Introduction
Lung cancer is the most common cancer in the world,
in terms of both morbidity (1.8 million cases, 12.9% of total) and
mortality (1.6 million deaths, 19.4% of total) (1). Bone is one of the most common sites of
metastasis in patients with lung cancer, which is often accompanied
by skeletal-related events which significantly reduce the quality
of life in patients, including bone pain, hypercalcemia,
pathological fractures, spinal cord compression and bone marrow
infiltration (2–4). Consequently, effective targeted
therapies for bone metastasis in lung cancer are urgently needed.
However, the mechanisms underlying the development of bone
metastasis are not well understood.
Dickkopf1 (DKK1) is known as a negative regulator of
the Wnt signaling pathway (5), which
regulates cell fate and proliferation. Dysregulation of this
pathway has been reported in many developmental defects and cancer
(6). Several studies suggest that
DKK1 has a role in malignant bone metastasis, such as in breast
cancer (6) and prostate cancer
(7,8).
Previously, a study from our group has demonstrated that the
expression levels of DKK1 were significantly higher in SBC-5 cells
compared with SBC-3 cells. SBC-5 cells have a greater propensity to
metastasize to bone. In addition, downregulation of DKK1 in SBC-5
cells inhibited cell proliferation, colony formation, migration and
cell invasion in vitro and bone metastasis in vivo;
nevertheless, it also promoted cell apoptosis (9). However, it remained unclear whether
upregulating DKK1 in SBC-3 cells could endow them with more
aggressive properties, or even reverse their inability to form
metastases in the bone. In the present study, DKK1 was
overexpressed in SBC-3 cells and consequently it effects were
examined in cell proliferation, colony formation, cell migration
in vitro, and tumorigenic ability in NOD-SCID mice. The
results further confirmed that DKK1, a critical tumor promoter, was
associated with skeleton metastasis of SCLC. These data suggested
that DKK1 may provide a potential novel strategy for therapeutic
intervention in SCLC patients with bone metastases.
Materials and methods
Cell culture
SBC-5 and SBC-3 cells were a kind gift from
Professors Sone and Yano (Tokushima University, Tokushima, Japan).
Cells were cultured in RPMI1640 medium (Gibco; Thermo Fisher
Scientific, Waltham, MA, USA) supplemented with 10% fetal bovine
serum (FBS; Biochrom GmbH, Berlin, Germany), 100 U/ml streptomycin
and 100 U/ml penicillin at 37°C with 5% CO2.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from cells using TRIzol
reagent (Thermo Fisher Scientific, Inc.) following the
manufacturer's instructions. Consequently, RNA (500 ng) was
reverse-transcribed into cDNA by QuantiNova™ Reverse
Transcription kit (QIAGEN GmbH, Hilden, Germany). qPCR was
preformed using SYBR Premix Ex TaqTM II (TakaraBio, Inc., Otsu,
Japan), the thermocycling conditions were denaturated at 95°C for
30 sec, followed by 40 cycles at 95°C for 5 sec and 60°C for 30
sec, real-time fluorescence signals were detected, and gene
expression was quantified using 2−ΔΔCq method (10). The primer sequences are listed in
Table I.
 | Table I.Primers used in quantitative
polymerase chain reaction analysis. |
Table I.
Primers used in quantitative
polymerase chain reaction analysis.
| Gene | Forward primer
(5′-3′) | Reverse primer
(5′-3′) |
|---|
| DKK1 |
TAGAGTCTAGAATGCAAGGATCTC |
CAAAAACTATCACAGCCTAAAGGG |
| β-actin |
TGGCACCCAGCACAATGAA |
CTAAGTCATAGTCCGCCTAGAAGCA |
Western blot analysis
The protein of cells was extracted by RIPA buffer
(Beyotime Biotechnology, Shanghai, China), after quantification
with BCA kit (Pierce, Thermo Fisher Scientific, Inc.), 20 µg of
protein was subjected to 10% SDS-PAGE and transferred to
nitrocellulose membranes. The members were then blocked in 5%
fat-free milk for 1 h at room temperature and incubated overnight
at 4°C with primary antibodies against DKK1 (1:15,00; BS7731;
Bioworld Technology, St. Louis Park, MN, USA) or β-actin (1:10,000;
051M4892; Sigma-Aldrich, Merck KGaA, Darmstadt, Germany). Next,
peroxidase-conjugated goat anti-rabbit IgG (1:5,000; ZB-2301;
Zhongshan, Beijing, China) or peroxidase-conjugated goat anti-mouse
IgG (1:5,000; ZB-2305; Zhongshan, Beijing, China) were incubated
for 1 h at room temperature. Finally, the target proteins were
visualized by chemiluminescence (Pierce, Thermo Fisher Scientific,
Inc.).
Cells stable transfection
DKK1-overexpressing plasmids were obtained from
BioworldTechnology, Inc. and 1.2 µg plasmids were transfected into
SBC-3 cells in 6-well plates with Lipofectamine 2000 (Invitriogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
instructions. A total of 48 h after transfection, cells were
selected with G418 sulfate (600 µg/ml). One month later,
G418-resistant colonies, stably overexpressing DKK1 (termed
SBC-3-DKK1 cells), were harvested and consequently subcultured in
medium containing 200 µg/ml of G418. Parental SBC-3 cells were used
as the control.
MTT assay
Cells in the logarithmic growth phase were harvested
and plated in 96-well plates (2,000 cells/well), and cell growth
was analyzed for the next days. Briefly, cells were incubated with
MTT solution (5 mg/ml, 20 µl/well) at 37°C for 3 h. Then, the
medium was removed and 150 µl DMSO was added to dissolve the
formazane. The absorbance value was detected at 490 nm using a
microplate reader. The assay was performed in triplicate.
Colony formation assay
Cells in the logarithmic growth phase were harvested
and plated in 6-well plates (200 cells/well). Two weeks
post-seeding, the cells were stained with 0.25% crystal violet and
the number of colonies was counted manually. The assay was
performed in triplicate.
Conditioned medium collection
SBC-5 cells were seeded in 6-well plate
(1×105 cells/well). At 24 h post-seeding, the cells were
washed twice with PBS, mixed with serum-free medium, and then
incubated for an additional 24 h. Then the conditioned medium (CM)
was collected and stored at −80°C for further use.
Cell migration and invasion assay
The invading potential of the cells was investigated
using 8-µm pore size transwell inserts (Corning Incorporated,
Corning, NY, USA). Briefly, cells were first resuspended in
serum-free RPMI-1640 and then seeded in triplicates in the upper
chamber of the transwells, which was pre-coated with 70 µl Matrigel
(1:8 dilution; Corning Incorporated). A total of 500 µl of
RPMI-1640 with 10% FBS was then added into the bottom chamber to
serve as a chemoattractant. To examine the function of DKK1,
anti-human DKK1-neutralizing antibody (10 µg/ml; R&D Systems,
Inc., Minneapolis, MN, USA), recombinant human DKK1 (16 µg/ml;
PeproTech, NJ, USA), or conditioned medium (CM) were also added to
the bottom chambers. Following 24 h of incubation, cells that
invaded to the lower chamber were fixed in 95% ethyl alcohol and
then stained with 0.5% crystal violet. Finally, the number of
invasive cells was counted in five random fields of each sample and
the average was determined.
For migration assays, the same protocol was
followed, with the only difference being that the inserts were not
coated with Matrigel.
In vivo metastasis experiment
A total of 10 female NOD-SCID mice, 4 weeks old,
weighing 20–25 g, were obtained from Beijing HFK Bioscience Co.
(Beijing, China). Animals were divided into two groups of 5 mice
each. SBC-3 cells and SBC-3-DKK1 cells were harvested, resuspended
with PBS in a concentration of 5×106 cells/ml and then
injected by tail vein in the mice (200 µl). The mice were kept
under germ-free conditions (temperature, 22°C; ventilation rate,
15/h, light/dark cycle, 12/12 h; food was sterilized with Cobalt-60
irradiation and water was autoclaved, and access to the food was
ad libitum). Five weeks post-injection, mice were
anesthetized and the bone metastases were investigated using an
X-ray device. The imaging data of the osteolytic bone metastases
were independently evaluated by two experienced investigators.
All animal studies (including the mice euthanasia
procedure) were done in compliance with the regulations and
guidelines of The Fourth Military Medical University Institutional
Animal Care and conducted according to the AAALAC and the IACUC
guidelines. The present study was approved by the Animal
Experimental Ethical Inspection Committee of The Fourth Military
Medical University Laboratory Animal Center (Xi'an, China).
Statistical analysis
One-way analysis of variance was used to analyze the
statistical differences in cell migration and invasion assays. The
other data was analyzed by t-test. Statistical tests were performed
with GraphPad Prism 6 (GraphPad Software, Inc., La Jolla, CA, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
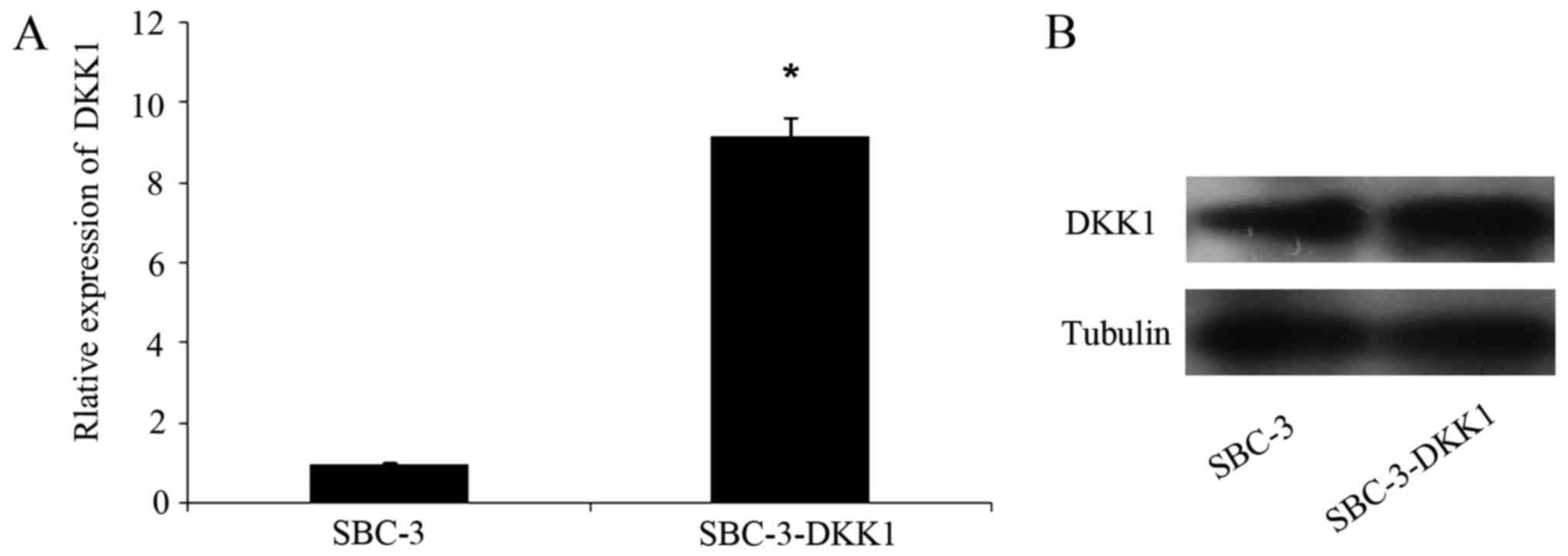
Expression levels of DKK1 were
increased in transfected cells
Transfection efficacy of DKK1 in SBC-3 cells was
analyzed by RT-qPCR and western blotting. Briefly, DKK1 mRNA
(Fig. 1A) and protein (Fig. 1B) expression levels in SBC-3-DKK1
cells were both stably increased compared with parental SBC-3
cells.
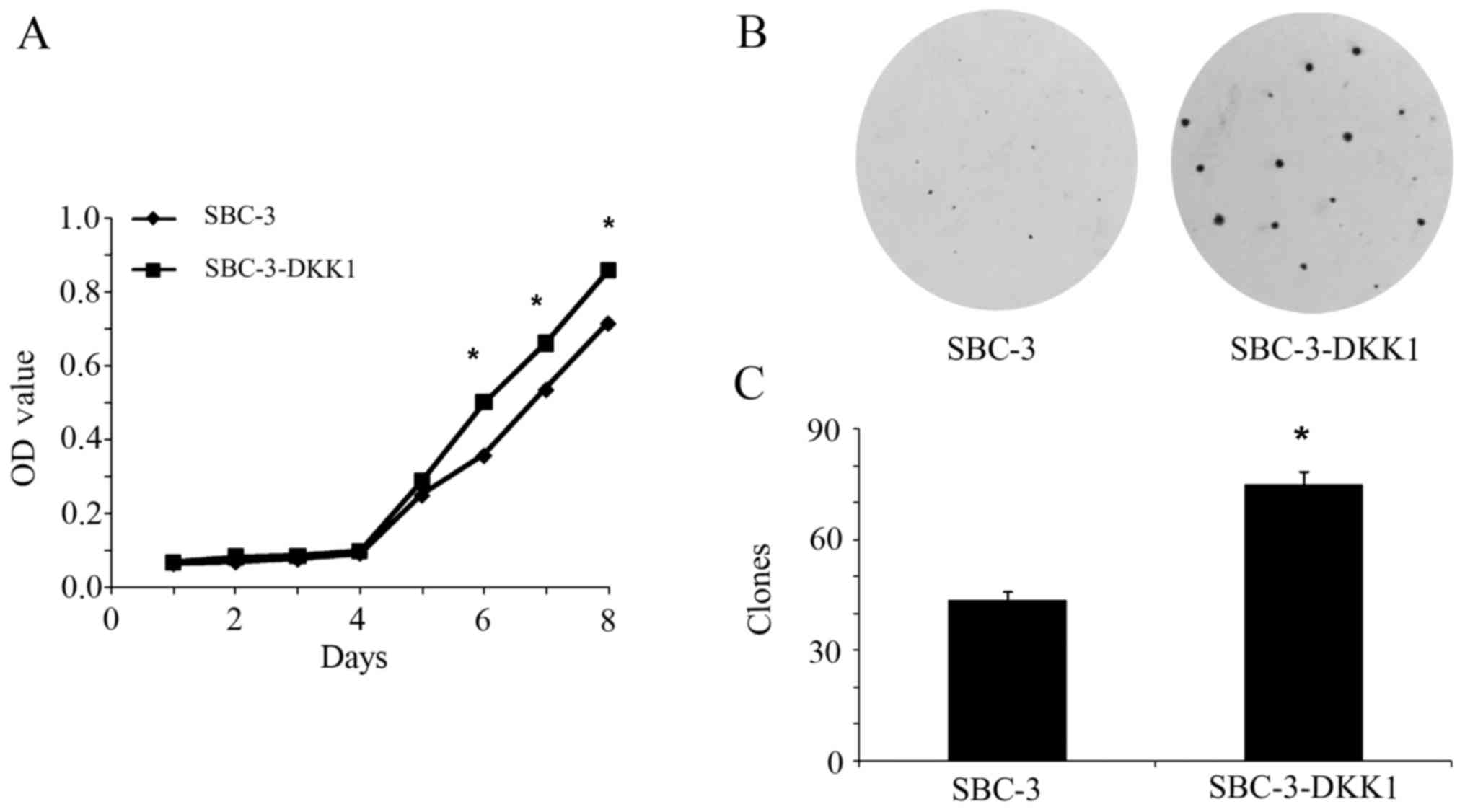
Overexpression of DKK1 increases the
proliferation ability of SBC-3 cells
The effect of DKK1 overexpression on cell
proliferation was tested by MTT and colony formation assays. From
the cell growth curve presented in Fig.
2A, it was demonstrated that DKK1 overexpression significantly
promoted proliferation of SBC-3 cells compared with the parental
SBC-3 cells (P<0.05). Consistently, by colony formation assay,
an increased number of colonies (which were also larger in
diameter) were observed in SBC-3-DKK1 cells compared with parental
SBC-3 cells (P<0.05; Fig. 2B and
C). To conclude, these results implied that DKK1 acted as a
promoter of cell proliferation inSBC-3 cells.
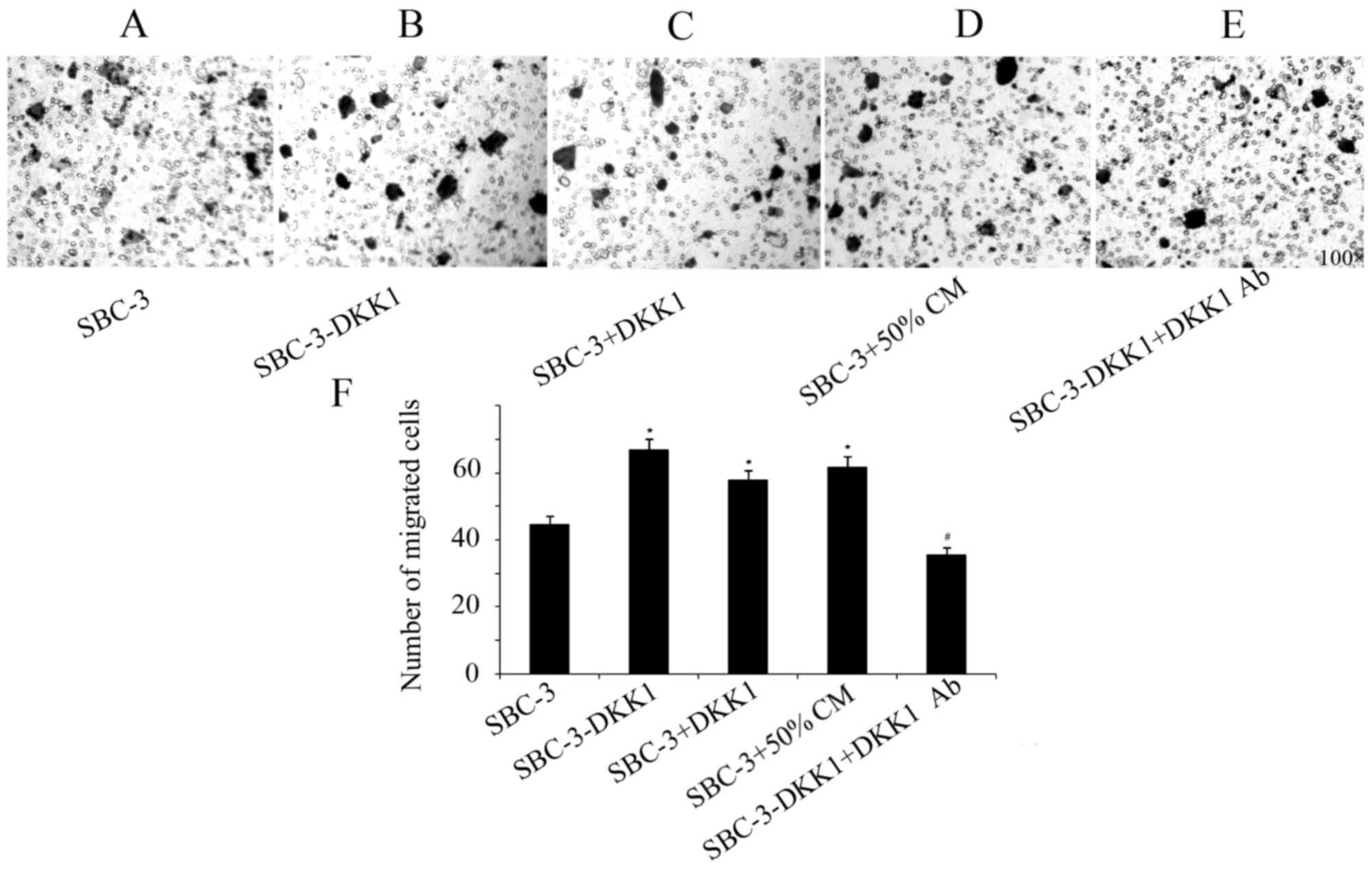
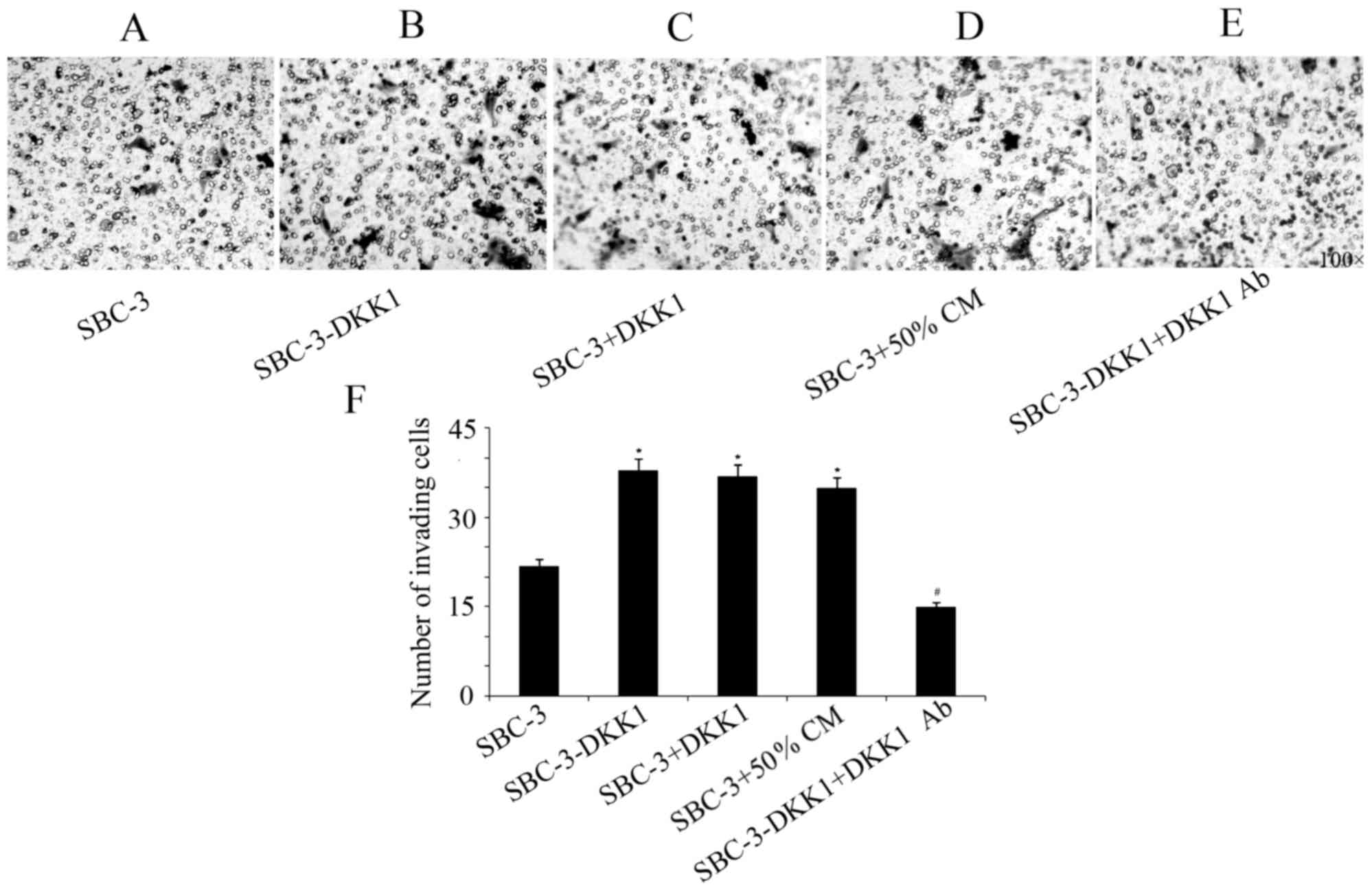
DKK1 overexpression increases
migration and invasion in SBC-3 cells
Results from the transwell assays indicated that
upregulation of DKK1 significantly increased the invasive and
migratory capabilities of SBC-3 cells (Figs. 3 and 4).
Furthermore, addition of recombinant DKK1 or CM from SBC-5 cells
had the same effect in increasing migration and invasion of SBC-3
cells (Figs. 3 and 4). By contrast, addition of the
DKK1-neutralizing antibody in the SBC-3-DKK1 cells reversed the
effect of DKK1 overexpression on cell invasion and migration
(Figs. 3 and 4).
Overexpression of DKK1 facilitates
bone metastasis in vivo
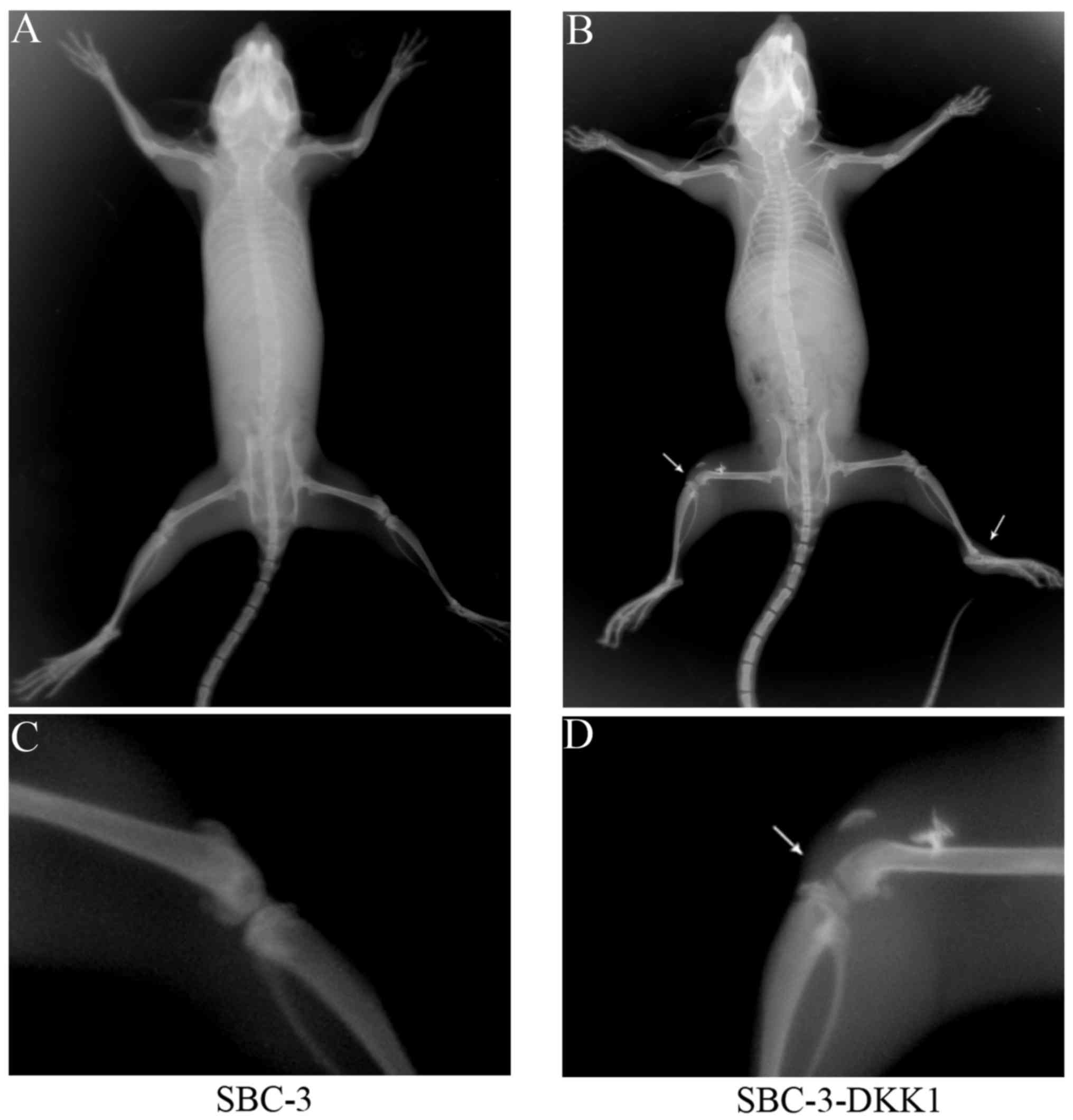
X-ray imaging was used to investigate whether
overexpression of DKK1 had an effect on in vivo metastasis.
As presented in Table II, bone
metastasis was observed in 3 of 5 mice in the SBC-3-DKK1 group,
while no metastasis (0/5) was detected in the control SBC-3 group.
Furthermore, the total number of bone metastasis lesions was
counted (Table II). The results
suggested that upregulation of DKK1 could enhance metastasis to
bone. Representative X-ray images of skeletal metastasis are shown
in Fig. 5.
 | Table II.Incidence of bone metastasis and the
number of metastasis lesions formed in NOD-SCID mice. |
Table II.
Incidence of bone metastasis and the
number of metastasis lesions formed in NOD-SCID mice.
| Cell line | Incidence | Numbers of bone
metastases |
|---|
| SBC-3 | 0/5 | 0.00±0.00 |
| SBC-3-DKK1 | 3/5 |
1.40±1.34a |
Discussion
DKK1, a secreted inhibitor of the Wnt/β-catenin
pathway, is a member of the human DKK family. Many of these
extensively studied members have been evolutionarily conserved and
include an intricate network of signaling molecules known to
participate in bone diseases, Alzheimer's disease and cancer
(11–15). Several reports associate DKK1
expression with skeletal lesions. DKK1 has a promoting role in the
formation of bone lesions in patients with multiple myeloma
(16,17), since DKK1 can prohibit osteoblastic
differentiation. Consistently, elevated circulating DKK-1 levels in
patients with multiple myeloma have been associated with osteolytic
lesions (18,19). Furthermore, DKK1 is expressed at a
higher level the MDA-MB-231-BO breast cancer cell line, which
metastasizes exclusively to bone and produces larger osteolytic
lesions compared to the parental MDA-MB-231 line. This advanced
malignant feature is caused by inhibition in osteoblast
differentiation and osteoprotegerin expression (6,20). In
addition, DKK1 overexpression significantly increases the
subcutaneous tumor volumes and the incidence of bone metastases of
the after intracardiac Ace-1 prostate cancer cells (8). It is speculated that DKK1 may reduce the
Ace-1 osteoblastic phenotype, resulting in increased phospho-46
c-Jun N-terminal kinase via the Wnt noncanonical pathway (8). A lung cancer study reported that the
serum levels of DKK1 in patients with bone metastases were higher
compared with NSCLC patients without bone metastasis, suggesting
that DKK1 secreted by lung cancer cells may be associated with
NSCLC bone metastasis (21).
A previous study from our group has demonstrated
that downregulation of DKK1 in bone-metastatic SCLC cells (SBC-5)
could partially inhibit cell proliferation, colony formation,
migration and invasion in vitro, and bone metastases in
vivo (9). In the present study,
DKK1 expression was upregulated in SBC-3 cells, which have
inherently low expression levels of DKK1 and no tendency to
metastasize to bone tissue. The obtained results indicated that
overexpression of DKK1 in SBC-3 cells was sufficient to enhance
their capacity of proliferation, colony formation, migration and
invasion in vitro, as well as their bone metastasis
potential in vivo. In conclusion, DKK1 was demonstrated to
be involved in the skeletal metastasis of SCLC cells. Furthermore,
DKK1 was identified as an indispensable tumor contributor for the
development of bone metastases, and therefore, it may serve as a
promising target for the prevention and effective treatment of bone
metastases in SCLC. Further studies will be needed in the future in
order to fully elucidate the molecular mechanisms underlying the
role of DKK1 on bone metastasis of lung cancer.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81572251 and
81402411) and the Natural Science Foundation of Shanxi, China
(grant no. 2016JM8036).
Availability of data and materials
The analyzed datasets generated during the study are
available from the corresponding author on reasonable request.
Authors' contributions
HP constructed stable transected cell lines and
collated data. NM detected cells proliferation, colony formation
and cell migration. WS was a major contributor in writing the
manuscript. QZ performed the cell invasion in vitro. WC, JW
and LD performed the mice experiments. NZ and ZZ were responsible
for the X-ray device and evaluated osteolytic bone metastases. LL
and HZ guided and reviewed the experiments. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Animal
Experimental Ethical Inspection Committee of The Fourth Military
Medical University Laboratory Animal Center (Xi'an, China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Oster G, Lamerato L, Glass AG, Richert-Boe
KE, Lopez A, Chung K, Richhariya A, Dodge T, Wolff GG, Balakumaran
A and Edelsberg J: Natural history of skeletal-related events in
patients with breast, lung, or prostate cancer and metastases to
bone: A 15-year study in two large US health systems. Support Care
Cancer. 21:3279–3286. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Silva SC, Wilson C and Woll PJ:
Bone-targeted agents in the treatment of lung cancer. Ther Adv Med
Oncol. 7:219–228. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Roato I: Bone metastases: When and how
lung cancer interacts with bone. World J Clin Oncol. 5:149–155.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Menezes ME, Devine DJ, Shevde LA and
Samant RS: Dickkopf1: A tumor suppressor or metastasis promoter?
Int J Cancer. 130:1477–1483. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bu G, Lu W, Liu CC, Selander K, Yoneda T,
Hall C, Keller ET and Li Y: Breast cancer-derived Dickkopf1
inhibits osteoblast differentiation and osteoprotegerin expression:
Implication for breast cancer osteolytic bone metastases. Int J
Cancer. 123:1034–1042. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hall CL, Daignault SD, Shah RB, Pienta KJ
and Keller ET: Dickkopf-1 expression increases early in prostate
cancer development and decreases during progression from primary
tumor to metastasis. Prostate. 68:1396–1404. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Thudi NK, Martin CK, Murahari S, Shu ST,
Lanigan LG, Werbeck JL, Keller ET, McCauley LK, Pinzone JJ and
Rosol TJ: Dickkopf-1 (DKK-1) stimulated prostate cancer growth and
metastasis and inhibited bone formation in osteoblastic bone
metastases. Prostate. 71:615–625. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pang H, Ma N, Jiao M, Shen W, Xin B, Wang
T, Zhang F, Liu L and Zhang H: The biological effects of Dickkopf1
on small cell lung cancer cells and bone metastasis. Oncol Res.
25:35–42. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cadigan KM and Nusse R: Wnt signaling: A
common theme in animal development. Genes Dev. 11:3286–3305. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Krupnik VE, Sharp JD, Jiang C, Robison K,
Chickering TW, Amaravadi L, Brown DE, Guyot D, Mays G, Leiby K, et
al: Functional and structural diversity of the human Dickkopf gene
family. Gene. 238:301–313. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Moon RT, Kohn AD, De Ferrari GV and Kaykas
A: WNT and beta-catenin signalling: Diseases and therapies. Nat Rev
Genet. 5:691–701. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
MacDonald BT, Tamai K and He X:
Wnt/beta-catenin signaling: Components, mechanisms, and diseases.
Dev Cell. 17:9–26. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rao TP and Kühl M: An updated overview on
Wnt signaling pathways: A prelude for more. Circ Res.
106:1798–1806. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tian E, Zhan F, Walker R, Rasmussen E, Ma
Y, Barlogie B and Shaughnessy JD Jr: The role of the Wnt-signaling
antagonist DKK1 in the development of osteolytic lesions in
multiple myeloma. N Engl J Med. 349:2483–2494. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Politou MC, Heath DJ, Rahemtulla A, Szydlo
R, Anagnostopoulos A, Dimopoulos MA, Croucher PI and Terpos E:
Serum concentrations of Dickkopf-1 protein are increased in
patients with multiple myeloma and reduced after autologous stem
cell transplantation. Int J Cancer. 119:1728–1731. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pinzone JJ, Hall BM, Thudi NK, Vonau M,
Qiang YW, Rosol TJ and Shaughnessy JD Jr: The role of Dickkopf-1 in
bone development, homeostasis, and disease. Blood. 113:517–525.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Giuliani N and Rizzoli V: Myeloma cells
and bone marrow osteoblast interactions: Role in the development of
osteolytic lesions in multiple myeloma. Leuk Lymphoma.
48:2323–2329. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yoneda T, Williams PJ, Hiraga T, Niewolna
M and Nishimura R: A bone-seeking clone exhibits different
biological properties from the MDA-MB-231 parental human breast
cancer cells and a brain-seeking clone in vivo and in vitro. J Bone
Miner Res. 16:1486–1495. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chu T, Teng J, Jiang L, Zhong H and Han B:
Lung cancer-derived Dickkopf1 is associated with bone metastasis
and the mechanism involves the inhibition of osteoblast
differentiation. Biochem Biophys Res Commun. 443:962–968. 2014.
View Article : Google Scholar : PubMed/NCBI
|