Introduction
The Galα1-3Galβ1-4GlcNAc-R (α-gal) epitope structure
is a carbohydrate epitope produced in virtually all non-primate
mammal species, including pigs, rats, and New World monkeys
(1). α-gal is formed by the
α1,3-galactosyltransferase (α-1,3GT)-mediated enzymatic transfer of
a galactose molecule onto terminal N-acetyllactosamine on
carbohydrate side chains of various glycoproteins and glycolipids
(2,3).
Owing to the inactivation of the α-1,3GT enzyme during evolution,
the α-gal epitope is absent in humans, apes, and Old World monkeys
(4–7).
Human blood therefore contains a large number of antibodies against
the α-gal epitope, including immunglobulin G (IgG), IgM, and IgA,
which are directly produced by B lymphocytes in response to
constant stimulation from normal gastrointestinal flora (8,9). These
α-gal-binding antibodies activate the classical complement pathway
to induce a hyperacute rejection (HAR) (10,11), which
is largely responsible for the failure of xenotransplantation.
Engineering tumor cells to express the heterologous
α-gal antigen to induce host HAR against tumor cells is a novel
antitumor strategy has been the focus of several prior studies
(12–16). Indeed, certain studies observed a
distinct complement-dependent cytolysis (CDC) of α-gal-expressing
tumor cells following treatment with normal human serum (NHS)
(12,16–18).
However, it was recently identified that A549 tumor cells
engineered to present α-gal exhibited strong resistance to this
α-gal induced CDC by NHS (19,20).
The complement system is a highly organized
proteolytic cascade whose activation can be achieved by three
different pathways (classical, mannan-binding lectin and
alternative) depending on the initiating molecules. These three
pathways all lead to the formation of the membrane attack complex
(MAC), which mediates cell lysis (21). Normally, the activation of complement
system is under the precise control of multiple factors, including
the C1 esterase inhibitor, C3b inactivator and membrane-bound
complement regulatory proteins (mCRPs), which avoid over-activation
or excessive complement consumption to achieve a balance between
immune activation and suppression (22). A substantial proportion of malignant
tumor cells overexpress mCRPs, including membrane cofactor protein
precursor (CD46), decay accelerating factor (CD55) (23,24), and
protectin (CD59) (24,25). In a retrospective study evaluating the
effectiveness of using the monoclonal antibody (mAb) therapy
trastuzumab against Her2-positive breast cancer, patients
overexpressing CD55 or CD59 had a higher relapse rate and a shorter
mean disease-free survival time than those with low expression of
CD55 or CD59; the expression of CD46 had no effect on prognosis
(26). In another study, it also
suggested that CD55 and CD59 served roles in blocking
trastuzumab-induced CDC in Her2-positive cells (27). Therefore, the overexpression of mCRPs
may be a main factor behind the observed divergences in sensitivity
to cell lysis by NHS among diverse α-gal-expressing tumor
cells.
The present study examined whether expression levels
of CD55 and CD59 in α-gal-expressing cell lines influenced their
CDC resistance. Following detection of CD55 and CD59 in various
tumor cell lines by flow cytometry (FCM), Lovo colonic cancer cells
which express low levels of CD55 and CD59, and A549 lung cancer
cells which express high levels of CD55 and CD59, were selected for
transfection with an α-1,3GT gene plasmid. CDC was observed in
α-gal-expressing Lovo and A549 cells by NHS treatment. We also
evaluated the resistant effects of CD55 and CD59 in A549 cells via
cleaving them by phosphatidylinositol-specific phospholipase C
(PI-PLC) and blocking them by mAbs.
Materials and methods
Cell lines
Human A549, SPC-A-1, GLC-82, and LTPEP-a-2 lung
cancer cells; Lovo colonic adenocarcinoma cells; SMMC-7721
hepatocarcinoma cells; SGC-7901 gastric cancer cells; MCF-7 and
BT549 breast cancer cells were obtained from The Cell Bank of Type
Culture Collection of the Chinese Academy of Sciences (Shanghai,
China). Pig iliac arterial endothelial cells (PIEC) were kindly
provided by the Key Laboratory of Transplant Engineering and
Immunology, Sichuan University. PIEC, which naturally express high
levels of α-gal, were used as a positive control to assess
complement activity in cytolysis assays.
Sera
Pooled NHS, used as the source of complement and
anti-α-gal specific antibodies provided by the Central Blood Bank
of West China Hospital, was stored at −80°C in aliquots until
assayed. Use of the human serum samples in the study was approved
by the Clinical Test and Biomedical Ethics Committee of West China
Hospital, Sichuan University.
Antibodies and reagents
Fluorescein isothiocyanate (FITC)-conjugated mouse
anti-human CD55 (cat. no. FHF055) and CD59 (cat. no. FHF059) mAbs
were purchased from Beijing 4A Biotech Co., Ltd. (Beijing, China).
The mAbs against CD55 (cat. no. SC-59092), CD59 (cat. no.
SC-59095), and β-actin (cat. no. SC-47778) for western blotting
were purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX,
USA). The horseradish peroxidase (HRP)-labeled goat anti-rat IgG
(cat. no. SC-2030) and goat anti-mouse IgG (cat. no. SC-2031) Abs
were purchased from Santa Cruz Biotechnology, Inc. The blocking
mAbs against CD55 (cat. no. CBL511) was purchased from EMDMillipore
(Billerica, MA, USA). The blocking mAbs against CD59 (cat. no.
AB9182) was purchased from Abcam (Cambridge, UK).
FITC-BS-IB4 lectin (FITC-conjugated Griffonia
simplicifolia isolectin B) which specifically binds α-gal was
purchased from Vector Laboratories, Inc. (Burlingame, CA, USA). The
100 U/ml PI-PLC and liposome transfection reagent kit Lipofectamine
2000 were purchased from Invitrogen (Thermo Fisher Scientific,
Inc., Waltham, MA, USA).
Eukaryotic expression plasmids
The eukaryotic pCMV-GT α-gal expression plasmid and
the control p1-GT plasmid, in which the cytomegalovirus promoter
did or did not regulate α-1,3GT gene expression, respectively, were
successfully constructed in a preliminary study (28).
Detection of CD55 and CD59 expression
by FCM
Cells were removed from the culture flask using
0.25% trypsin and 0.25% EDTA, and washed in 1% bovine serum albumin
(BSA; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) diluted in PBS
and centrifuged at 300 × g for 10 min. The cells were then
suspended in 100 µl 1% BSA and incubated with 10 µl FITC-CD55 or
FITC-CD59mAbs for 30 min at 37°C. FCM was performed using FACSAriaI
and data were analyzed using FACSDiva 6.0 software (both from BD
Biosciences, Franklin Lakes, NJ, USA).
Detection of CD55 and CD59 expression
by western blotting
Cells in the logarithmic growth phase were harvested
and lysed at 4°C in radioimmunoprecipitation lysis buffer (Beyotime
Institute of Biotechnology, Shanghai, China). Total protein
concentration was determined using a BCA kit (Beyotime Institute of
Biotechnology). A total of 30 µg protein from each sample was
separated by 12% SDS-PAGE and transferred to polyvinylidene
difluoride membranes. The membranes were blocked with 5% nonfat
milk in PBS-Tween (0.1% Tween in PBS). Membranes were incubated
overnight with the primary antibodies against CD55 (1:400), CD59
(1:800) and β-actin (1:8,000) in 5% nonfat milk at 4°C. After
washed with PBS-Tween 10 min × 3 times, Membranes were incubated 2
h with HRP-labeled goat anti-mouse IgG (dilution, 1:7,000) or goat
anti-rat IgG (dilution, 1:8,000) at room temperature. After washed,
the bands were visualized using chemiluminescent HRP substrate
(cat. no. WBKLS0100; EMDMillipore), and detected using the
ChemiDocXRS system. Data was analyzed by QuantityOne software
(Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Establishing stable α-gal-expressing
cell lines
The pCMV-GT or the control p1-GT plasmids 0.8 µg
mixed with 2 µl Lipofectamine2000 were diluted in 100 µl Opti-MEM
and transfected into the A549 and Lovo cell lines, then incubated
for 6 h. The transfected cells were further cultured in in
RPMI-1640 medium containing 10% fetal bovine serum for an
additional 48 h. The transfected cells were termed A549-GT (α-gal
expressing A549), A549-V (control), Lovo-GT (α-gal expressing
Lovo), and Lovo-V (control), respectively. The transfected cells
were then transferred at a 1:10 dilution into a 6-well plate where
stably transfected A549 and Lovo cells were selected following
cultivation in the presence of G418.
Following selection, stably transfected cells
expressing α-gal were identified by direct immunofluorescence
staining. A total of 50 µl FITC-BS-IB4 lectin (1:50 dilution in
RPMI-1640) per well was added into the transfected cells
(1×104), which had been plated for 24 h. After a 20-min
incubation in dark, the cells were analyzed under an inverted
fluorescence microscope.
Analysis of α-gal expression on stable transfected
cells was also performed by FCM. A total of 1×106 cells
from each cell line were incubated in 100 µl FITC-BS-IB4 lectin
(1:50 dilution in 1% BSA-PBS) for 1.5 h at 4°C in dark. Following
centrifugation at 300 × g for 10 min and immersion in 1 ml
paraformaldehyde fixative solution (1% BSA + 1% paraformaldehyde)
for 30 min at 4°C in the dark, the cells were then resuspended in
300 µl 1% BSA-PBS and analyzed by FCM, according to the
aforementioned method.
To determine α-1,3GT mRNA expression in transfected
cells, total RNA was extracted using an RNeasy Mini kit (cat. no.
74104) from (QiagenGmbH, Hilden, Germany). First-strand cDNAs were
synthesized from total RNA using 5X all-in-one RTMasterMix (G492;
Applied Biological Materials, Inc., Richmond, BC, Canada). PCR was
performed using Easy-load PCR Master Mix (cat. no. D7251; Beyotime
Institute of Biotechnology) in iCycler (Bio-Rad Laboratories,
Inc.). The PCR primer for α-1,3GT and GAPDH was synthesized by
Sangon Biotech (Shanghai, China) as following: α-1,3GT fragment
forward, 5′-TCAATGCTGCTTGTCTCA-3′ and reverse,
5′-TAAGTGCCTTCCCATA-3′; GAPDH fragment forward,
5′-GTCAGTGGTGGACCTGACCT-3′ and reverse, 5′-AGGGGAGATTCAGTGTGGTG-3′.
The following thermocycling conditions were used: 94°C for 2 min,
followed by 33 cycles of 94°C for 30 sec, 50°C for 30 sec, and 72°C
for 2 min, with a final extension step of 72°C for 10 min.
Amplification products were analyzed by 2% agarose gel
electrophoresis.
Trypan blue exclusion assay for CDC
induced by α-gal-expressing cells
A549, A549-V, A549-GT, Lovo, Lovo-V, Lovo-GT and
PIEC cells were resuspended to 1×106 per tube, and
incubated with various dilutions of NHS (0, 15, 30, and 50%) in a
total volume of 500 µl for 1 h at 37°C. Next, 50 µl of each group
of the incubated cells were mixed with the same volume of 0.4%
trypan blue. The number of living/dead cells were counted in a
hemocytometer under a light microscope, and the survival and lysis
rates were calculated as follows: survival rate (%) = number of
living cells/(number of living cells + number of dead cells) ×100;
lysis rate = 100%-survival rate.
Evaluating susceptibility of
α-gal-expressing A549 cells to CDC following pre-exposure to
PI-PLC
PBS diluted PI-PLC to concentrations of 0.001, 0.01,
0.05, 0.1, 0.2, or 0.5 U/ml were used to pre-treat A549, A549-V and
A549-GT cells at 37°C for 1 h. Cells were then incubated with 500
µl 50% NHS, and assessed using a trypan blue exclusion assay, as
aforementioned.
A concentration of 0.1 U/ml PI-PLC was selected to
determine the specific cleavage effects of PI-PLC on membrane CD55
and CD59 at 37°C for 1 h. CD55 and CD59 in A549, A549-V and A549-GT
cells were tested by western blot analysis as aforementioned.
Results were compared with those from cells that did not undergo
PI-PLC treatment.
Following treatment with 0.1 U/ml PI-PLC, levels of
CD55 and CD59 in A549-GT cells were assessed by FCM, and the
results compared with those obtained prior to PI-PLC treatment.
Evaluating susceptibility of
α-gal-expressing A549 cells to CDC following blocking CD55 and
CD59
A549, A549-V and A549-GT cells (1×106)
were pre-incubated with 10 µg/ml anti-CD55, anti-CD59 or anti-CD55
together with anti-CD59 at room temperature for 10 min, then 50%
NHS was added to give a final volume of 500 µl and cells were
incubated at 37°C for 1 h. Each sample was assessed using trypan
blue exclusion assay, as aforementioned.
Statistical analysis
Results were expressed as the mean ± standard
deviation. Statistical significance was tested using an independent
Student's t-test in two groups or one-way analysis of variance for
experiments consisting of more than two groups, with a
Student-Newman-Keuls test used as the post hoc test. P<0.05 was
considered to indicate a statistically significant difference. SPSS
software 16.0 (SPSS, Inc., Chicago, IL, USA) was used.
Results
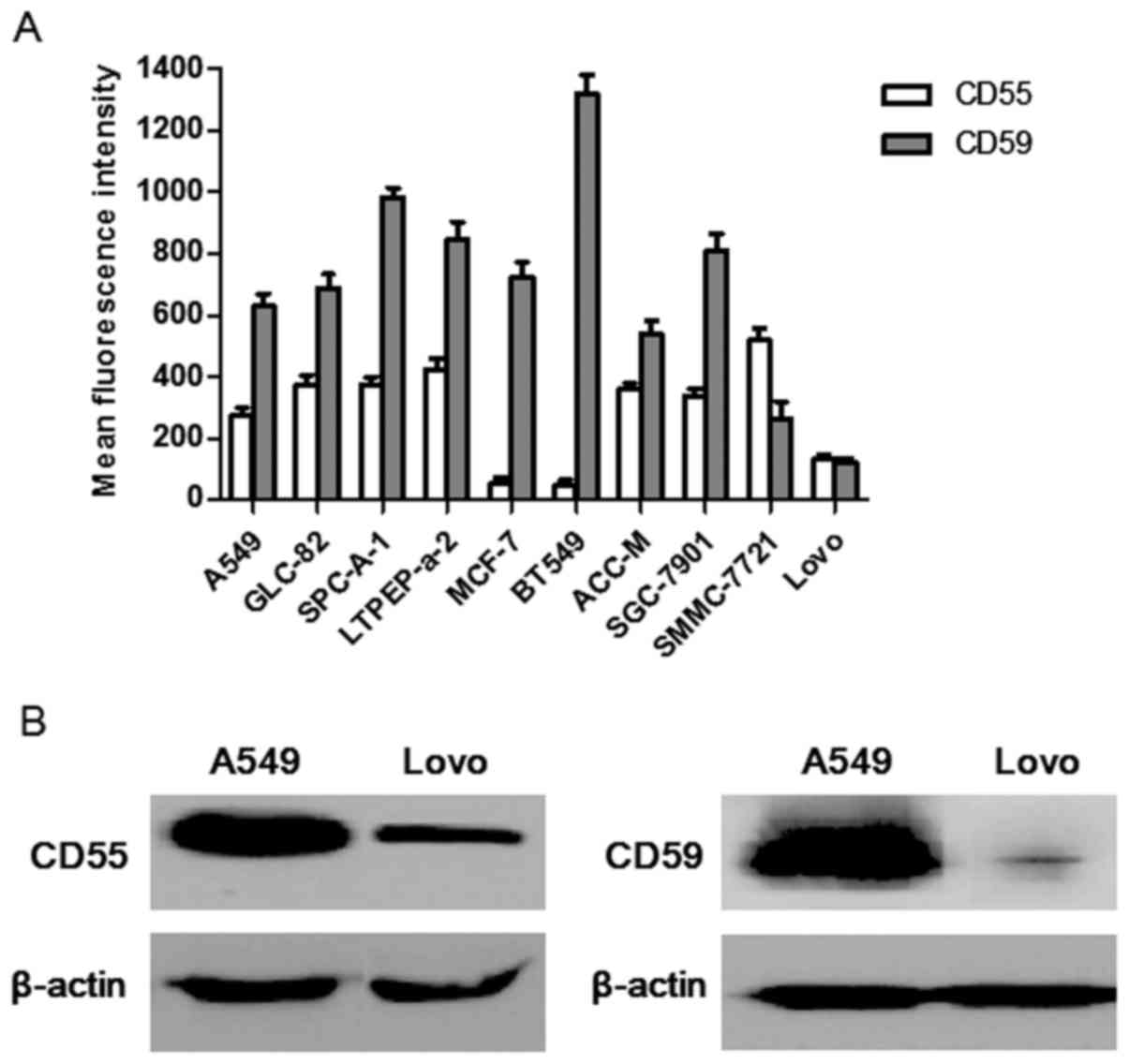
Expression of CD55 and CD59, detected
by FCM
A number of cell lines were evaluated for their
surface CD55 and CD59 protein levels by FCM. The results revealed
that these cell lines expressed distinct levels of surface CD55 and
CD59 (Fig. 1A). Specifically A549
cells expressed high levels of CD55 (mean fluorescence intensity
(MFI)=276±23) and CD59 (MFI=629±42), whereas Lovo cells expressed
relatively low levels of CD55 (MFI=134±12) and CD59 (MFI=119±17).
Since A549 and Lovo cells differed significantly in their
expression of CD55 and CD59, Lovo cells were chosen as the ideal
comparative cell line to assess whether CD55 and CD59 expression
influenced the α-gal-mediated cytolysis to compare with A549 cells
in the present study.
Similar results were observed in western blot
analysis (Fig. 1B). Comparing these
two cell lines, the CD55 and CD59 protein level in A549 cells were
evidently higher than Lovo cells.
Expression of α-1,3GT mRNA in stably
transfected A549 and Lovo cells
The results of the present study revealed that
α-1,3GT mRNA was successful expressed in stably transfected A549-GT
and Lovo-GT cells, but was absent from the control A549, A549-V,
Lovo, and Lovo-V cells, as detected by agarose gel electrophoresis
(Fig. 2A).
 | Figure 2.Establishing stable transfected
α-gal-expressing cell lines. (A) α-1,3GT mRNA expression in A549,
A549-V, A549-GT, Lovo, Lovo-V and Lovo-GT cells were detected by
reverse transcription-polymerase chain reaction. The amplified
product of α-1,3GT was detected by agarose gel electrophoresis in
lane 2, 4 and 6 of the two gels. GAPDH was used as loading control
in lane 1, 3 and 5 of the two gels. (B) Expression of α-gal epitope
in each group of cells were detected by direct immunofluorescence
(magnification, ×200). FITC-conjugated BS-IB4 lectin staining was
performed to probe α-gal epitope. (B-a) A549-GT, (B-b) A549, (B-c)
A549-V, (B-d) Lovo-GT, (B-e) Lovo, (B-f) Lovo-V and (B-g) positive
control PIEC cells. (C) Expression of α-gal epitope in each group
of cells were stained with FITC-BS-IB4 lectin, then analyzed by
flow cytometry. Error bars depict standard deviations. FITC,
fluorescein isothiocyanate; α-gal, Galα1-3Galβ1-4GlcNAc-R; α-1,3GT,
α1,3-galactosyltransferase; PIEC, pig iliac arterial endothelial
cells; A549-GT, α-gal expressing A549; A549-V, control. |
Expression of α-gal epitope on A549
and Lovo cells membranes
The stably transfected A549-GT and Lovo-GT cells
exhibited high α-gal epitope expression, as the observed staining
was similar to that of PIEC positive-control cells. No fluorescent
signals were observed on control A549, A549-V, Lovo, or Lovo-V cell
membranes, which indicated that α-gal epitope was specifically
expressed in A549-GT and Lovo-GT cells (Fig. 2B).
The percentage of α-gal-expressing cells in the
stably transfected A549-GT and Lovo-GT cells, as well as in the
PIEC cells were 80.1±3.2, 95.4±5.2, and 98.4±1.7%, respectively, as
detected by FCM (Fig. 2C).
Expression of CD55 and CD59 on
α-gal-expressing cells influences their sensitivity to CDC
A549, A549-V, A549-GT, Lovo, Lovo-V, Lovo-GT and
PIEC cells were incubated with 0, 15, 30 or 50% NHS, and survival
rates of these cells were calculated by trypan blue exclusion assay
(Fig. 3A). A549, A549-V and A549-GT
cells all exhibited resistance to CDC, and no evident changes were
observed when the cells were exposed to a series of NHS
concentrations. As the concentration of NHS increased from 0–50%,
the survival rate of Lovo-GT cells decreased gradually, reduced
from 96.4 to 0.2% (P<0.05). No significant cytolysis was
observed in either Lovo or Lovo-V cells at any NHS concentration.
In other words, 99.8% of Lovo-GT cells were killed when incubated
with 50% NHS. The survival rate of Lovo-GT and PIEC
positive-control cells exhibited similar concentration-dependency
in NHS-induced CDC.
 | Figure 3.Expression of CD55 and CD59 on
α-gal-expressing cells influences their sensitivity to CDC. (A)
A549, A549-V, A549-GT, Lovo, Lovo-V, Lovo-GT and positive control
PIEC cells were incubated with various dilutions of NHS (0, 15, 30,
50%) and survival rates were analyzed by trypan blue staining.
Error bars showed standard deviations (*P<0.05 vs. the control).
(B) A549, A549-V, A549-GT Cells were pre-treated with various
concentrations of PI-PLC (0.001, 0.01, 0.05, 0.1, 0.2, or 0.5
U/ml), incubated with 50% NHS, and survival rates were analyzed by
trypan blue staining. Error bars showed standard deviations.
*P<0.05, vs. the control. CD55, decay accelerating factor; CD59,
protectin; NHS, normal human serum; PI-PLC,
phosphatidylinositol-specific phospholipase C; A549-GT, α-gal
expressing A549; A549-V, control. |
Increased susceptibility to CDC
following cleavage of CD55 and CD59 in α-gal-expressing A549
cells
Incubating A549-GT cells with 0.001, 0.01, 0.05,
0.1, 0.2, or 0.5 U/ml PI-PLC resulted in cell survival rates of
84.2, 72.3, 65.6, 54.9, 56.4 and 53.8%, following treatment with
50% NHS (Fig. 3B). A549-GT cells
treated with PI-PLC of various dilutions experienced a significant
decrease in survival rates (P<0.05). At a PI-PLC dose of 0.1
U/ml, the survival rate of A549-GT cells decreased to 54.9%, and
did not decrease further at higher doses of PI-PLC. No significant
cell death was observed in the control A549 or A549-V cells in the
PI-PLC concentration series.
PI-PLC cleavage effect on CD55 and
CD59 in A549-GT cells
CD55 and CD59 expression in A549, A549-V and A549-GT
cells was almost completely abrogated following exposure to 0.1
U/ml PI-PLC (Fig. 4A).
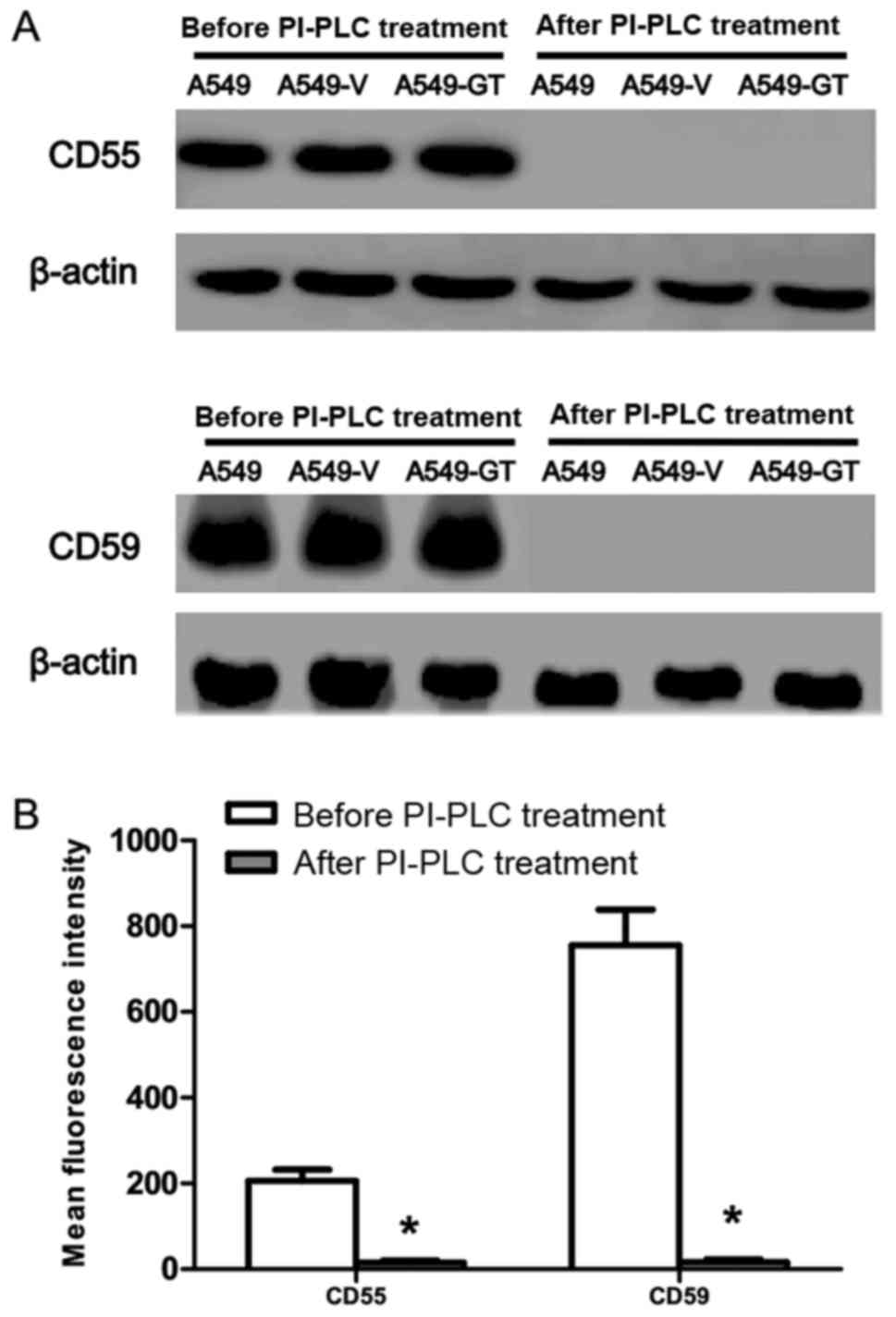 | Figure 4.Effects of PI-PLC treatment on CD55
and CD59 protein level in A549-GT cells. (A) Following 0.1 U/ml
PI-PLC treatment, CD55 and CD59 were tested by western blot in
A549, A549-V, A549-GT, Lovo, Lovo-V and Lovo-GT cells, compared
with that prior to PI-PLC treatment. (B) After 0.1 U/ml PI-PLC
treatment, A549-GT cells was incubated with fluorescein
isothiocyanate-conjugated anti-human monoclonal antibodies. CD55
and CD59 were analyzed by flow cytometry, compared with that prior
to PI-PLC treatment. Error bars showed standard deviations.
*P<0.05 vs. the control. CD55, decay accelerating factor; CD59,
protectin; PI-PLC, phosphatidylinositol-specific phospholipase C;
A549-GT, α-gal expressing A549; A549-V, control. |
The data obtained from FCM also confirmed these
results, where CD55 (MFI=15±5) and CD59 (MFI=16±7) expression
following PI-PLC treatment was significantly lower than that of
CD55 (MFI=206±26) and CD59 (MFI=755±84) expression prior to PI-PLC
treatment (Fig. 4B).
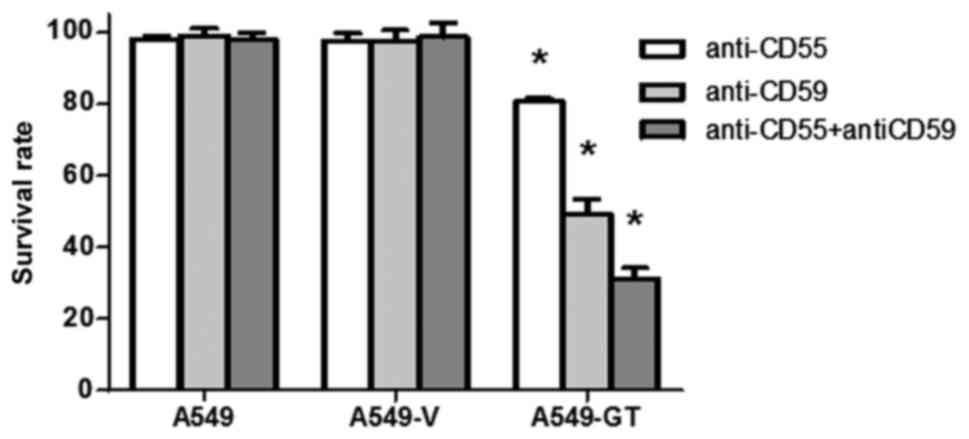
Blocking antibodies against CD55 and
CD59 enhanced susceptibility to CDC in A549-GT cells
Following treatment with anti-CD55 or anti-CD59
antibodies alone, the survival rates of A549-GT cells significantly
decreased to 80.5 and 49.3%, respectively (P<0.05). Combination
treatment with anti-CD55 and anti-CD59 further decreased the
survival rate to 31.2% (P<0.05). No significant decrease in cell
survival was observed in the control A549 or A549-V cells in the
anti-CD55-, anti-CD59- and anti-CD55 with anti-CD59-treated groups
(Fig. 5).
Discussion
In xenotransplantation across species, such as pig
to human, α-gal expression on the cell surface of non-primate
organs is the major xenoantigen responsible for HAR. A novel
promising therapeutic approach can elicit the host anti-α-gal
immune response against the tumor cells to kill and/or inhibit
tumor growth by expressing heterologous α-gal antigen. Unfer et
al (18) demonstrated that the
co-incubation of α-gal-expressing MC38 colon cancer cells with 50%
NHS led to 98% cell death. Other studies also found that
transfection of the α-1,3GT gene into the A375 melanoma cells
(12), MIA PaCa-2 pancreatic cancer
cells and Huh7 hepatocellular carcinoma cells (16), led to the differentiated
susceptibility to CDC, compared with untransfected cells.
Although these studies (12,16,18)
revealed that the expression of the α-gal xenoantigen sensitized
tumor cells to NHS-induced cytolysis, certain other studies
observed no statistical significance. Xing et al (14) examined the susceptibility of human
SGC-7901, SPC-A-1 and A375 cells to NHS-mediated cytolysis with an
α-gal epitope expressed by adenoviral vector-mediated transfer of
the pig α-1,3GT gene. No evident cell lysis was observed following
incubation with 10, 20 or 40% NHS, as analyzed using a trypan blue
exclusion assay. Jäger et al (29) revealed that α1,3GT-transfected HT1080α
tumor cells that expressed high levels of CD46, CD55, and CD59 were
not lysed by NHS, whereas cytolysis reached up to 75–80% following
PI-PLC-treatment.
More recently, the use of therapeutic antibodies is
among the most active fields of cancer research. The binding of
tumor antigens and the corresponding antibodies can initiate the
killing of tumor cells through CDC, antibody-dependent
cell-mediated cytotoxicity (ADCC) and signal-pathway alteration
(30). CDC is a mechanism that can
lead to tumor cell lysis and also stimulate the adaptive immune
response, as the fragments released upon CDC function to attract
and activate immune cells (31). A
number of types of tumor cells suppress the activation of the
complement system by overexpressing mCRPs to evade complement
attack (32–36). The main mCRPs are complement receptor
1 (CD35), CD46, CD55 and CD59 (37,38). CD55
is a membrane glycosyl-phosphatidyl inositol (GPI)-anchored
glycoprotein that accelerates the decay of C3 convertases and C5
convertases, leading to the suppression of MAC activation (35). CD59 is also a small GPI-anchored
glycoprotein that prevents the formation of a functional MAC by
inhibiting the incorporation of multiple copies of C9 on the target
cell membrane (37,39). In the present study, we hypothesized
that CD59 and CD55 expression may have a role in the resistance of
α-gal-expressing A549 cells to α-gal-mediated complement
attack.
Other factors which may confer tumor resistance to
anti-α-gal Ab-mediated cytolysis were proposed in previous studies,
such as blood type, anti-α-gal Ab titers, and concentration of
complementary factors. McMorrow et al (40) evaluated the anti-α-gal antibodies
levels in A, B, AB, and O serum samples using ELISA and flow
cytometry, and observed a significant reduction in α-gal reactive
IgG in serum samples from B-antigen-expressing donors (B, AB),
comparing with non-B-antigen-expressing donors (A, O). Wang et
al (41) revealed that the
proportion of elderly individuals with low-affinity anti-α-gal Abs
is six-fold higher than that in young individuals, verifying that
there might be an age-associated change in the affinity of
anti-α-gal antibodies. Koopmans et al (42) demonstrated that little α-gal induced
cytolysis occurred in pig mesencephalon cells cultured with NHS
containing low anti-α-gal IgM titers, whereas NHS containing
40-fold higher anti-α-gal IgM titers induced cell death in 65% of
cells. In the present study, the influence of blood type,
anti-α-gal Ab titers, and concentration of complementary factors in
the experiments was eliminated by: i) Using the same pooled serum
(frozen in aliquots at −80°C) from healthy and young human donors;
and ii) using the pig-derived PIEC cell line, which highly
expresses α-gal, as a positive control to assess antibodies and
complement activity in cytolysis assays, where serum could be used
only if the 50% pooled NHS concentration killed >95% of the PIEC
cells.
In the present study, A549 and Lovo cells were
chosen for further manipulation owing to their endogenous high and
low expression of both CD55 and CD59, respectively, with the
purpose of further confirmation of the role of mCRPs in the
α-gal/NHS CDC system. Lovo-GT cells were significantly more
susceptible to NHS-mediated cytolysis, as almost all Lovo-GT cells
were killed upon treatment with 50% NHS. By contrast, A549-GT cells
exhibited resistance to NHS-induced cytolysis with survival rates
as high as 95%. Consistent with prior speculation, in the
α-gal/NHS-mediated cytolysis system, tumor cells with low CD55 and
CD59 expression were more susceptible to CDC, whereas those with
high expression of CD55 and CD59 might inhibit CDC.
The non-human PI-PLC enzyme can cleave the
GPI-anchored mCRPs CD55 and CD59 at the cell membrane (43,44).
Therefore, a PI-PLC enzyme and corresponding blocking antibody were
used to assess the suspected inhibitory effect of CD55 and CD59 on
CDC. With an increase in PI-PLC dosage (0.001–0.1 U/ml), A549-GT
survival rates significantly decreased from 96.9 to 54.9%, which
indicated that the cell sensitivity to NHS increased along with the
enzyme concentration. When cells were treated with anti-CD55 or
anti-CD59 blocking antibodies alone, the survival rate of A549-GT
cells significantly decreased to 80.5 and 49.3%, respectively. The
combined use of anti-CD55 and anti-CD59 further decreased the
survival rate to 31.2%. The aforementioned results confirmed that
the destruction of CD55 and CD59 functions on tumor cell membrane
can reduce the resistance to α-gal/NHS-mediated cell lysis.
In the present study, the killing effect of NHS
induced CDC on A549-GT cells did not reach an ideal proportion,
which may be a result of the inhibitory effects mediated by other
complement regulatory proteins that were not assessed, such as
soluble complement regulatory proteins factor H, factor I,
C1-inhibitor or mCRPs CD35, CD46. Other than by the aberrant
expression of complement regulatory proteins, tumor cells can evade
attack by the complement system via other mechanisms, including the
repair of cell damage and preventing formation of the MAC. To make
full use of the α-gal/NHS therapy system to kill tumor cells,
further in depth investigation into the possible influencing
factors will be a necessity.
The in vitro cellular model presented in the
current study may be difficult to adapt to in vivo studies
owing to the lack of appropriate animal models. α-Gal was used as a
target antigen to induce the complement cascade reaction to
eliminate tumor cells, which may represent a prospective cancer
therapy for human being in the future.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Sciences Foundation of China (grant no. 30470762).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HZ and YPW conceived and designed the experiments.
YW, JL, YJY, ZW, FQ and SMZ performed the experiments. YW and JL
analyzed the data. YW and JL wrote the paper. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Clinical Test
and Biomedical Ethics Committee of West China Hospital, Sichuan
University (2012 130).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CDC
|
complement-dependent cytolysis
|
|
NHS
|
normal human serum
|
|
mCRPs
|
membrane-bound complement regulatory
proteins
|
|
HAR
|
hyperacute rejection
|
|
MAC
|
membrane attack complex
|
|
mAbs
|
monoclonal antibodies
|
|
PI-PLC
|
phosphatidylinositol-specific
phospholipase C
|
|
FCM
|
flow cytometry
|
|
PIEC
|
pig iliac arterial endothelial
cells
|
|
ADCC
|
antibody-dependent cell-mediated
cytotoxicity
|
|
MFI
|
mean fluorescence intensity
|
References
|
1
|
Kobayashi T and Cooper DK: Anti-Gal,
alpha-Gal epitopes and xenotransplantation. Subcell Biochem.
32:229–257. 1999.PubMed/NCBI
|
|
2
|
Eto T, Ichikawa Y, Nishimura K, Ando S and
Yamakawa T: Chemistry of lipid of the posthemyolytic residue or
stroma of erythrocytes. XVI. Occurrence of ceramide pentasaccharide
in the membrane of erythrocytes and reticulocytes of rabbit. J
Biochem. 64:205–213. 1968. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Galili U, Shohet SB, Kobrin E, Stults CL
and Macher BA: Man, apes, and Old World monkeys differ from other
mammals in the expression of alpha-galactosyl epitopes on nucleated
cells. J Biol Chem. 263:17755–17762. 1988.PubMed/NCBI
|
|
4
|
Koike C, Fung JJ, Geller DA, Kannagi R,
Libert T, Luppi P, Nakashima I, Profozich J, Rudert W, Sharma SB,
et al: Molecular basis of evolutionary loss of the alpha
1,3-galactosyltransferase gene in higher primates. J Biol Chem.
277:10114–10120. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Roy BB, Jinno-Oue A, Shinagawa M, Shimizu
A, Tamura K, Shimizu N, Tanaka A and Hoshino H: Isolation of the
feline alpha1,3-galactosyltransferase gene, expression in
transfected human cells and its phylogenetic analysis. J Exp Zool B
Mol Dev Evol. 306:59–69. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lanteri M, Giordanengo V, Vidal F, Gaudray
P and Lefebvre JC: A complete alpha1,3-galactosyltransferase gene
is present in the human genome and partially transcribed.
Glycobiology. 12:785–792. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Galili U: The alpha-gal epitope and the
anti-Gal antibody in xenotransplantation and in cancer
immunotherapy. Immunol Cell Biol. 83:674–686. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Peng SY and Wang WJ: The alpha-gal epitope
(Gal alpha 1–3 Gal beta 1–4 GlcNAc-R) in xenotransplantation. Sheng
Li Ke Xue Jin Zhan. 34:248–250. 2003.(In Chinese). PubMed/NCBI
|
|
9
|
Galili U, Anaraki F, Thall A, Hill-Black C
and Radic M: One percent of human circulating B lymphocytes are
capable of producing the natural anti-Gal antibody. Blood.
82:2485–2493. 1993.PubMed/NCBI
|
|
10
|
Kobayashi T: Problems and perspectives for
clinical organ xenotransplantation. Nihon Jinzo Gakkai Shi.
47:83–93. 2005.PubMed/NCBI
|
|
11
|
Walpen AJ, Mohacsi P, Frey C, Roos A, Daha
MR and Rieben R: Activation of complement pathways in
xenotransplantation: an in vitro study. Transpl Immunol. 9:271–280.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Link CJ Jr, Seregina T, Atchison R, Hall
A, Muldoon R and Levy JP: Eliciting hyperacute xenograft response
to treat human cancer: alpha (1,3) galactosyltransferase gene
therapy. Anticancer Res. 18:2301–2308. 1998.PubMed/NCBI
|
|
13
|
Aubert M, Crotte C, Bernard JP, Lombardo
D, Sadoulet MO and Mas E: Decrease of human pancreatic cancer cell
tumorigenicity by alpha1,3galactosyltransferase gene transfer. Int
J Cancer. 107:910–918. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xing L, Xia GH, Fei J, Huang F and Guo LH:
Adenovirus-mediated expression of pig alpha (1, 3)
galactosyltransferase reconstructs Gal alpha (1, 3) gal epitope on
the surface of human tumor cells. Cell Res. 11:116–124. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Deriy L, Chen ZC, Gao GP and Galili U:
Expression of alpha-gal epitopes on HeLa cells transduced with
adenovirus containing alpha1,3galactosyltransferase cDNA.
Glycobiology. 12:135–144. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yoshimura N, Sawada T, Furusawa M and
Fuchinoue S: Expression of xenoantigen transformed human cancer
cells to be susceptible to antibody-mediated cell killing. Cancer
Lett. 164:155–160. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Takeuchi Y, Porter CD, Strahan KM, Preece
AF, Gustafsson K, Cosset FL, Weiss RA and Collins MK: Sensitization
of cells and retroviruses to human serum by (alpha 1–3)
galactosyltransferase. Nature. 379:85–88. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Unfer RC, Hellrung D and Link CJ Jr:
Immunity to the alpha (1,3)galactosyl epitope provides protection
in mice challenged with colon cancer cells expressing alpha
(1,3)galactosyl-transferase: A novel suicide gene for cancer gene
therapy. Cancer Res. 63:987–993. 2003.PubMed/NCBI
|
|
19
|
Liu M, Zhu SM, Zheng H, Wang Y, Wang Z,
Yang YJ, Wu YX, Zeng YZ and Wang YP: Cloning of splicing variants
of alpha1,3-galactosyltransferase cDNA of Chinese Banna Minipig
inbred line and its expression in human cells. Sichuan Da Xue Xue
Bao Yi Xue Ban. 43:145–150. 2012.PubMed/NCBI
|
|
20
|
Qin F, Zhu SM, Zheng H, Wang Z, Wang Y,
Zuo Y, Chen J, Sun WL and Wang YP: Establishment and identification
of human lung adenocarcinoma cell line stably expressing the
alpha1, 3-galaetosyltransferase gene from pig. Sichuan Da Xue Xue
Bao Yi Xue Ban. 41:194–198. 2010.PubMed/NCBI
|
|
21
|
Bajic G, Degn SE, Thiel S and Andersen GR:
Complement activation, regulation and molecular basis for
complement-related diseases. EMBO J. 34:2735–2757. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jurianz K, Ziegler S, Donin N, Reiter Y,
Fishelson Z and Kirschfink M: K562 erythroleukemic cells are
equipped with multiple mechanisms of resistance to lysis by
complement. Int J Cancer. 93:848–854. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Loberg RD, Day LL, Dunn R, Kalikin LM and
Pienta KJ: Inhibition of decay-accelerating factor (CD55)
attenuates prostate cancer growth and survival in vivo. Neoplasia.
8:69–78. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ziller F, Macor P, Bulla R, Sblattero D,
Marzari R and Tedesco F: Controlling complement resistance in
cancer by using human monoclonal antibodies that neutralize
complement-regulatory proteins CD55 and CD59. Eur J Immunol.
35:2175–2183. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Treon SP, Mitsiades C, Mitsiades N, Young
G, Doss D, Schlossman R and Anderson KC: Tumor cell expression of
CD59 is associated with resistance to CD20 Serotherapy in patients
with B-cell malignancies. J Immunother (1991). 24:263–271. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu M, Yang YJ, Zheng H, Zhong XR, Wang Y,
Wang Z, Wang YG and Wang YP: Membrane-bound complement regulatory
proteins are prognostic factors of operable breast cancer treated
with adjuvant trastuzumab: A retrospective study. Oncol Rep.
32:2619–2627. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang Y, Yang YJ, Wang Z, Liao J, Liu M,
Zhong XR, Zheng H and Wang YP: CD55 and CD59 expression protects
HER2-overexpressing breast cancer cells from trastuzumab-induced
complement-dependent cytotoxicity. Oncol Lett. 14:2961–2969. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhu S, Wang Y, Zheng H, Cheng J, Lu Y,
Zeng Y, Wang Y and Wang Z: Cloning of Chinese Banna minipig
inbred-line alpha1,3-galactosyltransferase gene and construction of
its recombinant eukaryotic expression vector. Sheng Wu Yi Xue Gong
Cheng Xue Za Zhi. 26:360–365. 2009.PubMed/NCBI
|
|
29
|
Jäger U, Takeuchi Y and Porter C:
Induction of complement attack on human cells by Gal (alpha1,3)Gal
xenoantigen expression as a gene therapy approach to cancer. Gene
Ther. 6:1073–1083. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hendriks D, Choi G, de Bruyn M, Wiersma VR
and Bremer E: Antibody-based cancer therapy: successful agents and
novel approaches. Int Rev Cell Mol Biol. 331:289–383. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shuptrine CW, Surana R and Weiner LM:
Monoclonal antibodies for the treatment of cancer. Semin Cancer
Biol. 22:3–13. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hakulinen J, Junnikkala S, Sorsa T and
Meri S: Complement inhibitor membrane cofactor protein (MCP; CD46)
is constitutively shed from cancer cell membranes in vesicles and
converted by a metalloproteinase to a functionally active soluble
form. Eur J Immunol. 34:2620–2629. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bellone S, Roque D, Cocco E, Gasparrini S,
Bortolomai I, Buza N, Abu-Khalaf M, Silasi DA, Ratner E, Azodi M,
et al: Downregulation of membrane complement inhibitors CD55 and
CD59 by siRNA sensitises uterine serous carcinoma overexpressing
Her2/neu to complement and antibody-dependent cell cytotoxicity in
vitro: Implications for trastuzumab-based immunotherapy. Br J
Cancer. 106:1543–1550. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kesselring R, Thiel A, Pries R,
Fichtner-Feigl S, Brunner S, Seidel P, Bruchhage KL and Wollenberg
B: The complement receptors CD46, CD55 and CD59 are regulated by
the tumour microenvironment of head and neck cancer to facilitate
escape of complement attack. Eur J Cancer. 50:2152–2161. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Devarapu SK, Mamidi S, Plöger F, Dill O,
Blixt O, Kirschfink M and Schwartz-Albiez R: Cytotoxic activity
against human neuroblastoma and melanoma cells mediated by IgM
antibodies derived from peripheral blood of healthy donors. Int J
Cancer. 138:2963–2973. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fishelson Z, Donin N, Zell S, Schultz S
and Kirschfink M: Obstacles to cancer immunotherapy: expression of
membrane complement regulatory proteins (mCRPs) in tumors. Mol
Immunol. 40:109–123. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gorter A and Meri S: Immune evasion of
tumor cells using membrane-bound complement regulatory proteins.
Immunol Today. 20:576–582. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mamidi S, Cinci M, Hasmann M, Fehring V
and Kirschfink M: Lipoplex mediated silencing of membrane
regulators (CD46, CD55 and CD59) enhances complement-dependent
anti-tumor activity of trastuzumab and pertuzumab. Mol Oncol.
7:580–594. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Loberg RD, Wojno KJ, Day LL and Pienta KJ:
Analysis of membrane-bound complement regulatory proteins in
prostate cancer. Urology. 66:1321–1326. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
McMorrow IM, Comrack CA, Nazarey PP, Sachs
DH and DerSimonian H: Relationship between ABO blood group and
levels of Gal alpha,3Galactose-reactive human immunoglobulin G.
Transplantation. 64:546–549. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang L, Anaraki F, Henion TR and Galili U:
Variations in activity of the human natural anti-Gal antibody in
young and elderly populations. J Gerontol A Biol Sci Med Sci.
50:M227–M233. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Koopmans J, de Haan A, Bruin E, van der
Gun I, van Dijk H, Rozing J, de Leij L and Staal M: Individual
human serum differs in the amount of antibodies with affinity for
pig fetal ventral mesencephalic cells and the ability to lyse these
cells by complement activation. Cell Transplant. 13:631–637. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
White MJ, Boyd JM, Horswill AR and Nauseef
WM: Phosphatidylinositol-specific phospholipase C contributes to
survival of Staphylococcus aureus USA300 in human blood and
neutrophils. Infect Immun. 82:1559–1571. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Cummerson JA, Flanagan BF, Spiller DG and
Johnson PM: The complement regulatory proteins CD55 (decay
accelerating factor) and CD59 are expressed on the inner acrosomal
membrane of human spermatozoa as well as CD46 (membrane cofactor
protein). Immunology. 118:333–342. 2006. View Article : Google Scholar : PubMed/NCBI
|