Introduction
Ovarian carcinoma is one of the most common
malignancies of the female reproductive system and remains a major
global health problem. In 2012, 238,700 new cases were diagnosed,
and 151,900 females succumbed to this disease worldwide (1). One of the most common types of ovarian
cancer is epithelial ovarian cancer (EOC), which accounts for ~90%
of all cases (2). Due to the lack of
early diagnostic methods EOC is usually diagnosed at a late stage,
reducing the chance of successful treatment (3). The primary factor that limits the
efficacy of treatment in EOC is chemotherapy resistance. In
previous years, there have been advances in medical treatments and
several novel chemotherapeutic regimens have been identified in
EOC; however, these continue to result in poor prognoses.
Therefore, there is a requirement to characterize the pathogenesis
of EOC and identify novel and efficient prognostic markers.
Interferon regulatory factor-4 binding protein
(IBP), which had been isolated from the human cDNA library, was
first identified during the search for the potential partner of the
lymphoid-restricted transcription factor interferon regulatory
(IRF-4) in 2003 (4). IBP is primarily
expressed in lymphocytes and located in the cytoplasm. As IBP is a
type of protein that participates in the formation of T
cell-mediated immunity synapses under T-cell receptor signal
stimulation and associates with the activity of T-helper cell (Th)2
cells, early studies of IBP focused on the lymphatic system
(4,5).
Through the inhibition of IRF-4 transcription factor activity, IBP
may also decrease the generation of interleukin-17/21, and affect
the function of Th17 cells (6,7). In
addition, IBP participates in the regulation of the Toll-like
receptor signal transduction pathway, in which mitogen-activated
protein kinase and nuclear factor-κB are involved. Therefore, IBP
serves an important function in maintaining immune homeostasis.
IBP-knockout mice exhibited symptoms of autoimmune disease
(8). Gene expression profiles
demonstrate that IBP is one of the top five differentially
expressed genes in extraskeletal myxoid chondrosarcoma (9). IBP may also be considered as a guanine
nucleotide exchange factor (GEF), which activates the ρ GTPase
family members, including ras-related C3 botulinum toxin substrate
1 (Rac1), cell division control protein 42 homolog and ras homolog
gene family, member A (10). The ρ
GTPase family serves an important function in the metabolism of
tumor cells, and the progression of tumor proliferation, migration
and invasion (11). In addition, the
high expression of GEF and the guanosine triphosphate (GTP) ρ
enzyme family are often associated with tumor occurrence (11). Therefore, IBP molecules may have a
direct or indirect effect on cytoskeleton remodeling, and cell
transmission mechanism, which serves a certain function in tumor
development. Consequently, IBP may serve as a novel and efficient
prognostic marker. At present, previous studies investigating IBP
and its effects in tumors are limited. Existing data suggests that
IBP may promote the development of breast cancer and colorectal
cancer, and increases the invasiveness of oral squamous cell
carcinoma (12–14). However, the expression and prognostic
value of IBP in ovarian cancer remain unknown.
The present study aimed to reveal the expression of
IBP in epithelial ovarian cancer and its association with
clinicopathological features, then determine whether IBP may
perform as a novel prognostic factor of EOC.
Materials and methods
Patients and tissue samples
All the paraffin-embedded samples and clinical data
were selected from the Department of Gynecologic Oncology, Third
Affiliated Hospital of Harbin Medical University (Harbin, China)
between January 2008 and January 2011. The inclusion criteria of
ovarian cancer patients were as follows: i) Diagnosed with ovarian
cancer via pathology; ii) did not suffer from other malignant
diseases; and iii) patients had not undergone any preoperative
treatment, including chemotherapy or radiotherapy. Exclusion
criteria were listed as: i) Was diagnosed with serious systemic
disease; ii) the tumor had distant metastasis; iii) the patient had
not received a radical resection. A total of 107 patients who met
the aforementioned criteria were enrolled in the present study aged
22–76 years old (with a median age of 54 years old). All patients
with ovarian cancer were treated with cytoreductive surgery, which
was followed by six courses of standard platinum-based combination
chemotherapy, consisting of six courses of treatment with three
weeks between each course.
The surgical staging of all patients was confirmed
based on The International Federation of Gynecology and Obstetrics
stage (FIGO) and subsequently classified into early (I–II) and
advanced stages (III–IV) (15).
Histological type and tumor grade were based on the World Health
Organization classification standard (16). Histological grade (tumor
differentiation) was determined in accordance with the standard
Silverberg grading system (17), and
classified into low (G1) and high levels (G2-G3). A complete set of
all parameters was obtained; these parameters included patient age,
histological grade, clinical stage, CA-125 level, ascites volume,
lymph-node metastasis and peritoneal carcinomatosis, and whether
recurrence, overall survival (OS), and recurrence-free survival
(RFS) occurred. The study protocol was approved by the Ethics
Committee of the Third Affiliated Hospital of Harbin Medical
University and all patients provided written informed consent.
For survival analysis, all enrolled patients with
ovarian cancer were followed up periodically until mortality or up
to 5 years following surgery. The median follow-up time was 48
months (ranging from 3–60 months). Within 2 years following
surgery, examinations, including serum cancer antigen (CA)-125,
pelvic magnetic resonance imaging, chest X-ray and color Doppler
ultrasound of abdomen, were performed every 3 months. During the
postoperative period of 3–5 years, the aforementioned tests were
repeated once every 6 months and annually thereafter. RFS was
defined as the time span between the date of surgery and diagnosed
recurrence or distant metastasis. OS was measured as the time
interval from the date of surgery to mortality, which included
all-cause mortality or the termination of the follow-up period of
the present study.
Immunohistochemical (IHC)
staining
Slices (4-µm thick) were cut from paraffin-embedded
specimens stored by the pathology department of The Third
Affiliated Hospital of Harbin Medical University (Harbin, China),
and hematoxylin and eosin (HE) staining was conducted on these
samples. IHC staining was performed on tissue slices adjacent to
the HE-stained section using an avidin-biotin immunoperoxidase
technique, as follows. These sections were incubated at 80°C for 30
min, deparaffinized with xylene, and rehydrated with different
alcohol concentrations (Analytically pure ethanol concentrations
were: 100, 95 and 80%, each for 5–10 min). All dewaxed slices were
immersed in 0.01 mmol/l citrate buffer (pH 6.0) and maintained in
high-pressure steam at 121°C for 4 min to fix antigenicity.
Subsequently, the slices were cooled to room temperature. To remove
the endogenous peroxidase activity, the slices were placed in 3%
hydrogen peroxide for 10 min at room temperature. Tissue sections
were incubated with IBP-specific mouse monoclonal antibodies
(dilution 1:100; Abcam, Cambridge, UK; cat. no. ab57228) at 4°C
overnight, washed three times (5 min each) with PBS, and finally
incubated at 37°C with biotin-labeled secondary antibody (goat
anti-mouse immunoglobulin G; undiluted; cat. no. Pv6000; OriGene
Technologies, Inc., Rockville, MD, USA) and horseradish
peroxidase-conjugated streptavidin for 20 min. All slices were
immersed in 3,3 diaminobenzidine tetrahydrochloride (Dako; Agilent
Technologies, Inc., Santa Clara, CA, USA) at 37°C, which was
followed by Meyer hematoxylin (undiluted) at room temperature for 1
min to counterstain. The negative control sections were incubated
at 4°C overnight with rabbit serum (undiluted; cat. no. AR0010;
Boster Biological Technology Co., Ltd., Wuhan, China), instead of
primary antibodies.
Evaluation of IBP expression by
IHC
All slices were independently assessed by two
experienced pathologists who were blind to the clinical pathology
results and other patient information. IBP expression level was
evaluated via the proportion of positively stained cells and
intensity of tumor cells. The semi-quantitative evaluation criteria
were as follows: The score of all sections was based upon the
intensities and proportions of IBP staining in the tumor cells. The
percentage of positive tumor cells was scored according to the
following: 1, 0–10% of positive cells; 2, 11–50% of positive cells;
3, 51–80% of positive cells; and 4, >80% of the positive cells.
The IBP protein expression levels were scored as follows: 0,
unstained; 1, weak positive staining; 2, moderate staining; and 3,
marked staining. The final score was calculated by multiplying the
percentage score with the expression level score (0, 1, 2, 3, 4, 6,
8, 9 or 12). Scores of 0 were categorized as negative IBP staining,
scores between 1–4 were considered weak positives for IBP staining,
and scores >4 were classified as strong positives for IBP
staining. Slices with inconsistent results were re-examined by the
original two pathologists and a senior pathologist until a
consensus was reached.
Statistical analysis
All data are presented as the mean ± the standard
deviation. A χ2 test was used to analyze the association
between IBP overexpression and clinicopathological variables. OS
and RFS were calculated according to the Kaplan-Meier method, and a
univariate analysis of the log-rank test was used to evaluate the
differences among the levels of potential prognostic factors. In
the multivariate analysis, a Cox proportional hazard regression
model was used to assess the independent predictive factors of OS
and RFS. P<0.05 was considered to indicate a statistically
significant difference. All statistical analyses were performed
using SPSS version 19.0 software (IBM Corp., Armonk, NY, USA).
Results
IBP is highly expressed in patients
with epithelial ovarian carcinoma
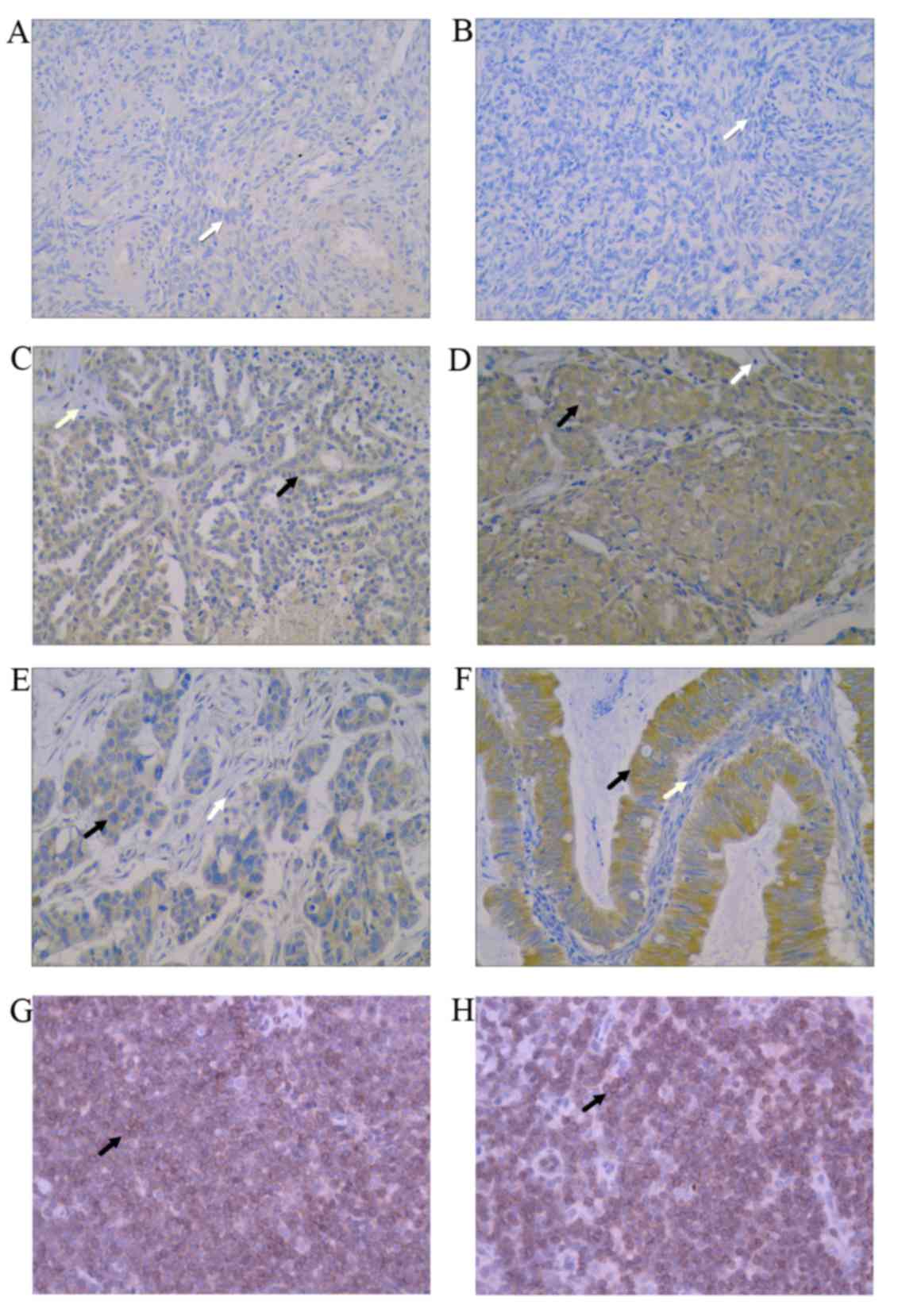
Representative immunostaining of IBP is demonstrated
in Fig. 1. The positive cells were
stained brown. Fig. 1A indicates the
negative expression of IBP in normal ovarian tissue and Fig. 1B exhibits the negative control in
ovarian carcinoma. Fig. 1C and D
demonstrate a low expression of IBP in low-level and high-level
serous epithelial ovarian carcinoma, respectively. Fig. 1E and F indicate a strong positive
expression of IBP in high-level serous epithelial ovarian carcinoma
and endometrioid epithelial ovarian carcinoma. Fig. 1G and H demonstrate positive expression
of IBP in lymphoid tissue. In the present study, IBP was not
expressed in normal ovary tissues (Fig.
1A). However, in the epithelial ovarian carcinoma tissue
samples, 39/107 (36.4%) slices exhibited low IBP expression, and
68/107 (63.6%) sections indicated high IBP expression. In
epithelial ovarian carcinoma tissue samples, as indicated in
Fig. 1, IBP staining appeared as
brown particles.
Association between IBP protein
expression and clinicopathological parameters
Table I summarizes the
associations between the expression of IBP in ovarian cancer and
clinicopathological variables. High IBP overexpression was
associated with high FIGO stage (P<0.001), histologic grade
(P=0.002), relapse rate (P<0.001), lymphatic metastasis
(P=0.023), and peritoneal carcinomatosis (P<0.001). In contrast,
no statistically significant associations between IBP and age
(P=0.120), histological type (P=0.054), preoperative serum CA-125
(P=0.301), ascites (P=0.587) or other pathological parameters were
observed.
 | Table I.Association between interferon
regulatory factor-4 binding protein overexpression and
clinicopathological characteristics of ovarian carcinoma. |
Table I.
Association between interferon
regulatory factor-4 binding protein overexpression and
clinicopathological characteristics of ovarian carcinoma.
|
|
| IBP expression |
|
|---|
|
|
|
|
|
|---|
| Variable | No. | Low, n (%) | High, n (%) | P-valuea |
|---|
| Age, years |
|
|
|
|
| ≤50 | 34 | 16 (47.1) | 18 (52.9) |
|
|
>50 | 73 | 23 (31.5) | 50 (68.5) | 0.120 |
| Histological
type |
|
|
|
|
|
Serous | 62 | 23 (37.1) | 39 (62.9) |
|
|
Mucinous | 19 | 5 (26.3) | 14 (73.7) |
|
|
Endometrioid | 21 | 11 (52.4) | 10 (47.6) |
|
| Clear
cell | 5 | 0 (0.0) | 5 (100.0) | 0.054 |
| Histological
grade |
|
|
|
|
| G1 | 16 | 11 (68.8) | 5 (31.2) |
|
|
G2/G3 | 91 | 26 (28.6) | 65 (71.4) | 0.002 |
| FIGO stage |
|
|
|
|
| I+II | 23 | 21 (91.3) | 2 (8.7) |
|
|
III+IV | 84 | 18 (21.4) | 66 (78.6) | <0.001 |
| Serum CA-125 level,
U/ml |
|
|
|
|
|
≤35 | 12 | 6 (50.0) | 6 (50.0) |
|
|
>35 | 95 | 33 (34.7) | 62 (65.3) | 0.301 |
| Lymph node
metastasis |
|
|
|
|
|
Yes | 62 | 17 (28.8) | 42 (71.2) |
|
| No | 45 | 22 (45.8) | 26 (54.2) | 0.023 |
| Peritoneal
metastasis |
|
|
|
|
|
Yes | 72 | 18 (25.0) | 54 (75.0) |
|
| No | 35 | 21 (60.0) | 14 (40.0) | <0.001 |
| Ascites, ml |
|
|
|
|
|
<100 | 43 | 17 (39.5) | 26 (60.5) |
|
|
≥100 | 64 | 22 (34.4) | 42 (65.6) | 0.587 |
| Recurrence |
|
|
|
|
|
Yes | 26 | 20 (76.9) | 6 (23.1) |
|
| No | 81 | 19 (23.5) | 62 (76.5) | <0.001 |
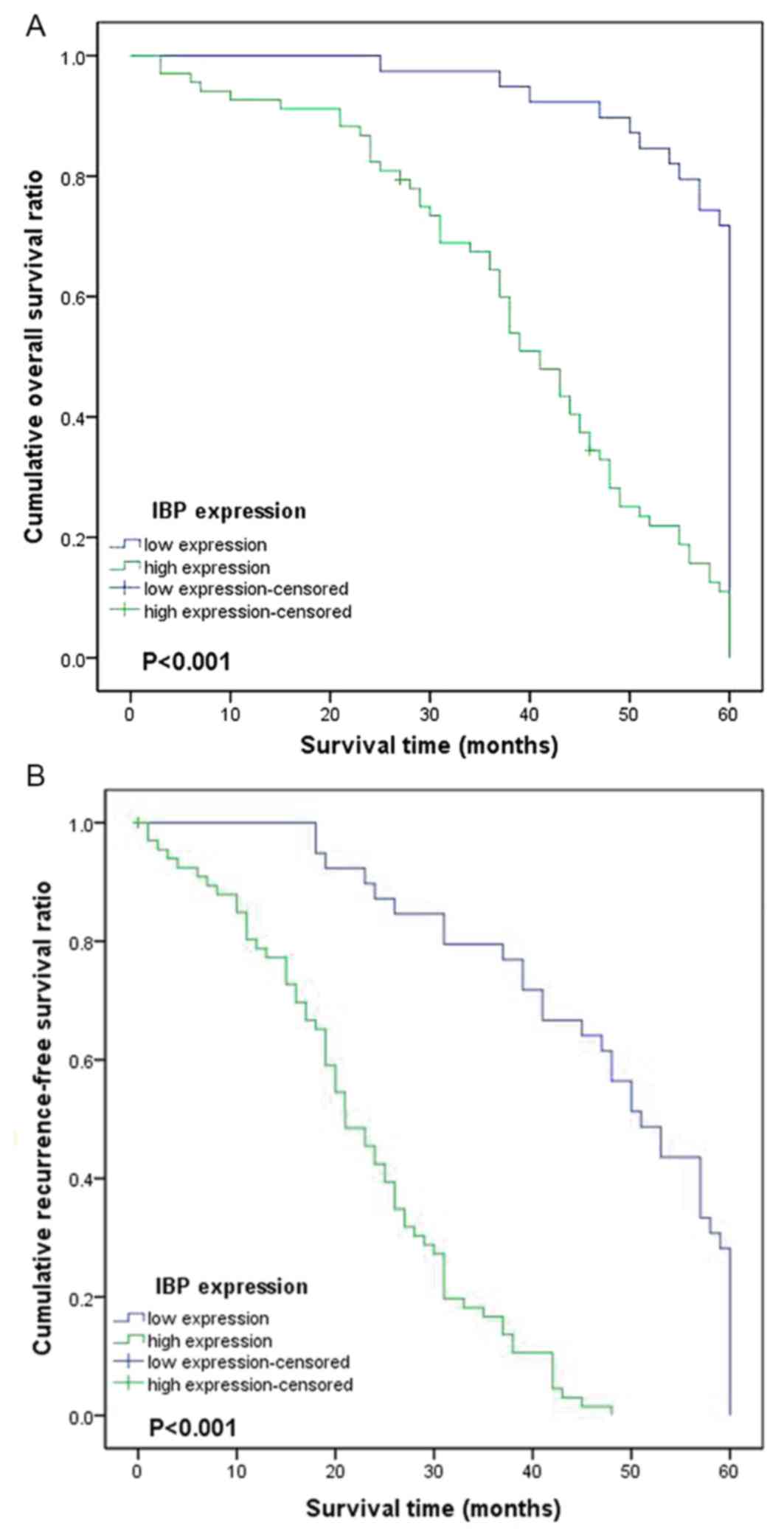
To investigate the potential clinical utility of IBP
overexpression, the association between IBP and OS, and RFS
outcomes in 107 cases of epithelial ovarian carcinoma specimens was
assessed. Kaplan-Meier and log-rank test methods were used, and it
was identified that a high expression of IBP was associated with
relatively short RFS and OS times compared with the low expression
group (P<0.001 and P<0.001, respectively; Table II; Fig.
2). In addition, alongside IBP overexpression, FIGO stage,
histological type, histological grade, lymph node metastasis and
peritoneal metastasis were associated with epithelial ovarian
cancer prognosis.
 | Table II.Univariate survival analysis of OS
and RFS in 107 patients with ovarian carcinoma. |
Table II.
Univariate survival analysis of OS
and RFS in 107 patients with ovarian carcinoma.
|
| OS, months | RFS, months |
|
|---|
|
|
|
|
|
|---|
| Variable | No. (n=107) | Mean ± SE | 95% CI |
P-valuea | Mean ± SE | 95% CI |
P-valuea |
|---|
| Age, years |
|
|
| 0.145 |
|
| 0.395 |
|
≤50 | 34 | 48.029±2.581 | 42.970–53.089 |
| 34.353±3.061 | 28.353–40.353 |
|
|
>50 | 73 | 44.715±1.827 | 41.133–48.296 |
| 30.535±2.014 | 26.589–34.482 |
|
| Histological
type |
|
|
| <0.001 |
|
| 0.005 |
|
Serous | 62 | 45.450±1.956 | 41.617–49.283 |
| 30.967±2.157 | 26.739–35.196 |
|
|
Mucinous | 19 | 48.105±3.589 | 41.070–55.140 |
| 33.474±4.239 | 25.164–41.783 |
|
|
Endometrioid | 21 | 49.125±3.145 | 42.961–55.289 |
| 36.700±3.844 | 29.165–44.235 |
|
| Clear
cell | 5 | 27.000±2.811 | 21.491–32.509 |
| 15.400±3.750 | 8.051–22.749 |
|
| Histological
grade |
|
|
| 0.002 |
|
| 0.003 |
| G1 | 16 | 57.188±1.239 | 54.759–59.616 |
| 46.688±3.454 | 39.917–53.458 |
|
|
G2/G3 | 91 | 43.761±1.656 | 40.514–47.007 |
| 29.090±1.748 | 25.663–32.516 |
|
| FIGO stage |
|
|
| <0.001 |
|
| <0.001 |
|
I+II | 23 | 59.000±1.000 | 57.040–60.960 |
| 53.261±1.951 | 49.437–57.085 |
|
|
III+IV | 84 | 42.108±1.676 | 38.823–45.392 |
| 25.744±1.526 | 22.752–28.735 |
|
| Serum CA-125 level,
U/ml |
|
|
| 0.451 |
|
| 0.519 |
|
≤35 | 12 | 49.667±3.722 | 42.371–56.962 |
| 34.417±5.391 | 23.849–44.984 |
|
|
>35 | 95 | 45.289±1.614 | 42.125–48.453 |
| 31.430±1.779 | 27.944–34.916 |
|
| Lymph node
metastasis |
|
|
| 0.001 |
|
| 0.001 |
|
Yes | 62 | 41.455±2.060 | 37.148–45.492 |
| 26.672±2.103 | 22.551–30.793 |
|
| No | 45 | 51.696±1.814 | 48.141–55.251 |
| 38.841±2.415 | 34.108–43.574 |
|
| Peritoneal
metastasis |
|
|
| <0.001 |
|
| <0.001 |
|
Yes | 72 | 41.376±1.861 | 37.729–45.023 |
| 25.757±1.705 | 22.415–29.099 |
|
| No | 35 | 54.714±1.685 | 51.411–58.017 |
| 43.800±2.805 | 38.303–49.297 |
|
| Ascites, ml |
|
|
| 0.102 |
|
| 0.070 |
|
<100 | 43 | 48.958±2.055 | 44.931–52.986 |
| 35.341±2.752 | 29.948–40.735 |
|
|
≥100 | 64 | 43.672±2.050 | 39.654–47.690 |
| 29.484±2.097 | 25.375–33.594 |
|
| IBP expression |
|
|
| <0.001 |
|
| <0.001 |
|
Low | 39 | 56.718±1.208 | 54.350–59.086 |
| 47.103±2.244 | 42.705–51.500 |
|
|
High | 68 | 39.401±1.852 | 35.771–43.031 |
| 22.712±1.445 | 19.880–25.544 |
|
Parameters identified as significant in the
univariate Kaplan-Meier analysis were included in the multivariate
Cox regression model. The multivariate Cox regression analysis
indicated that IBP may serve as an independent prognostic marker
for OS [hazard ratio (HR)=2.317; 95% confidence interval (CI),
1.484–3.617; P<0.001] and RFS (HR=4.099; 95% CI, 2.209–7.606;
P<0.001) in EOC (Table III).
Furthermore, FIGO stage (P=0.029 and P=0.002, respectively), lymph
node (P=0.022 and P=0.013, respectively), and peritoneal metastasis
(P=0.014 and P=0.020, respectively) were significantly associated
with OS and RFS. However, histological grade and histological type
were not independent predictive factors for RFS and OS.
 | Table III.Multivariate Cox regression analysis
for various potential prognostic characteristics of OS and RFS in
107 patients with ovarian carcinoma. |
Table III.
Multivariate Cox regression analysis
for various potential prognostic characteristics of OS and RFS in
107 patients with ovarian carcinoma.
|
| OS | RFS |
|---|
|
|
|
|
|---|
| Variable | Exp (B) | 95% CI |
P-valuea | Exp (B) | 95% CI |
P-valuea |
|---|
| FIGO stage | 1.803 | 1.062–3.059 | 0.029 | 2.623 | 1.426–4.823 | 0.002 |
| Histological
type | 1.119 | 0.886–1.412 | 0.337 | 1.120 | 0.891–1.407 | 0.332 |
| Histological
grade | 1.240 | 0.701–2.193 | 0.460 | 1.651 | 0.942–2.893 | 0.080 |
| Lymph node
metastasis | 1.587 | 1.067–2.359 | 0.022 | 1.679 | 1.114–2.530 | 0.013 |
| Peritoneal
metastasis | 1.725 | 1.118–2.660 | 0.014 | 1.732 | 1.089–2.755 | 0.020 |
| IBP | 2.317 | 1.484–3.617 | <0.001 | 4.099 | 2.209–7.606 | <0.001 |
Discussion
To the best of our knowledge, the present study was
the first to reveal the expression of IBP in primary untreated
epithelial ovarian carcinoma and normal ovarian tissue through IHC
using paraffin-embedded samples. In addition, the present study
investigated the association of IBP overexpression with
clinicopathological factors and prognosis of patients with ovarian
cancer. Analysis on the experimental data of 107 patients with
ovarian cancer indicated that high IBP expression was associated
with tumor recurrence, metastasis and a shorter OS or RFS time
These data indicate that IBP may be an independent prognostic
factor for epithelial ovarian carcinoma.
In previous years, the expression of IBP has been
explored: Several studies have identified that IBP was
overexpressed, and served an important role in several types of
carcinomas, including breast cancer (13,18),
colorectal cancer (12) and oral
squamous cell carcinoma (OSCC) (14).
However, prior to the present study, the expression and prognostic
value of IBP in epithelial ovarian carcinoma was unclear. In the
present study, the association between IBP expression and
clinicopathological features in EOC was analyzed. It was observed
that IBP was markedly expressed in EOC specimens, but not in normal
ovarian tissue. High IBP expression was significantly associated
with high FIGO stage, poor differentiation, high relapse rate,
lymphatic metastasis, histological type and peritoneal
carcinomatosis. In addition, the Kaplan-Meier method and log-rank
test data also suggested that the patients with high IBP expression
exhibited significantly poor OS, and RFS in comparison with
patients with low expression of IBP. The Cox proportional hazard
regression model demonstrated that IBP was an independent
prognostic factor for OS and RFS in patients with EOC. This data
suggested that IBP serves an important role in the carcinogenesis
and tumor progression of EOC, and therefore may be a promising
prognostic marker. These results are in accordance with previous
studies demonstrating the roles of IBP in tumor progression in
breast cancer (13,18), colorectal cancer (12) and oral squamous cell carcinoma
(14), and suggest an association
between high IBP expression and unfavorable biological behavior in
EOC including poor histological grade, high FIGO stage, lymph node
and peritoneal metastasis. All these results suggest that IBP
serves an important biological role in carcinogenesis and tumor
progression.
At present, certain indicators attempt to explain
the mechanisms by which IBP promotes cancer development.
Specifically, IBP and GEF family molecules have similar structures.
Therefore, IBP may exhibit GEF-like characteristics. GEF is a
member of the diffuse B-cell lymphoma protein family that promotes
the GDP/GTP exchange reaction converting inactivated GDP-ρ into
GTP-ρ, and participating in the regulation of ρ GTPase family
(19). The ρ GTPase family is
involved in the regulation of a number of physiological process,
including the motility and polarity of the cell, cell
proliferation, formation of cytoskeleton, cell cycle and
cross-membrane signal transfer (20–22). ρ
GTPase also serves an important function in the apoptosis,
division, metabolism, proliferation, migration and invasion
processes of tumor cells by regulating the gene transcriptional
activity (23). In addition, it may
regulate the interaction between tumor cells and surrounding
stromal cells (23,24). Furthermore, the tumorigenic activity
of ρ GTPase family is reflected in its excessive expression in
various types of tumors (23,25,26).
Saurin et al (27)
demonstrated that the ρ GTPase family was involved in the
migration, invasion and resistance of colon cancer. Zhang et
al (18) suggested that D4-GDI, a
type of ρ GTPase family regulatory factor, may promote the invasion
of breast cancer. In addition, further studies indicated that GEF
and ρ GTPase were expected to become a target for cancer treatment
(11,24).
IBP has a similar structure and function to members
of the GEF family, and may participate in the activation and
regulation of ρ GTPase family molecules (10,28). IBP
may also work together with activated Rac1 to regulate cell
morphology (29) and affect cell
differentiation via its interaction with integrins (30). An intramolecular basic amino acid-rich
region K328-R340 (KRREQREQRERRR) exists within the IBP molecule,
thereby suggesting that this molecule may be transposed into the
nucleus to regulate gene expression (31). These studies indicated that IBP has a
significant role in tumorigenesis and development.
Jian et al (14) identified that IBP was ectopically
expressed in certain cases of OSCC, and that its expression was
significantly correlated with tumor size, clinical stage,
differentiation and distant metastasis. In addition, the
upregulation of IBP expression markedly promoted the proliferation
of OSCC cells and its knockdown inhibited the proliferation of OSCC
cells. They suggested that IBP shortened the G1 phase, and enhanced
the proportion of tumor cells entering the S phase, potentially
through increasing the expression of cyclin D1. Li et al
(13) revealed that IBP was highly
expressed in breast cancer, but expressed at low levels in normal
breast tissues, and that the ectopic expression of IBP was
correlated with the malignant behavior of human breast cancer
cells. Furthermore, Chen et al (32) suggested that IBP-mediated suppression
of autophagy promotes the growth and metastasis of breast cancer
cells by activating the mechanistic target of rapamycin kinase
complex 2/protein kinase B/forkhead box O3a signaling pathway. Yang
et al (33) demonstrated that
IBP was a target gene of tumor protein 53 and that it suppresses
cisplatin-induced apoptosis of breast cancer cells. Zhang et
al (12) also indicated that IBP
was overexpressed in colorectal cancer, but not in normal tissues;
such observations were associated with the occurrence and
development of colorectal cancer.
In conclusion, the present study demonstrated that
IBP was overexpressed in the majority of patients with ovarian
carcinoma, and that increased IBP expression was significantly
associated with advanced tumor aggressiveness and poor prognosis.
Therefore, these data may be used as evidence for the additional
study of IBP as a novel biomarker to predict the prognosis of
epithelial ovarian carcinoma.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81472028).
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Katso RM, Manek S, Ganjavi H, Biddolph S,
Charnock MF, Bradburn M, Wells M and Ganesan TS: Overexpression of
H-Ryk in epithelial ovarian cancer: Prognostic significance of
receptor expression. Clin Cancer Res. 6:3271–3281. 2000.PubMed/NCBI
|
|
3
|
Kolwijck E, Lybol CJ, Bulten J, Vollebergh
JH, Wevers RA and Massuger LF: Prevalence of cysts in epithelial
ovarian cancer. Eur J Obstet Gynecol Reprod Biol. 151:96–100. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gupta S, Fanzo JC, Hu C, Cox D, Jang SY,
Lee AE, Greenberg S and Pernis AB: T cell receptor engagement leads
to the recruitment of IBP, a novel guanine nucleotide exchange
factor, to the immunological synapse. J Biol Chem. 278:541–549.
2003. View Article : Google Scholar
|
|
5
|
Tanaka Y, Bi K, Kitamura R, Hong S, Altman
Y, Matsumoto A, Tabata H, Lebedeva S, Bushway PJ and Altman A:
SWAP-70-like adapter of T cells, an adapter protein that regulates
early TCR-initiated signaling in Th2 lineage cells. Immunity.
18:403–414. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Canonigo-Balancio AJ, Fos C, Prod'homme T,
Bécart S and Altman A: SLAT/Def6 plays a critical role in the
development of Th17 cell-mediated experimental autoimmune
encephalomyelitis. J Immunol. 183:7259–7267. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen Q, Gupta S and Pernis AB: Regulation
of TLR4-mediated signaling by IBP/Def6, a novel activator of Rho
GTPases. J Leukoc Biol. 85:539–543. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fanzo JC, Yang W, Jang SY, Gupta S, Chen
Q, Siddiq A, Greenberg S and Pernis AB: Loss of IRF-4-binding
protein leads to the spontaneous development of systemic
autoimmunity. J Clin Invest. 116:703–714. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Subramanian S, West RB, Marinelli RJ,
Nielsen TO, Rubin BP, Goldblum JR, Patel RM, Zhu S, Montgomery K,
Ng TL, et al: The gene expression profile of extraskeletal myxoid
chondrosarcoma. J Pathol. 206:433–444. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gupta S, Lee A, Hu C, Fanzo J, Goldberg I,
Cattoretti G and Pernis AB: Molecular cloning of IBP, a SWAP-70
homologous GEF, which is highly expressed in the immune system. Hum
Immunol. 64:389–401. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lazer G and Katzav S: Guanine nucleotide
exchange factors for RhoGTPases: Good therapeutic targets for
cancer therapy? Cell Signal. 23:969–979. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang Z, Wang Q, Li P, Zhou Y, Li S, Yi W,
Chen A, Kong P and Hu C: Overexpression of the interferon
regulatory factor 4-binding protein in human colorectal cancer and
its clinical significance. Cancer Epidemiol. 33:130–136. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li P, Zhang Z, Wang Q, Li S, Zhang Y, Bian
X, Chen A and Hu C: The ectopic expression of IFN regulatory factor
4-binding protein is correlated with the malignant behavior of
human breast cancer cells. Int Immunopharmacol. 9:1002–1009. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jian CX, Yang MZ, Li P, Xiong J, Zhang ZJ,
Li CJ, Chen A, Hu CM, Zhou JX and Li SH: Ectopically expressed IBP
promotes cell proliferation in oral squamous cell carcinoma. Cancer
Invest. 30:748–756. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pecorelli S: Revised FIGO staging for
carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol
Obstet. 105:103–104. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tavassoli FA and Devilee P: World health
organization classification of tumor. Histopathology. 30:274–276.
2002.
|
|
17
|
Ioffe OB and Silverberg SG: A universal
grading system for ovarian cancer. Contemporary Ob/gyn. 2000.
|
|
18
|
Zhang Y and Zhang B: D4-GDI, a Rho GTPase
regulator, promotes breast cancer cell invasiveness. Cancer Res.
66:5592–5598. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Raimondi F, Felline A and Fanelli F:
Catching functional modes and structural communication in Dbl
family Rho guanine nucleotide exchange factors. J Chem Inf Model.
55:1878–1893. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ridley AJ: Rho family proteins:
Coordinating cell responses. Trends Cell Biol. 11:471–477. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bokoch GM: Regulation of cell function by
Rho family GTPases. Immunol Res. 21:139–148. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bar-Sagi D and Hall A: Ras and Rho
GTPases: A family reunion. Cell. 103:227–238. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vega FM and Ridley AJ: Rho GTPases in
cancer cell biology. FEBS Lett. 582:2093–2101. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Karlsson R, Pedersen ED, Wang Z and
Brakebusch C: Rho GTPase function in tumorigenesis. Biochim Biophys
Acta. 1796:91–98. 2009.PubMed/NCBI
|
|
25
|
Lin M and van Golen KL: Rho-regulatory
proteins in breast cancer cell motility and invasion. Breast Cancer
Res Treat. 84:49–60. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Banyard J, Anand-Apte B, Symons M and
Zetter BR: Motility and invasion are differentially modulated by
Rho family GTPases. Oncogene. 19:580–591. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Saurin JC, Fallavier M, Sordat B, Gevrey
JC, Chayvialle JA and Abello J: Bombesin stimulates invasion and
migration of Isreco1 colon carcinoma cells in a Rho-dependent
manner. Cancer Res. 62:4829–4835. 2002.PubMed/NCBI
|
|
28
|
Mavrakis KJ, McKinlay KJ, Jones P and
Sablitzky F: DEF6, a novel PH-DH-like domain protein, is an
upstream activator of the Rho GTPases Rac1, Cdc42, and RhoA. Exp
Cell Res. 294:335–344. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Oka T, Ihara S and Fukui Y: Cooperation of
DEF6 with activated Rac in regulating cell morphology. J Biol Chem.
282:2011–2018. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Samson T, Will C, Knoblauch A, Sharek L,
von der Mark K, Burridge K and Wixler V: Def-6, a guanine
nucleotide exchange factor for Rac1, interacts with the skeletal
muscle integrin chain alpha7A and influences myoblast
differentiation. J Biol Chem. 282:15730–15742. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Christophe D, Christophe-Hobertus C and
Pichon B: Nuclear targeting of proteins. How many different
signals? Cell Signal. 12:337–341. 2000.PubMed/NCBI
|
|
32
|
Chen S, Han Q, Wang X, Yang M, Zhang Z, Li
P, Chen A, Hu C and Li S: IBP-mediated suppression of autophagy
promotes growth and metastasis of breast cancer cells via
activating mTORC2/Akt/FOXO3a signaling pathway. Cell Death Dis.
4:e8422013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang M, Yuan F, Li P, Chen Z, Chen A, Li S
and Hu C: Interferon regulatory factor 4 binding protein is a novel
p53 target gene and suppresses cisplatin-induced apoptosis of
breast cancer cells. Mol Cancer. 11:542012. View Article : Google Scholar : PubMed/NCBI
|