Introduction
Acute myeloid leukemia (AML) is a malignancy
characterized by abnormal excessive proliferation of hematopoietic
cells in the bone marrow (1).
Cytotoxic chemotherapy and bone marrow hematopoietic stem cell
transplantation are among the primary therapeutic treatments for
AML (2). However, the majority of
patients do not have access to treatment options other than
chemotherapy, due to the limited medical access and economic
conditions in China.
Arsenic trioxide (ATO) was the first successful
treatment for acute promyelocytic leukemia (APL), and has improved
the clinical outcomes and prolonged the survival of patients with
APL (3–8). Physicians have begun to use ATO to treat
types of hematological malignancies other than APL, with AML being
the most common (9). However, the
suitability of treating hematological malignancies other than APL
with ATO, and the molecular mechanism underlying ATO therapy,
remains unknown. An improved understanding of the mechanisms
associated with ATO may reveal its potential therapeutic value for
treating AML.
A number of malignant tumor types exhibit abnormal
expression of ROS proto-oncogene 1 receptor tyrosine kinase (ROS1),
a gene associated with the Wnt/β-catenin signaling pathway
(10–14). ROS1 is considered a potential target
for cancer treatment and targeted anticancer therapies are under
development (15). If ROS1 inhibitors
may be used in combination with ATO to treat patients with AML, the
therapeutic efficacy may be improved and adverse effects may be
decreased. In addition, novel applications of approved drugs may
decrease the costs associated with researching and developing novel
agents. Thus, in the present study, the function of ROS1 in the
ATO-treatment of AML cells has been evaluated.
Materials and methods
Cell lines
The AML cell lines used in this study include THP-1
(organism, Homo sapien/human; cell type, monocyte; tissue,
peripheral blood; disease, acute monocytic leukemia; product
format, frozen), HL60 (organism, Homo sapien/human; cell
type, promyeloblast; tissue, peripheral blood; disease, acute
promyelocytic leukemia; product format, frozen) and Kasumi-6
(organism, Homo sapien/human; cell type: myeloblast; tissue,
peripheral blood; disease, acute myeloid leukemia subtype M2;
product format, frozen). All cell lines were purchased from
Shenyang Yike Biological Technology Co., Ltd. (Shenyang,
China).
Transfection
AML cells were transfected with human ROS1-specific
or control small interfering (si)RNAs using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), according to the
manufacturer's protocol. Functionally-validated siRNA (1 nmol)
against ROS1 gene with target sequence of
5′-AAGGTAATTGCTCTAACTTTA-3′. The ROS1-silenced (ROS1-si) and
siRNA-transfected control (ROS1-c) cells were harvested 48 h
following transfection, and the efficacy of ROS1 silencing was
determined using western blot analysis.
Western blot analysis
ROS1-si and ROS1-c AML cells were collected 48 h
after transfection. GAPDH and β-actin were used for control. The
protein concentration in the lysates was quantified using an
enhanced bicinchoninic acid protein assay kit (Thermo Fisher
Scientific, Inc.) with bovine serum albumin (BSA; Invitrogen;
Thermo Fisher Scientific, Inc.) as a standard; 30 µg protein was
added per lane. The protein extracts were subjected to 7.5%
SDS-PAGE at 200 V for 40 min and the resolved proteins were
electro-transferred for 12 min at 18 V to nitrocellulose membranes
(Thermo Fisher Scientific, Inc.) using a western blotting semi-dry
transfer unit (Hoefer, San Francisco, CA, USA). The membranes were
blocked for 1 h at room temperature with TBST (10X buffer; 200 mM
Tris base, 1.5 M NaCl in MiliQ water; 0.1% Tween-20 was added and
pH was adjusted to 7.5 for 1X buffer) containing 5% BSA The
membranes were then incubated with one of the primary antibodies
(1:200) [rabbit anti-ROS 1 (cat no. MA5-26180; Invitrogen; Thermo
Fisher Scientific, Inc.), rabbit anti-Axin (cat no. 34-5900;
Invitrogen; Thermo Fisher Scientific, Inc.), rabbit anti-GSK-3β
(cat no. A170132; Invitrogen; Thermo Fisher Scientific, Inc.),
rabbit anti-APC (cat no. 17-9956-42; Invitrogen; Thermo Fisher
Scientific, Inc.), rabbit anti-β-catenin (cat no. 71-2700;
Invitrogen; Thermo Fisher Scientific, Inc.), rabbit anti-c-Myc (cat
no. MA1-980; Invitrogen; Thermo Fisher Scientific, Inc.), rabbit
anti-Cyclin-D1 (cat no. PA5-16777; Invitrogen; Thermo Fisher
Scientific, Inc.), rabbit anti-PPAR-α (cat no. PA1-820; Invitrogen;
Thermo Fisher Scientific, Inc.), rabbit anti-MMP-7 (cat no.
PA1-9069; Invitrogen; Thermo Fisher Scientific, Inc.), rabbit
anti-GAPDH (cat no. PA1-988; Invitrogen; Thermo Fisher Scientific,
Inc.) and rabbit anti-β-actin (cat no. PA1-183; Invitrogen; Thermo
Fisher Scientific, Inc.)] in TBST with 5% BSA overnight at 4°C with
gentle shaking. Membranes were washed twice with TBST for 10 min
each and additionally incubated with secondary antibody (goat
anti-rabbit; cat no. A11008; 1:5,000; Invitrogen; Thermo Fisher
Scientific, Inc.) for 1 h at room temperature. Then the membrane
was washed 2 times for 10 min each with TBST to remove any
non-bound secondary antibody. The membrane was incubated with
Pierce ECL western-blotting Detection Reagent (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) at room temperature for 5
min. Excess detection reagent was removed and the membrane was
exposed to X-ray film placed on top of the membrane for 6 min in an
X-ray film cassette (Amersham; GE Healthcare, Chicago, IL, USA).
Membranes were also assessed for equal loading. The degree of
target protein downregulation in ROS1-si cells relative to the
control cells was determined by gray level analysis, which was
calculated by area and pixel values, using Image J software
(National Institutes of Health, Bethesda, MD, USA).
Measurement of cell growth
THP-1, HL60 and Kasumi-6 cells (3×103
cells/well) were transferred to 96-well plates and treated with 2.5
µM ATO (Harbin Pharmaceutical Group Co., Ltd., Harbin, China) for
72 h at 37°C in an atmosphere containing 5% CO2. The
effect of ROS1 knockdown on cell proliferation in THP-1, HL60 and
Kasumi-6 cells was determined using a Cell Counting Kit-8 (Dojindo
Molecular Technologies, Inc., Kumamoto, Japan), as described
previously (16,17).
For cell migration assays, transfected THP-1, HL60
and Kasumi-6 cells (5×105 cells/well) were seeded in 250
µl RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.),
without fetal bovine serum (FBS), into the upper chamber of a
Transwell plate (with 8 µm pores; Corning Incorporated, Corning,
NY, USA). RPMI-1640 (Invitrogen; Thermo Fisher Scientific, Inc.)
with 10% FBS (Invitrogen; Thermo Fisher Scientific, Inc.) was added
into the lower chamber. These cells were incubated at 37°C in an
atmosphere containing 5% CO2 for between 48 and 72 h.
Cells were stained at room temperature for ~10 min and observed
using light microscopy at magnification, ×100. Each experiment was
repeated in triplicate.
Statistical analysis
All data were analyzed using SPSS 19.0 (IBM Corp.,
Armonk, NY, USA). Data from the three different samples are
presented as the mean ± standard deviation, and the differences
among them were compared by analysis of variance, followed by. a
Student Newman Keuls post-hoc test, and P<0.05 was considered to
indicate a statistically significant difference.
Results
ROS1 is silenced successfully in
THP-1, HL60 and Kasumi-6 cells
In order to determine the efficacy of gene
silencing, the expression levels of ROS1 in THP-1, HL60 and
Kasumi-6 cell lines were determined using western blot analysis.
ROS1 expression was markedly decreased in cells following
transfection with human ROS1-specific siRNA compared with control
cells (Fig. 1).
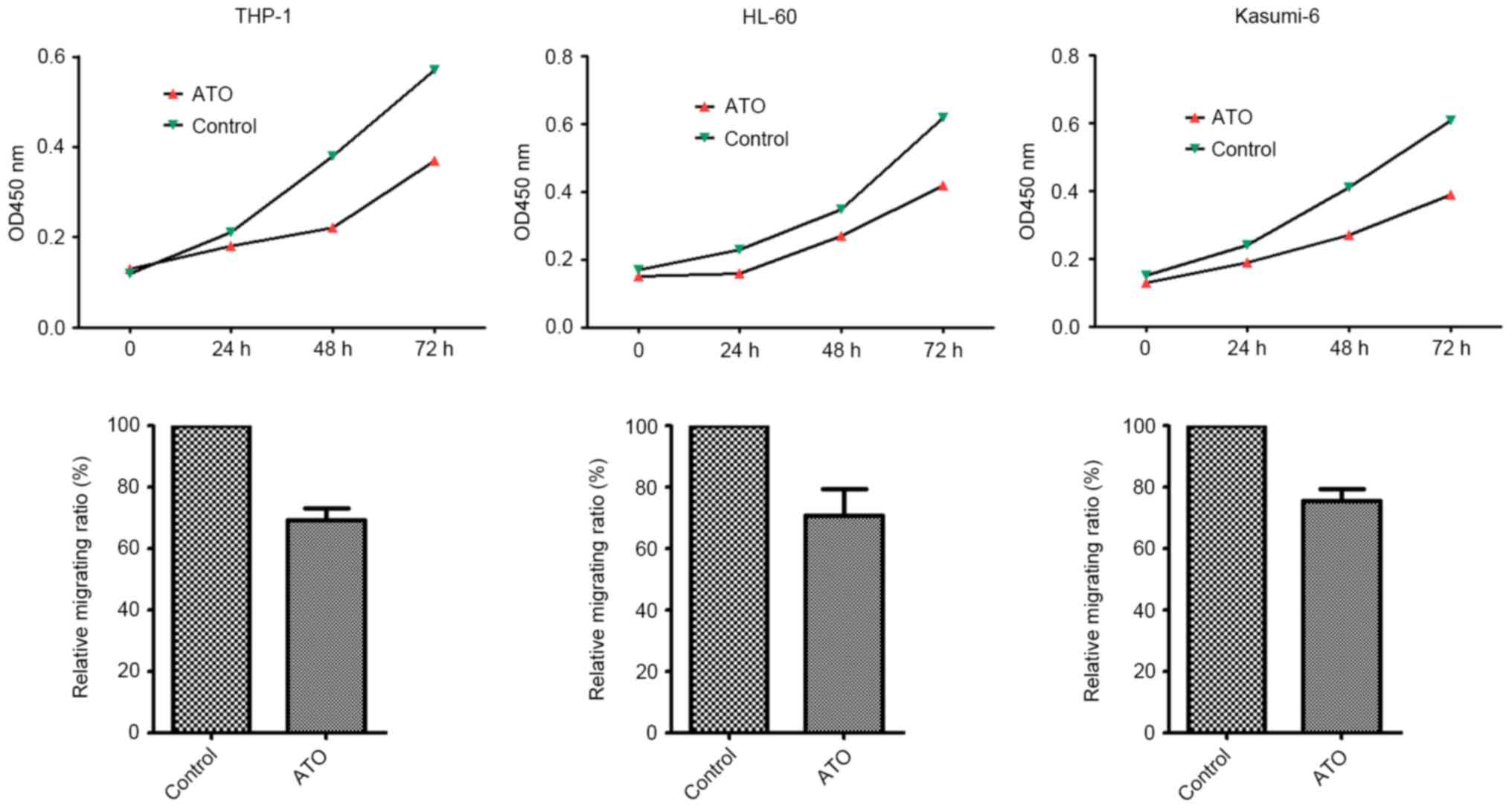
ATO inhibits the migratory and
proliferative abilities of THP-1, HL60 and Kasumi-6 cells
The effects of ATO on the migratory and
proliferative capabilities of THP-1, HL60 and Kasumi-6 cells was
determined in vitro. The results demonstrated that ATO
treatment significantly inhibited cell migration and proliferation
in these three cell lines (P= 0.003, 0.007, and 0.014,
respectively) (Fig. 2).
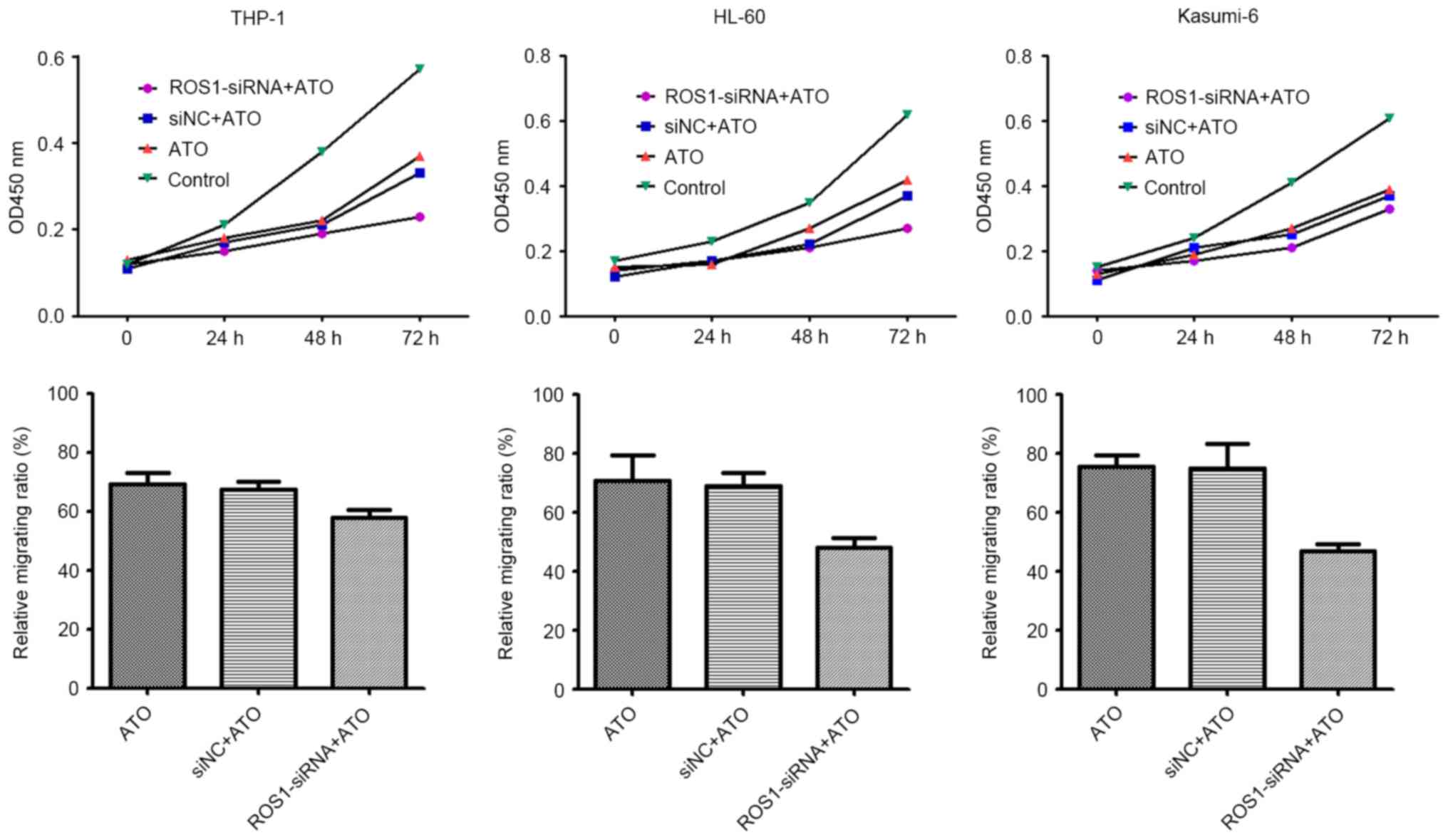
ROS1 knockdown increases the
sensitivity of AML cells to ATO treatment
Following ROS1 knockdown by siRNA, THP-1, HL60 and
Kasumi-6 cells were treated with 2.5 µM ATO. The results revealed
that cell migration and proliferation was significantly inhibited
by the combination of ROS1 knockdown and ATO treatment, compared
with ATO treatment alone (all P<0.001) (Fig. 3). This result suggests that ROS1
knockdown sensitized AML cells to the effects of ATO, and that ROS1
is involved in the migration and proliferation of leukemia
cells.
ROS1 silencing inhibits Wnt/β-catenin
signaling in AML cells
Proliferation and migration of AML cells are
regulated by a number of signaling pathways, including the
Wnt/β-catenin pathway. Therefore, in the present study, the effect
of ROS1 silencing on Wnt/β-catenin activation in AML cells was
investigated using western blot analysis (Fig. 4). The relative levels of Axin,
glycogen synthase kinase-3β, Adenomatous polyposis coli and
peroxisome proliferator-activator receptor α were markedly
increased in ROS1-si cells, compared with in ROS1-c cells. The
relative levels of β-catenin, c-Myc, cyclin D1, and matrix
metalloproteinase-7 were markedly decreased in ROS1-si cells,
compared with in ROS1-c cells. These data indicate that ROS1
silencing inhibits Wnt/β-catenin activation, which is crucial for
the proliferation and migration of AML cells.
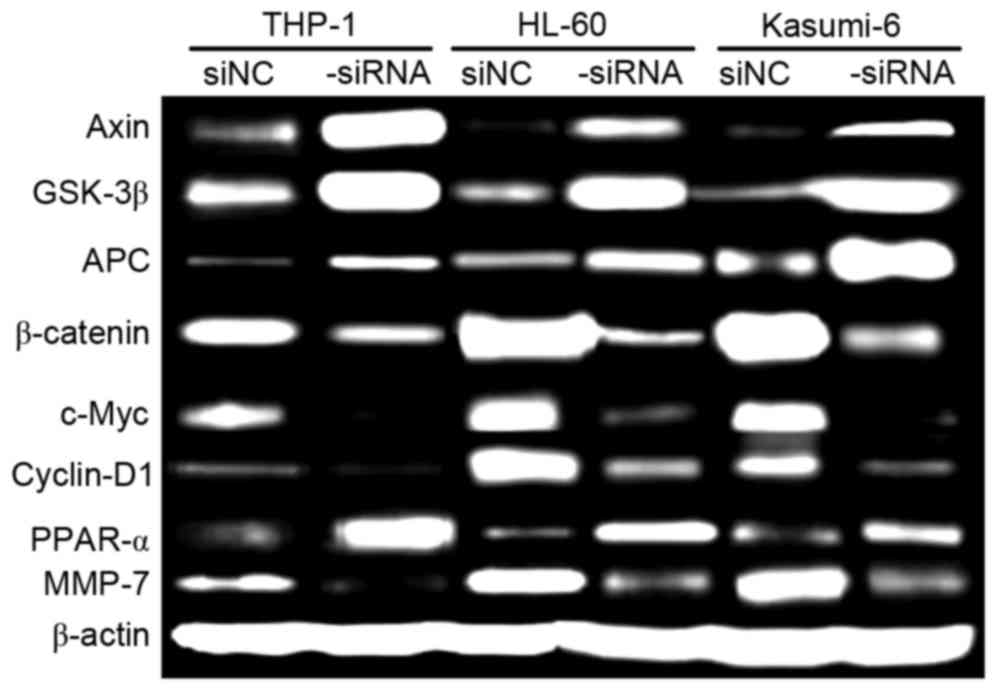 | Figure 4.Relative levels of target proteins in
ROS1-si and ROS1-c AML cells, cultured for 72 h, as determined
using western blot analysis. β-catenin was the loading control.
ROS1, ROS proto-oncogene 1 receptor tyrosine kinase; -si,
ROS1-silenced; -c, siRNA-transfected control; AML, acute myeloid
leukemia; GSK-3β, glycogen synthase kinase-3β; APC, adenomatous
polyposis coli; PPAR-α, peroxisome proliferator-activator receptor
α; MMP-7, matrix metalloproteinase-7. |
Discussion
In the present study, it was identified that ATO
treatment inhibited the migration and proliferation of THP-1, HL60
and Kasumi-6 leukemia cell lines, which was mediated by ROS1.
Knockdown of ROS1 expression sensitized the three leukemia cell
lines to ATO treatment and inhibited the Wnt/β-catenin signaling
pathway. Knockdown of ROS1 expression alone decreased the migration
and proliferation of the three cell lines, but not significantly
(data not presented).
ATO promotes apoptosis and other biological
responses in tumor cells, which has prompted its use in the
treatment of APL and other types of hematological malignancies,
including multiple myeloma (16–20), and
cancer of the breast, brain, liver, stomach, prostate, kidney and
bladder (21,22). However, toxicity to the liver, heart
and level 3/4 peripheral nerves rises with increasing ATO dosage
(19,23), which effects therapeutic efficacy and
patient compliance. Therefore, increasing the therapeutic window of
ATO is important. In this respect, ROS1 downregulation represents a
potential therapeutic strategy that requires additional
investigation.
A number of signal transduction pathways regulate
the biology of leukemia. Among them, the Wnt signaling pathway is
one of the best characterized. Wnt signaling serves functions in
the development of leukemia and is important for the survival and
self-renewal of leukemia cells (24–26).
Notably, the Wnt signaling pathway is associated with the
maintenance of leukemia stem cells, which is directly associated
with disease progression (27,28).
β-catenin is maintained in an activated state in these leukemia
cells and is expressed in a number of types of tumor (29–32).
Previous studies have suggested that ROS1 may be involved in the
regulation of Wnt signaling (33).
The results of the present study provided insights into the
regulation of Wnt signaling, although additional studies are
required. For example, in the present study, it was observed that
alterations in ROS1 expression resulted in alterations to the
expression of proteins associated with Wnt signaling. However, the
association between ROS1 and Wnt signaling remains unknown and, in
particular, the functions ROS1 serves in the regulatory network of
Wnt signaling. In addition, the regulation of Wnt signaling by ROS1
and sensitization of AML cells to ATO treatment by ROS1
downregulation requires validation in animal studies.
The present study demonstrated that knockdown of
ROS1 expression enhanced the migration and proliferation of
leukemia cell lines, and suppressed Wnt/β-catenin activation. The
results of the present study suggested that ROS1 may be a
therapeutic target and a sensitizer for ATO treatment in patients
with AML. Therefore, the present study provided novel insight into
the function of ROS1 in regulating AML progression and ATO
treatment.
Acknowledgements
Not applicable.
Funding
Funding information is not applicable.
Availability of data and materials
The datasets analyzed during the current study are
available from the corresponding author on reasonable request.
Authors' contributions
JL designed the study, conducted the statistical
analysis and drafted of the article, and read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The author declares that he has no competing
interests.
References
|
1
|
Estey E and Döhner H: Acute myeloid
leukaemia. Lancet. 368:1894–1907. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Estey EH: Acute myeloid leukemia: 2013
update on risk-stratification and management. Am J Hematol.
88:318–327. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Coombs CC, Tavakkoli M and Tallman MS:
Acute promyelocytic leukemia: Where did we start, where are we now,
and the future. Blood Cancer J. 5:e3042015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cicconi L and Lo-Coco F: Current
management of newly diagnosed acute promyelocytic leukemia. Ann
Oncol. 27:1474–1481. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lo-Coco F, Cicconi L and Breccia M:
Current standard treatment of adult acute promyelocytic leukaemia.
Br J Haematol. 172:841–854. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sanz MA and Lo-Coco F: Modern approaches
to treating acute promyelocytic leukemia. J Clin Oncol. 29:495–503.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhu J, Chen Z, Lallemand-Breitenbach V and
de Thé H: How acute promyelocytic leukaemia revived arsenic. Nat
Rev Cancer. 2:705–713. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
de Thé H and Chen Z: Acute promyelocytic
leukaemia: Novel insights into the mechanisms of cure. Nat Rev
Cancer. 10:775–783. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Takahashi S: Combination therapy with
arsenic trioxide for hematological malignancies. Anticancer Agents
Med Chem. 10:504–510. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shaw AT, Hsu PP, Awad MM and Engelman JA:
Tyrosine kinase gene rearrangements in epithelial malignancies. Nat
Rev Cancer. 13:772–787. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
El-Deeb IM, Yoo KH and Lee SH: ROS
receptor tyrosine kinase: A new potential target for anticancer
drugs. Med Res Rev. 31:794–818. 2011.PubMed/NCBI
|
|
12
|
Ye M, Zhang X, Li N, Zhang Y, Jing P,
Chang N, Wu J, Ren X and Zhang J: ALK and ROS1 as targeted therapy
paradigms and clinical implications to overcome crizotinib
resistance. Oncotarget. 7:12289–12304. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Arnaoutakis K: Crizotinib in
ROS1-rearranged non-small-cell lung cancer. N Engl J Med.
372:6832015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gold KA: ROS1-targeting the one percent in
lung cancer. N Engl J Med. 371:2030–2031. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lambros L, Guibourg B and Uguen A:
ROS1-rearranged non-small cell lung cancers with concomitant
oncogenic driver alterations: About some rare therapeutic dilemmas.
Clin Lung Cancer. 19:e73–e74. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Murgo AJ: Clinical trials of arsenic
trioxide in hematologic and solid tumors: Overview of the national
cancer institute cooperative research and development studies.
Oncologist. 6 Suppl 2:S22–S28. 2001. View Article : Google Scholar
|
|
17
|
Tallman MS: What is the role of arsenic in
newly diagnosed APL? Best Pract Res Clin Haematol. 21:659–666.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Berenson JR and Yeh HS: Arsenic compounds
in the treatment of multiple myeloma: A new role for a historical
remedy. Clin Lymphoma Myeloma. 7:192–198. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Evens AM, Tallman MS and Gartenhaus RB:
The potential of arsenic trioxide in the treatment of malignant
disease: Past, present, and future. Leuk Res. 28:891–900. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Baj G, Arnulfo A, Deaglio S, Mallone R,
Vigone A, De Cesaris MG, Surico N, Malavasi F and Ferrero E:
Arsenic trioxide and breast cancer: Analysis of the apoptotic,
differentiative and immunomodulatory effects. Breast Cancer Res
Treat. 73:61–73. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen Z, Chen GQ, Shen ZX, Sun GL, Tong JH,
Wang ZY and Chen SJ: Expanding the use of arsenic trioxide:
Leukemias and beyond. Semin Hematol. 39 2 Suppl 1:S22–S26. 2002.
View Article : Google Scholar
|
|
22
|
Dilda PJ and Hogg PJ: Arsenical-based
cancer drugs. Cancer Treat Rev. 33:542–564. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Verstovsek S, Giles F, Quintás-Cardama A,
Perez N, Ravandi-Kashani F, Beran M, Freireich E and Kantarjian H:
Arsenic derivatives in hematologic malignancies: A role beyond
acute promyelocytic leukemia? Hematol Oncol. 24:181–188. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Eaves CJ and Humphries RK: Acute myeloid
leukemia and the Wnt pathway. N Engl J Med. 362:2326–2327. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Caye A, Strullu M, Guidez F, Cassinat B,
Gazal S, Fenneteau O, Lainey E, Nouri K, Nakhaei-Rad S, Dvorsky R,
et al: Juvenile myelomonocytic leukemia displays mutations in
components of the RAS pathway and the PRC2 network. Nat Genet.
47:1334–1340. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang H, Peng C, Hu Y, Li H, Sheng Z, Chen
Y, Sullivan C, Cerny J, Hutchinson L, Higgins A, et al: The Blk
pathway functions as a tumor suppressor in chronic myeloid leukemia
stem cells. Nat Genet. 44:861–871. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kühnl A, Valk PJ, Sanders MA, Ivey A,
Hills RK, Mills KI, Gale RE, Kaiser MF, Dillon R, Joannides M, et
al: Downregulation of the Wnt inhibitor CXXC5 predicts a better
prognosis in acute myeloid leukemia. Blood. 125:2985–2994. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang Y, Krivtsov AV, Sinha AU, North TE,
Goessling W, Feng Z, Zon LI and Armstrong SA: The Wnt/beta-catenin
pathway is required for the development of leukemia stem cells in
AML. Science. 327:1650–1653. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Heidel FH, Bullinger L, Feng Z, Wang Z,
Neff TA, Stein L, Kalaitzidis D, Lane SW and Armstrong SA: Genetic
and pharmacologic inhibition of β-catenin targets
imatinib-resistant leukemia stem cells in CML. Cell Stem Cell.
10:412–424. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kleppe M and Levine RL: Targeting
β-catenin in CML: Leukemia stem cells beware! Cell Stem Cell.
10:351–353. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hoffmeyer K, Raggioli A, Rudloff S, Anton
R, Hierholzer A, Del Valle I, Hein K, Vogt R and Kemler R:
Wnt/β-catenin signaling regulates telomerase in stem cells and
cancer cells. Science. 336:1549–1554. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Morikawa T, Kuchiba A, Yamauchi M,
Meyerhardt JA, Shima K, Nosho K, Chan AT, Giovannucci E, Fuchs CS
and Ogino S: Association of CTNNB1 (beta-catenin) alterations, body
mass index, and physical activity with survival in patients with
colorectal cancer. JAMA. 305:1685–1694. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang K, Li N, Yeung CH, Cooper TG, Liu XX,
Liu J, Wang WT, Li Y, Shi H and Liu FJ: Comparison of gene
expression of the oncogenic Wnt/β-catenin signaling pathway
components in the mouse and human epididymis. Asian J Androl.
17:1006–1011. 2015. View Article : Google Scholar : PubMed/NCBI
|