 sec, annealing at 50° for 30 sec and extension at 68°C
for 90 sec, followed by a final extension at 68°C for 5 min. The
two fragments were purified using a Gel Extraction Kit (Omega
Bio-Tek, Inc., Norcross, GA, USA). The splicing CSPΔNLS (1257 bp)
fragment was amplified using the following primers: forward,
5′-CTGTGGTTCTGGTAACAAGCAACCAG-3′ and reverse,
5′-CTGGTTGCTTGTTACCAGAACCACAG-3′. The overlapping PCR conditions
consisted of an initial pre-denaturation at 94°C for 3 min; 35
cycles of denaturation at 94°C for 30 sec, annealing at 42° for 30
sec and extension at 68°C for 90 sec, followed by a final extension
at 68°C for 5 min. The correct orientation of all the recombinant
plasmids was confirmed using DNA sequencing.
Cell culture
A549 cell line was purchased from the Japanese
Cancer Research Bank (JCRB, Tokyo, Japan). A549 cells were
maintained in 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.) supplemented Dulbecco's modified Eagle's medium
(Nacalai Tesque, Kyoto, Japan), 100 U/ml penicillin and 100 µg/ml
streptomycin. The culture conditions were at 37°C with a 5%
CO2 atmosphere and 85% humidity were achieved by
adjusting the incubator (ASTEC, Fukuoka, Japan).
Preparation of rabbit anti-CSP
serum
The B cell epitope QGP GAQ GPG AQG PGA P of the CSP
was synthesized and purified via high-performance liquid
chromatography (HPLC) by using Shimadzu Inertsil ODS-SP column
(4.6×250 mm ×5 µm; Shimadzu Corporation) on a Shimadzu Corporation
Prominence HPLC system (Kyoto, Japan). The column was operated at
30°C, and proteins were detected at 214 nm. Samples were analyzed
at a volume of 60 µl. The composition of mobile phase (two
solvents) was as follows: buffer A: 0.1% TFA (A501480, Sangon
Biotech, China) in 100% water and buffer B: 0.1% TFA in 100%
acetonitrile (A506811, Sangon Biotech, China). The flow rate was
1.0 ml/min, and the gradient elution condition was as follows: the
running time was 27.01 min, the initial mobile phase was consist of
92% buffer A and 8% buffer B, and the final mobile phase was
consist of 5% buffer A and 95% buffer B. The consist of mobile
phase was changed by the system with time in the gradient
elution.
Then the peptide was conjugated with keyhole limpet
hemocyanin (KLH). The peptide was produced by Beijing Biosynthesis
Biotechnology Co., Ltd., (Beijing, China; cat. no., 080825). The
resulting conjugated peptide was emulsified with Freund's adjuvant
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and immunized in a
specific-pathogen-free grade rabbit at 0, 2 and 4 weeks. After the
final immunization, rabbit serum was collected, and the titer of
antibody against the epitope was detected using ELISA (cat. no.,
44-2404-21, Nunc MaxiSorp™ flat-bottom, Nalge Nunc International,
Penfield, NY, USA). Ethical approval for animal experiments was
obtained from the Animal Ethical and Welfare Committee of The Third
Military Medical University (Chongqing, China).
Indirect immunofluorescence assay
(IFA)
Following transfection with 0.8 µg pFLAG-CMV8-CSP or
a control plasmid using Lipofectamine 2000TM for 24 h, A549 cells,
which were grown on a coverslip in 24-well plate, were incubated
with rabbit anti-CSP serum (1:10) for 1 h. After two washes with
phosphate-buffered saline (PBS), the cells were labeled with goat
anti-rabbit FITC-immunoglobulin G (H+L) for 20 min and observed
under fluorescence microscopy.
Alamar Blue assay
Alamar blue is a sensitive oxidation reduction
indicator that fluoresces and undergoes a color change upon
reduction in living cells. A549 cells were transfected with or
without pFLAG-CMV8-CSP (0.2, 0.4, 0.8, 1.2 or 1.6 µg), and after 24
h 120 µl SunBioTMAm-Blue was added to each well and incubated for 2
h at 37°C. The fluorescence was read at 570 and 600 nm. Cell
proliferation was expressed as optical density (OD) ratio of
OD570/OD600.
Dual luciferase assay
A549 cells were transfected using Lipofectamine™
2000 in 24-well plates. Each well received 200 ng pBIIx-luc report
vector containing two κB sites upstream of the c-fos promoter (gift
from Dr Sankar Ghosh, Yale University, Connecticut, USA) and 2 ng
TK-RL (Promega Corporation, Madison, WI, USA), together with 600 ng
Pflag-CMV8-CSP, pFLAG-CMV8-CSP NLS or pFLAG-CMV8-CSPΔNSL. After 24
h, the cells were stimulated in the presence or absence of 100
ng/ml human recombinant TNF-α (hTNF-α; PeproTech, New Jersey, USA)
and/or lipopolysaccharide (Invitrogen; Thermo Fisher Scientific,
Inc.) for 6 h. Next, the cells were lysed, and the activity of
firefly and Renilla luciferase was determined using the Dual
Luciferase Assay kit (Promega Corporation). The data were expressed
as the ratio of firefly luciferase to Renilla
luciferase.
Annexin V-fluorescein isothiocyanate
(FITC) apoptosis assay
After A549 cells were transfected with or without
0.8 µg pFLAG-CMV8-CSP for 24 h, the cells were stimulated with 100
ng/ml hTNF-α for 6 h. Next, the cells were collected and incubated
with Annexin V-FITC (Beyotime Institute of Biotechnology, Haimen,
China) and propidium iodide (PI) for 10 min on ice and analyzed
using flow cytometry. The data were analyzed by FlowJo software
10.4 (FlowJo LLC, Ashland, OR, USA).
Western blotting
Following transfection with or without 4 µg
pFLAG-CMV8-CSP NLS for 24 h, A549 cells were re-stimulated with 100
ng/ml hTNF-α for 30 min. The cells (2×106) were washed
with cold PBS and suspended in 0.4 ml hypotonic lysis buffer
containing protease inhibitors for 30 min. The cells were lysed
with 12.5 µl 10% Nonidet P-40 (Beyotime Institute of
Biotechnology). The homogenate was centrifuged, and the supernatant
containing the cytoplasmic extracts was stored at −80°C. The
nuclear pellet was resuspended in 25 µl ice-cold nuclear extraction
buffer. After 30 min of intermittent mixing, the extract was
centrifuged at 15,000 × g for 30 min, and the supernatants
containing nuclear extracts were collected. Protein concentration
in cytoplasmic extracts and nuclear extracts were then determined
with the BCA protein assay kit (Beyotime Institute of
Biotechnology). Equal amounts 20 µg of cytoplasmic and nuclear
extracts were separated using SDS-PAGE (10% gel), transferred to a
PVDF membrane, then washed by TBST buffer (TBS buffer with 0.1%
tween-20) and blocked by 5% non-fat-dried milk in TBST buffer at
room temperature for 1 h. After blocking, the membrane was probed
with primary antibodies against NF-κB p105/p50 (1:2,000; cat. no.,
12540; Cell Signaling Technology, Inc., Danvers, MA, USA) and GAPDH
(1:4,000; cat. no., 5174; Cell Signaling Technology, Inc.) for 16 h
at 4°C, and secondary goat anti-rabbit HRP-conjugated antibody
(1:20,000; cat. no., sc-2004; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) for 1 h at room temperature, then the bands were
detected by using ECL reagent (Pierce; Thermo Fisher Scientific,
Inc.).
Statistical analysis
All data were analyzed using Student's t-test or
one-way analysis of variance, and P<0.05 was considered
statistically significant. SPSS 19.0 software (IBM Corp., Armonk,
NY) was used for the statistical analysis.
Results
CSP suppresses the proliferation of
A549 cells
For CSP to be able to promote the development of
sporozoite in hepatocytes, it has to be transferred from the
parasitophorous vacuole into the cytoplasm (18). To mimic this process, the full length
of the CSP coding sequence was amplified from the Plasmodium
yoelii 265BY sporozoite, and cloned into the pFLAG-CMV8
plasmid. The resulting recombinant plasmid, pFLAG-CMV8-CSP, was
then transfected into the human lung cancer cell line A549, and its
intracellular distribution was determined using indirect
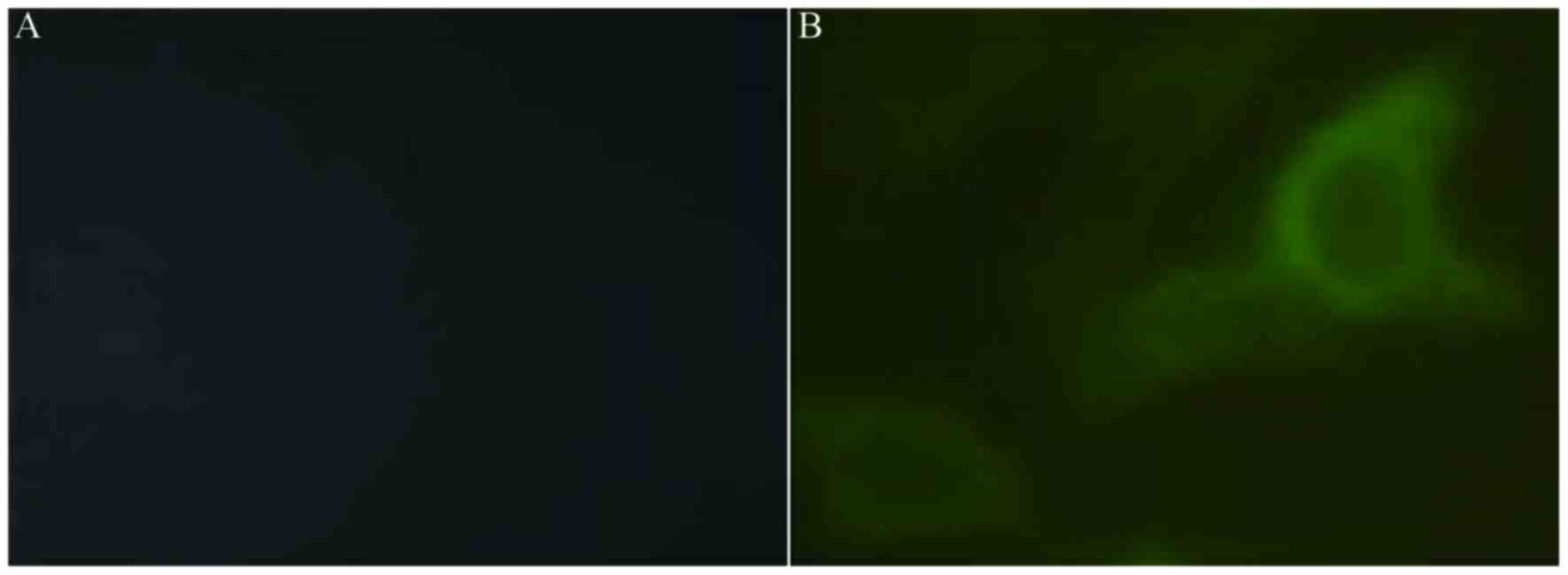
immunofluorescence assay using anti-CSP serum. CSP was expressed
primarily in the cytoplasm. CSP was not present in A549 cells
transfected with pFLAG-CMV8 (Fig.
1A), but was present in the membrane of A549 cells following
transfection of recombinant plasmid pFLAG-CMV8-CSP (Fig. 1B).
CSP in the cytoplasm is important for the
development of the malaria sporozoite (18). However, the effects of CSP on cancer
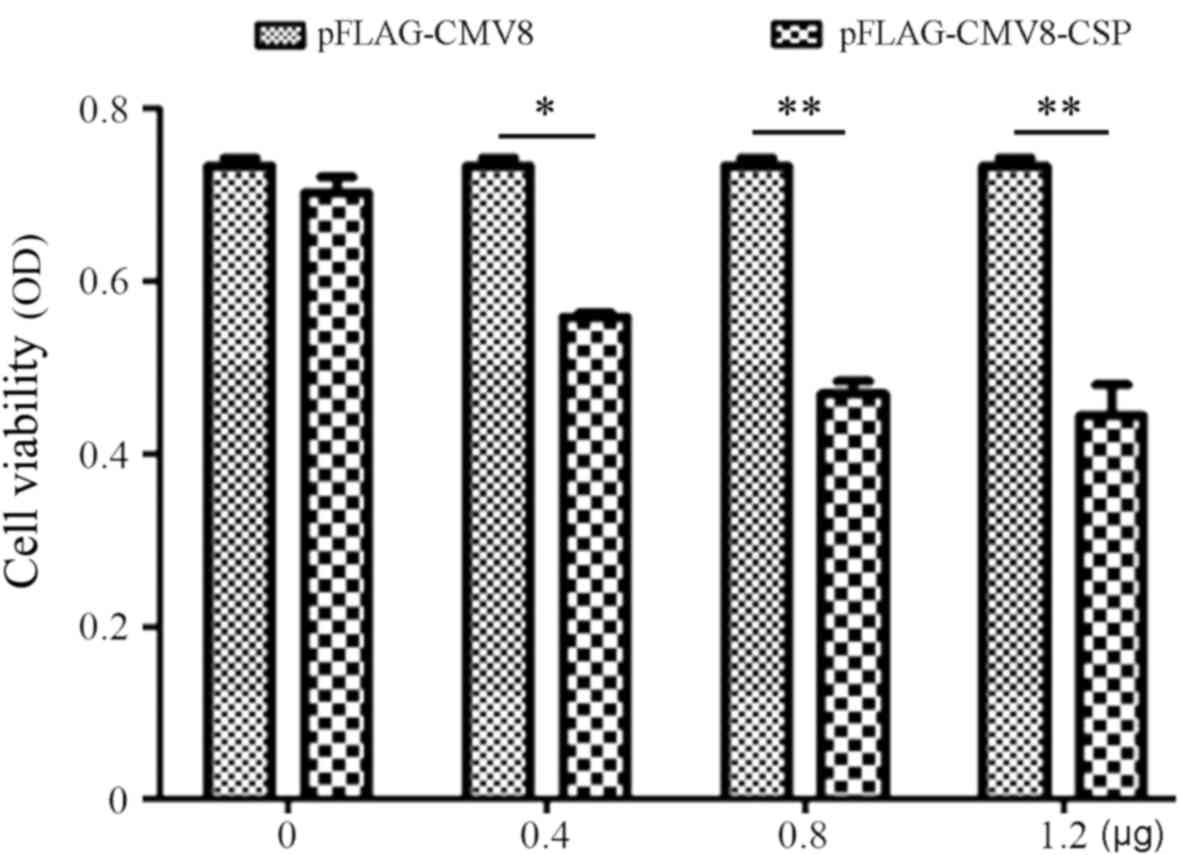
cell proliferation are unclear. Thus, the Alamar Blue assay was
used to investigate the effect of the CSP on proliferation of A549
cells. As shown in Fig. 2,
transfection with 0.4 µg pFLAGCMV8-CSP was able to significantly
suppress the proliferation of A549, compared with proliferation
following transfection with the control pFLAG-CMV8. When the
concentration of the transfected pFLAG-CMV8-CSP was increased from
0.4 to 1.2 µg, cell viability and proliferation decreased in a
dose-dependent manner. Following transfection with 1.2 µg
pFLAG-CMV8-CSP, cell proliferation was <30% (OD value, 0.105 vs.
0.375). Thus, the data demonstrated the inhibitory role of CSP on
the proliferation of A549 cells.
CSP induces the apoptosis of A549
cells
To investigate whether CSP is also able to induce
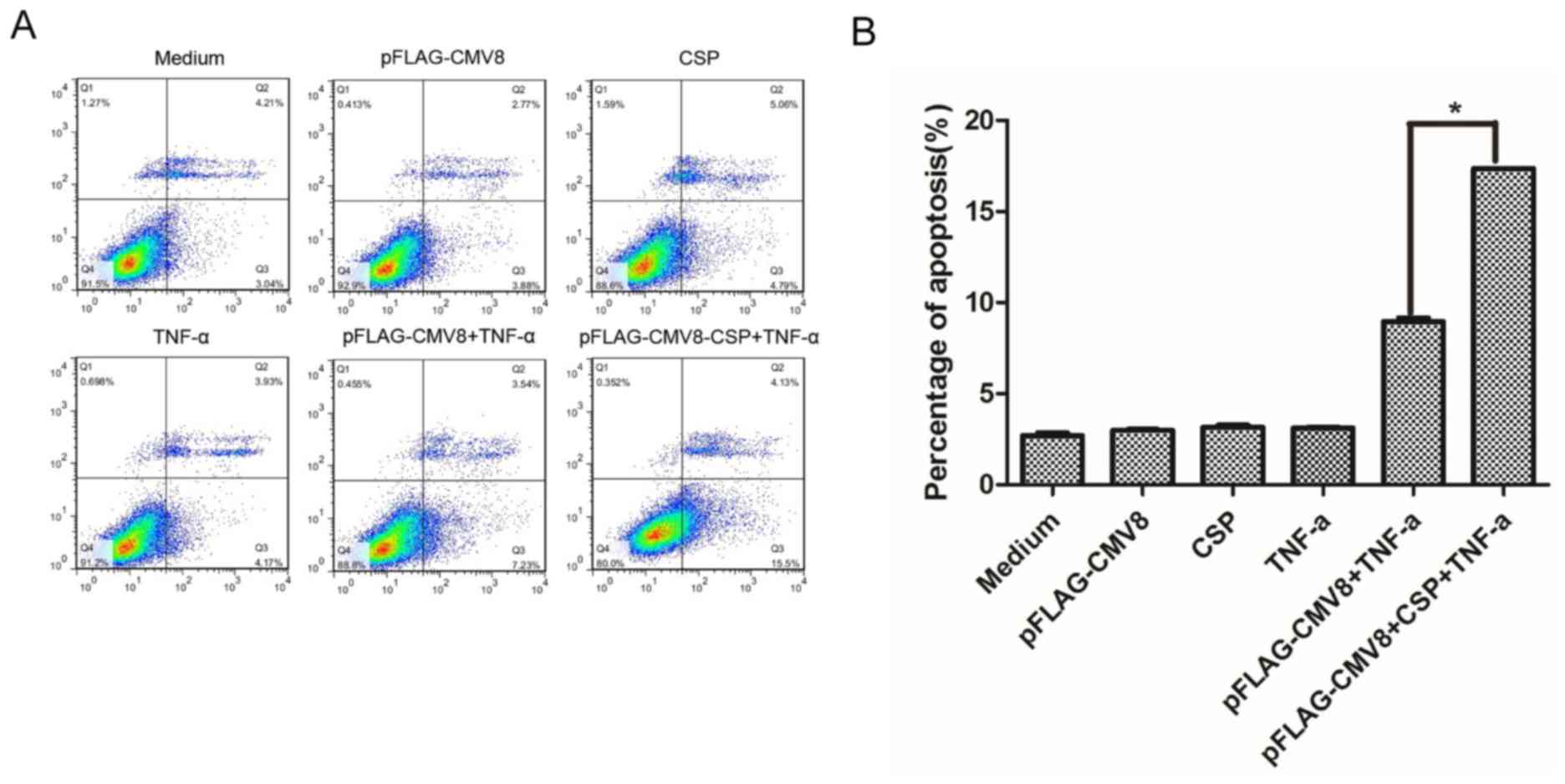
the apoptosis of A549 cells, an Annexin V-FITC apoptosis assay was
performed following the transfection of cells with 0.8 µg
pFLAG-CMV8-CSP and then stimulated with hTNF-α. As indicated in
Fig. 3, the apoptotic rate of A549
cells transfected with the control plasmid pFLAG-CMV8 was 3.5%,
comparable with the rate of cells that were transfected with
pFLAG-CMV8 or pFLAG-CMV8-CSP, or stimulated with hTNF-α alone.
However, the apoptotic rate of hTNF-α-stimulated A549 cells
transfected with pFLAG-CMV8-CSP was significantly higher compared
with the rate of cells that were transfected with pFLAG-CMV8 (17.4
vs. 3.5%, P<0.01). Therefore, the data indicated that CSP was
able to induce the apoptosis of A549 cells.
CSP suppresses TNF-α-induced
activation of NF-κB in A549 cells through its NLS domain
As NF-κB is important for survival and proliferation
of tumor cells (8), the effect of the
CSP on NF-κB activation in A549 cells was investigated. A NF-κB
reporter gene assay indicated that transfection with pFLAG-CMV8-CSP
was able to suppress the activation of NF-κB in hTNF-α-induced A549
cells (P=0.008; Fig. 4A). CSP is a
critical protein for the invasion of sporozoites into hepatocytes
and includes important motifs, including region I, region II+ and
region III (Fig. 3B) (19). Previously, a NLS motif with a sequence
of VRVRKNVN was reported in CSP (18)
(Fig. 4B). To determine the region
responsible for NF-κB inactivation by CSP, the recombinant plasmids
pFLAGCMV8-CSP NLS (containing only the NLS motif) and
pFLAG-CMV8-CSPΔNLS (CSP lacking the NLS motif) were constructed
(Fig. 3B), and their inhibitory role
on the activation of NF-κB in hTNF-α-induced A549 cells was
observed. As shown in Fig. 4C,
transfection of pFLAGCMV8-CSPΔNLS was not able to inhibit NF-κB
activation in hTNF-α-stimulated A549 cells, compared with that
transfected with pFLAG-CMV8 or pFLAG-CMV8-CSP. However,
transfection with pFLAG-CMV8-CSP or pFLAG-CMV8-CSP NLS was able to
reduce NF-κB activity of A549 cell by >3.5 fold, when compared
with pFLAG-CMV8 plasmid. Therefore, the data supported the
essential role of NLS for the inactivation of NF-κB by CSP.
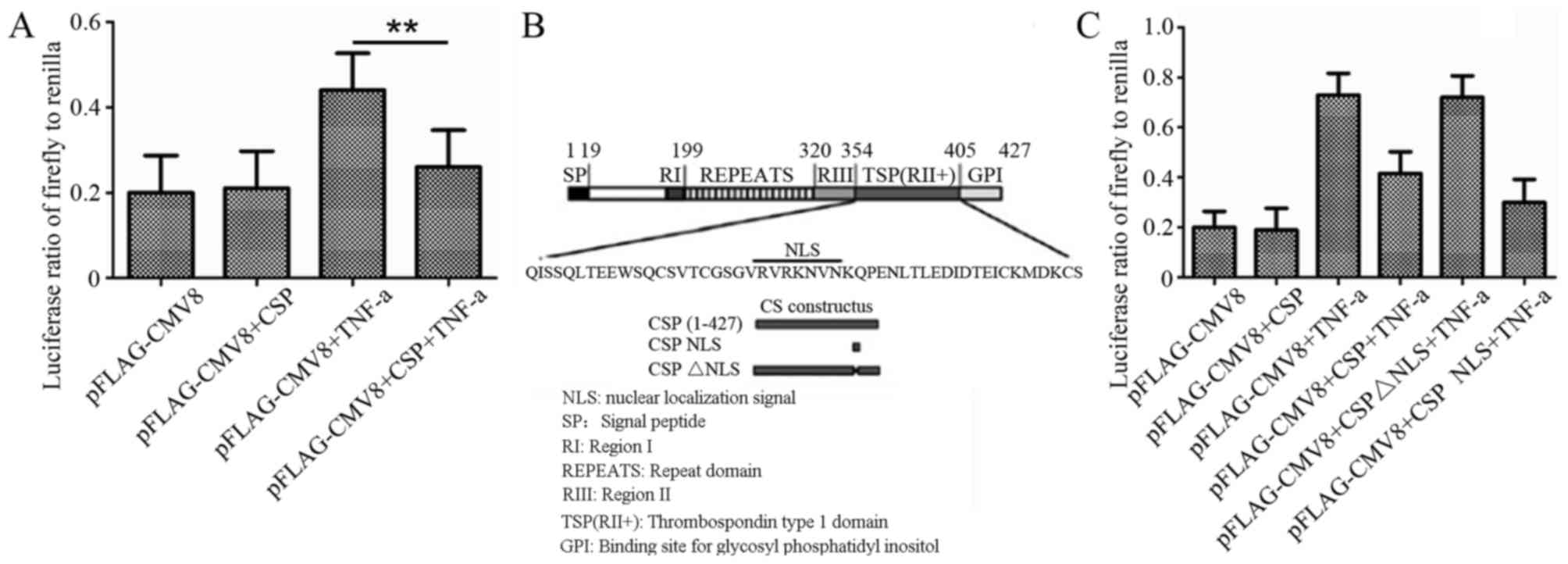 | Figure 4.Effects of CSP on NF-κB activation in
A549 cells. (A) Effects of inhibition of CSP on the activation of
NF-κB in TNF-α-stimulated A549 cells. The data are expressed as the
ratio of firefly luciferase to Renilla luciferase.
**P<0.01. (B) Schematic representation of the P. yoelii
CSP. SP, RI, RII+, RIII and REPEATS are conserved regions of CS.
The numbers indicate the position of amino acids. The bottom panel
indicates the location of the NLS in the CSP and a representation
of the constructed recombinant plasmids. (C) Following transfection
of A549 cells with pBIIx-luc and TK-RL, together with or without
pFLAG-CMV8, pFLAG-CMV8-CSP or pFLAG-CMV8-CSP NLS or
pFLAG-CMV8-CSPΔNLS for 24 h, the cells were incubated with or
without 100 ng/ml hTNF-α for 6 h. The activity of firefly and
Renilla luciferase was detected. The data are expressed as
the ratio of firefly luciferase to Renilla luciferase. CSP,
circumsporozoite protein; NF-κB, nuclear factor κB; NLS, nuclear
localization signal; SP, signal peptide; TNF, tumor necrosis
factor; Δ, deletion; GPI, glycosylphosphatidylinositol attachment
site. |
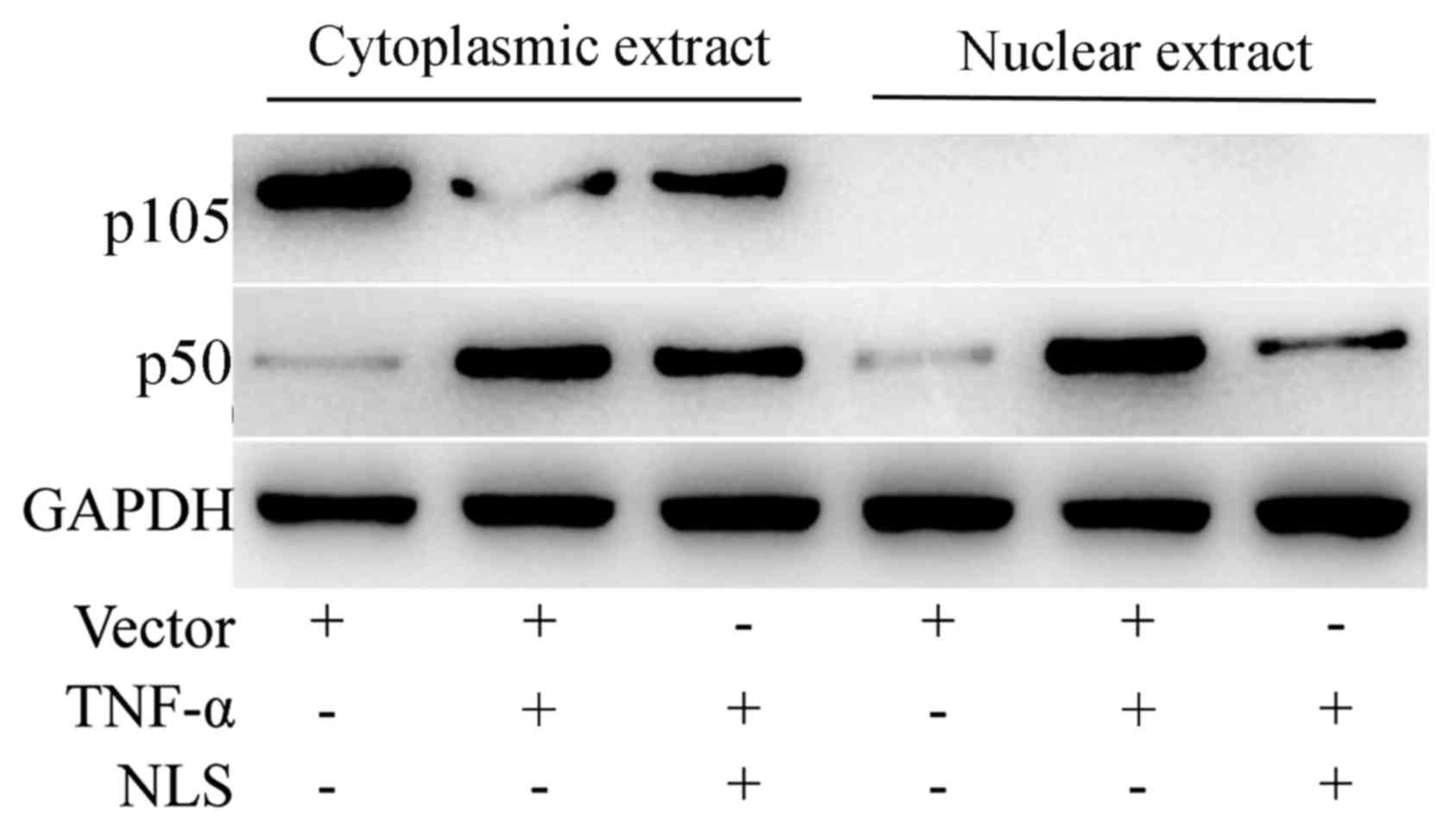
NLS of CSP outcompetes the nuclear
translocation of NF-κB in A549 cells following TNF-α induction
Proteins with NLS are imported into the cell nucleus
through the nuclear pore complex (20). It was hypothesized that CSP may
inhibit the nuclear translocation of NF-κB through its NLS motif.
The cytoplasmic and nuclear extracts were probed with NF-κB
p105/p50 antibody following separation on SDS-PAGE. As indicated in
Fig. 5, although the cytoplasmic
NF-κB p50 was at a comparable level in A549 cells transfected with
either pFLAG-CMV8 or pFLAG-CMV-CSPNLS, the level of NF-κB p50 in
the nuclear extract of A549 cells transfected with pFLAG-CMV-CSP
NLS was markedly lower compared with cells transfected with
pFLAG-CMV8. Therefore, the data suggested that NLS did not affect
the phosphorylation of IκB, and the subsequent release of NF-κB
from the inhibitory complex, but it may inhibit the nuclear
translocation of the activated NF-κB.
Discussion
The aim of the present study was to determine
whether CSP was able to inhibit the growth of the human cancer cell
A549. The data demonstrated that CSP was observed to be primarily
distributed in the cytoplasm of A549 cells following transfection,
and that CSP was able to suppress the proliferation and induce the
apoptosis of A549 cells. The inhibitory role of CSP on the growth
of A549 cells may be dependent on whether the CSP NLS motif is able
to outcompete the nuclear translocation of NF-κB.
CSP has an important role in the sporozoite invasion
of hepatocytes and is regarded as an immunodominant protective
antigen of irradiation-attenuated sporozoite (21). It was found that CSP was able to
significantly suppress the growth of human lung cancer cell line
A549 in a dose-dependent manner (Fig.
2) and induce its apoptosis (Fig.
3). It remains to be established whether antiapoptotic genes
(22), including B-cell lymphoma 2,
cellular FLICE-like inhibitory protein, survivin and IAP-1, are
also involved in this process, and whether CSP is also able to
suppress the growth of other human lung cancer cell lines. However,
the present study has demonstrated a potential novel role of CSP in
inhibiting the growth of tumor cells.
NF-κB activation is critical for the proliferation,
survival and metastasis of cancer cells, thus it was investigated
whether the inhibition of CSP on growth of A549 cells was
associated with CSP-mediated blockage of NF-κB nuclear
translocation. It was indicated that the transfection of
pFLAG-CMV-CSP or pFLAG-CMV-CSP NLS was able to markedly reduce the
activity of NF-κB, at comparable levels to the activity of
hTNF-α-induced A549 cells (Fig. 3B).
Furthermore, the NLS motif of the CSP, but not other sequences,
inhibited the nuclear translocation of NF-κB in tumor cells
(Fig. 4). Thus, the data indicated
that the inhibitory role of CSP on the growth of A549 cells may be
attributed to the ability of the CSP NLS motif to outcompete the
nuclear translocation of NF-κB in tumor cells.
However, other mechanisms of CSP on tumor growth
could not be excluded, as CSP was previously reported to modulate
non-NF-κB target genes (18) and
inhibit protein synthesis by binding to the ribosome (23).
Inhibition of NF-κB activation is a promising
antitumor strategy (24,25). Several NF-κB inhibitors have been
reported to treat various tumors. Curcumin, which inhibits at a
step prior to IκB phosphorylation, may suppress the growth of human
multiple myeloma cells, and head and neck squamous cell carcinoma
(26,27), as well as sensitize colorectal cancer
and breast cancer cells to radiation and chemotherapy (28–31). It
was reported that curcumin does not specifically target tumor cells
and also have a toxic effect on normal tissues, including the
immune system, when administrated in vivo (26). Recently, a small molecule inhibitor of
IKKβ, KINK-1, was reported to increase the susceptibility of
melanoma cells to chemotherapy through specifically inhibiting the
canonical pathway of NF-κB activation involved in tumor progression
(32). Although KINK-1 has no
unwanted side effects on adaptive immunity mediated by the
non-canonical pathway, its side effects on innate immunity cannot
be completely avoided (33). In the
present study, a novel protein-based NF-κB inhibitor, CSP, which is
a main surface protein of the malarial sporozoite, was reported to
suppress the growth of A549 cells. Evidence has suggested that the
specificity of gene therapy on tumor cells can be achieved by
placing the therapeutic gene downstream of a tumor-specific
promoter, such as hTERT (34,35). Thus, it would be possible to
specifically suppress the NF-κB activation in tumor cell, but not
the immune system through engineering the CSP into a vector
containing a tumor-specific promoter. However, whether the design
would work requires further research.
In summary, the data of the present study
demonstrated that the Plasmodium yoelii BY265 CSP was able
to suppress the growth of A549 human lung cancer cells, potentially
via its NLS domain, which outcompetes the nuclear translocation of
NF-κB. Although further investigations regarding the effects of CSP
on the invasive ability of lung cancer cells as well as its
antitumor efficiency in vivo are necessary, the potential
ability of the CSP to act as a novel NF-κB inhibitor for the
treatment of lung cancer should be investigated.
Acknowledgements
The present study was supported by funding from the
General Program of National Natural Science Foundation of China
(grant nos. 81172238 and 81472188). The present study was also
supported by the Third Military Medical University.
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 36:E359–E386. 2015.
View Article : Google Scholar
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gilmore TD and Wolenski FS: NF-κB: Where
did it come from and why? Immunol Rev. 246:14–35. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hayden MS and Ghosh S: NF-κB in
immunobiology. Cell Res. 21:223–244. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hayden MS and Ghosh S: Regulation of NF-κB
by TNF family cytokines. Semin Immunol. 26:253–266. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hayden MS and Ghosh S: Signaling to
NF-kappaB. Genes Dev. 18:2195–2224. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Maeda S and Omata M: Inflammation and
cancer: Role of nuclear factor-kappaB activation. Cancer Sci.
99:836–842. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pikarsky E, Porat RM, Stein I, Abramovitch
R, Amit S, Kasem S, Gutkovich-Pyest E, Urieli-Shoval S, Galun E and
Ben-Neriah Y: NF-kappaB functions as a tumour promoter in
inflammation-associated cancer. Nature. 431:461–466. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Greten FR, Eckmann L, Greten TF, Park JM,
Li ZW, Egan LJ, Kagnoff MF and Karin M: IKKbeta links inflammation
and tumorigenesis in a mouse model of colitis-associated cancer.
Cell. 118:285–296. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nakshatri H, Bhat-Nakshatri P, Martin DA,
Goulet RJ Jr and Sledge GW Jr: Constitutive activation of NF-kappaB
during progression of breast cancer to hormone-independent growth.
Mol Cell Biol. 17:3629–3639. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lindholm PF, Bub J, Kaul S, Shidham VB and
Kajdacsy-Balla A: The role of constitutive NF-kappaB activity in
PC-3 human prostate cancer cell invasive behavior. Clin Exp
Metastasis. 18:471–479. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang W, Abbruzzese JL, Evans DB, Larry L,
Cleary KR and Chiao PJ: The nuclear factor-kappa B RelA
transcription factor is constitutively activated in human
pancreatic adenocarcinoma cells. Clin Cancer Res. 5:119–127.
1999.PubMed/NCBI
|
|
13
|
Sakamoto K, Maeda S, Hikiba Y, Nakagawa H,
Hayakawa Y, Shibata W, Yanai A, Ogura K and Omata M: Constitutive
NF-kappaB activation in colorectal carcinoma plays a key role in
angiogenesis, promoting tumor growth. Clin Cancer Res.
15:2248–2258. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hagemann T, Wilson J, Kulbe H, Li NF,
Leinster DA, Charles K, Klemm F, Pukrop T, Binder C and Balkwill
FR: Macrophages induce invasiveness of epithelial cancer cells via
NF-kappa B and JNK. J Immunol. 175:1197–1205. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Stathopoulos GT, Sherrill TP, Han W,
Sadikot RT, Yull FE, Blackwell TS and Fingleton B: Host nuclear
factor-kappaB activation potentiates lung cancer metastasis. Mol
Cancer Res. 6:364–371. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Luo JL, Maeda S, Hsu LC, Yagita H and
Karin M: Inhibition of NF-kappaB in cancer cells converts
inflammation-induced tumor growth mediated by TNFalpha to
TRAIL-mediated tumor regression. Cancer Cell. 6:297–305. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang J, Pan WH, Clawson GA and Richmond A:
Systemic targeting inhibitor of kappaB kinase inhibits melanoma
tumor growth. Cancer Res. 67:3127–3134. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Singh AP, Buscaglia CA, Wang Q, Levay A,
Nussenzweig DR, Walker JR, Winzeler EA, Fujii H, Fontoura BM and
Nussenzweig V: Plasmodium circumsporozoite protein promotes the
development of the liver stages of the parasite. Cell. 131:492–504.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kappe SH, Buscaglia CA and Nussenzweig V:
Plasmodium sporozoite molecular cell biology. Annu Rev Cell Dev
Biol. 20:29–59. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kalderon D, Roberts BL, Richardson WD and
Smith AE: A short amino acid sequence able to specify nuclear
location. Cell. 39:499–509. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kumar KA, Sano G, Boscardin S, Nussenzweig
RS, Nussenzweig MC, Zavala F and Nussenzweig V: The
circumsporozoite protein is an immunodominant protective antigen in
irradiated sporozoites. Nature. 444:937–940. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Karin M: Nuclear factor-kappaB in cancer
development and progression. Nature. 441:431–436. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Frevert U, Galinski MR, Hügel FU, Allon N,
Schreier H, Smulevitch S, Shakibaei M and Clavijo P: Malaria
circumsporozoite protein inhibits protein synthesis in mammalian
cells. EMBO J. 17:3816–3826. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Karin M and Greten FR: NF-kappaB: Linking
inflammation and immunity to cancer development and progression.
Nat Rev Immunol. 5:749–759. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Baud V and Karin M: Is NF-kappaB a good
target for cancer therapy? Hopes and pitfalls. Nat Rev Drug Discov.
8:33–40. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bharti AC, Donato N, Singh S and Aggarwal
BB: Curcumin (diferuloylmethane) down-regulates the constitutive
activation of nuclear factor-kappa B and IkappaBalpha kinase in
human multiple myeloma cells, leading to suppression of
proliferation and induction of apoptosis. Blood. 101:1053–1062.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
LoTempio MM, Veena MS, Steele HL,
Ramamurthy B, Ramalingam TS, Cohen AN, Chakrabarti R, Srivatsan ES
and Wang MB: Curcumin suppresses growth of head and neck squamous
cell carcinoma. Clin Cancer Res. 11:6994–7002. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kunnumakkara AB, Guha S, Krishnan S,
Diagaradjane P, Gelovani J and Aggarwal BB: Curcumin potentiates
antitumor activity of gemcitabine in an orthotopic model of
pancreatic cancer through suppression of proliferation,
angiogenesis, and inhibition of nuclear factor-kappaB-regulated
gene products. Cancer Res. 67:3853–3861. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sandur SK, Deorukhkar A, Pandey MK, Pabón
AM, Shentu S, Guha S, Aggarwal BB and Krishnan S: Curcumin
modulates the radiosensitivity of colorectal cancer cells by
suppressing constitutive and inducible NF-kappaB activity. Int J
Radiat Oncol Biol Phys. 75:534–542. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kunnumakkara AB, Diagaradjane P, Guha S,
Deorukhkar A, Shentu S, Aggarwal BB and Krishnan S: Curcumin
sensitizes human colorectal cancer xenografts in nude mice to
gamma-radiation by targeting nuclear factor-kappaB-regulated gene
products. Clin Cancer Res. 14:2128–2136. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Aggarwal BB, Shishodia S, Takada Y,
Banerjee S, Newman RA, Bueso-Ramos CE and Price JE: Curcumin
suppresses the paclitaxel-induced nuclear factor-kappaB pathway in
breast cancer cells and inhibits lung metastasis of human breast
cancer in nude mice. Clin Cancer Res. 11:7490–7498. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Schön M, Wienrich BG, Kneitz S,
Sennefelder H, Amschler K, Vöhringer V, Weber O, Stiewe T,
Ziegelbauer K and Schön MP: KINK-1, a novel small-molecule
inhibitor of IKKbeta, and the susceptibility of melanoma cells to
antitumoral treatment. J Natl Cancer Inst. 100:862–875. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Moschos SJ, Chaudhary PM and Kirkwood JM:
Resolving ‘kinks’ of chemotherapy in melanoma. J Natl Cancer Inst.
100:833–835. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bilsland AE, Anderson CJ,
Fletcher-Monaghan AJ, McGregor F, Evans TR, Ganly I, Knox RJ, Plumb
JA and Keith WN: Selective ablation of human cancer cells by
telomerase-specific adenoviral suicide gene therapy vectors
expressing bacterial nitroreductase. Oncogene. 22:370–380. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wirth T, Zender L, Schulte B, Mundt B,
Plentz R, Rudolph KL, Manns M, Kubicka S and Kühnel F: A
telomerase-dependent conditionally replicating adenovirus for
selective treatment of cancer. Cancer Res. 63:3181–3188.
2003.PubMed/NCBI
|