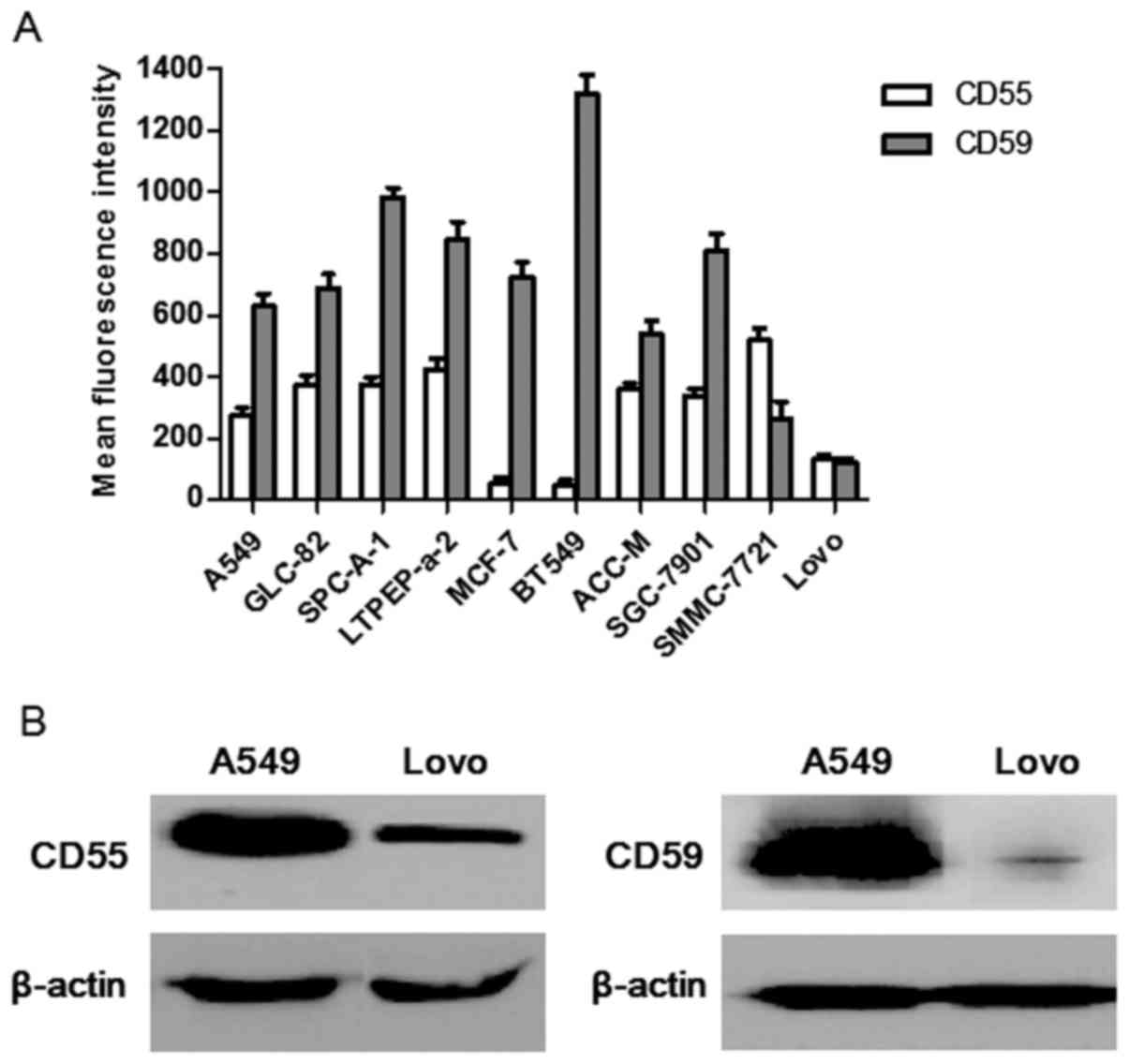
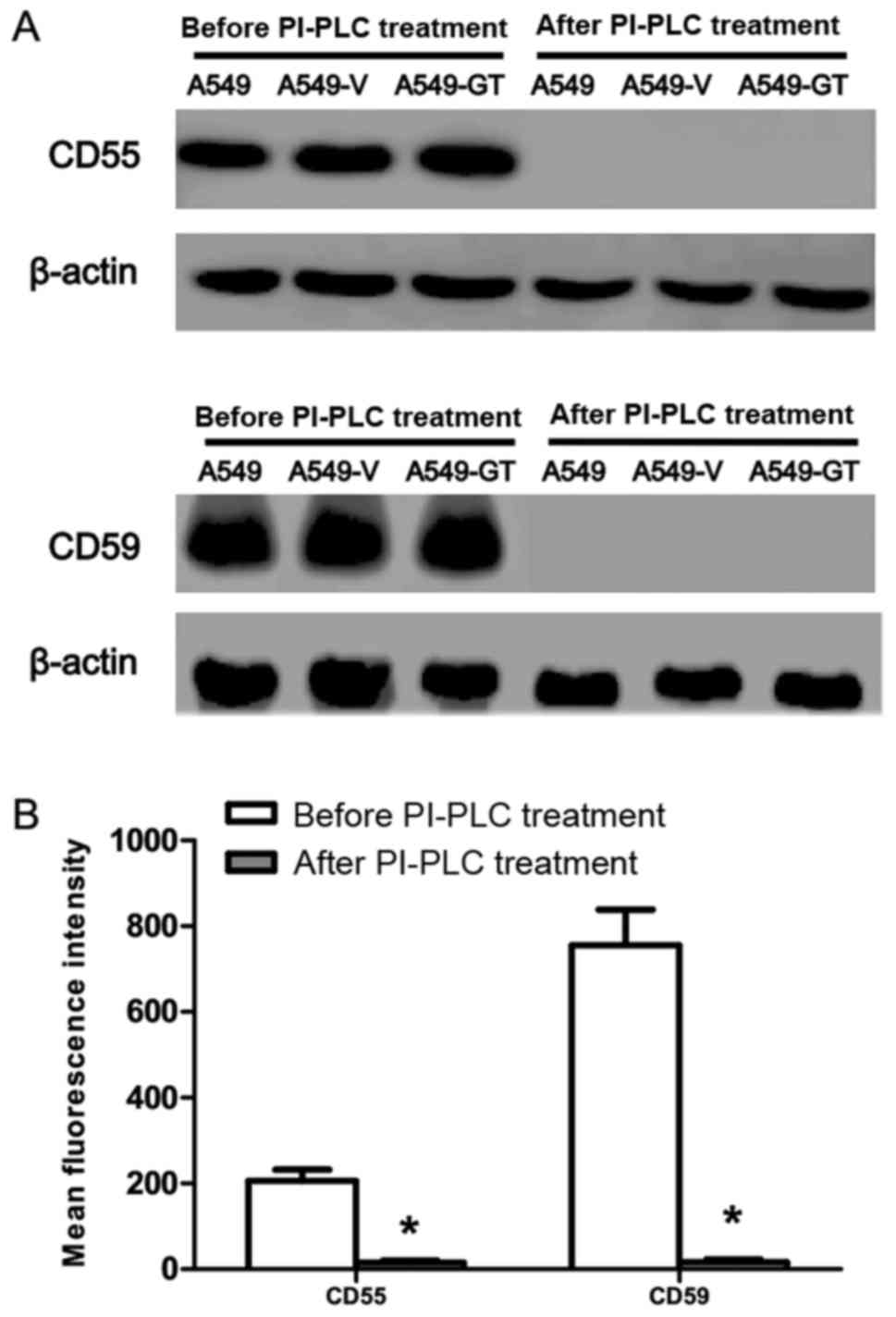
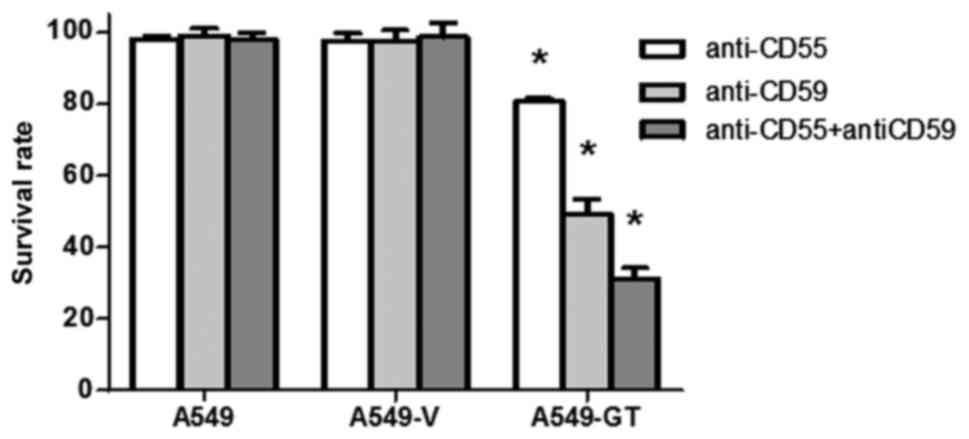
|
1
|
Kobayashi T and Cooper DK: Anti-Gal,
alpha-Gal epitopes and xenotransplantation. Subcell Biochem.
32:229–257. 1999.PubMed/NCBI
|
|
2
|
Eto T, Ichikawa Y, Nishimura K, Ando S and
Yamakawa T: Chemistry of lipid of the posthemyolytic residue or
stroma of erythrocytes. XVI. Occurrence of ceramide pentasaccharide
in the membrane of erythrocytes and reticulocytes of rabbit. J
Biochem. 64:205–213. 1968. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Galili U, Shohet SB, Kobrin E, Stults CL
and Macher BA: Man, apes, and Old World monkeys differ from other
mammals in the expression of alpha-galactosyl epitopes on nucleated
cells. J Biol Chem. 263:17755–17762. 1988.PubMed/NCBI
|
|
4
|
Koike C, Fung JJ, Geller DA, Kannagi R,
Libert T, Luppi P, Nakashima I, Profozich J, Rudert W, Sharma SB,
et al: Molecular basis of evolutionary loss of the alpha
1,3-galactosyltransferase gene in higher primates. J Biol Chem.
277:10114–10120. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Roy BB, Jinno-Oue A, Shinagawa M, Shimizu
A, Tamura K, Shimizu N, Tanaka A and Hoshino H: Isolation of the
feline alpha1,3-galactosyltransferase gene, expression in
transfected human cells and its phylogenetic analysis. J Exp Zool B
Mol Dev Evol. 306:59–69. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lanteri M, Giordanengo V, Vidal F, Gaudray
P and Lefebvre JC: A complete alpha1,3-galactosyltransferase gene
is present in the human genome and partially transcribed.
Glycobiology. 12:785–792. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Galili U: The alpha-gal epitope and the
anti-Gal antibody in xenotransplantation and in cancer
immunotherapy. Immunol Cell Biol. 83:674–686. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Peng SY and Wang WJ: The alpha-gal epitope
(Gal alpha 1–3 Gal beta 1–4 GlcNAc-R) in xenotransplantation. Sheng
Li Ke Xue Jin Zhan. 34:248–250. 2003.(In Chinese). PubMed/NCBI
|
|
9
|
Galili U, Anaraki F, Thall A, Hill-Black C
and Radic M: One percent of human circulating B lymphocytes are
capable of producing the natural anti-Gal antibody. Blood.
82:2485–2493. 1993.PubMed/NCBI
|
|
10
|
Kobayashi T: Problems and perspectives for
clinical organ xenotransplantation. Nihon Jinzo Gakkai Shi.
47:83–93. 2005.PubMed/NCBI
|
|
11
|
Walpen AJ, Mohacsi P, Frey C, Roos A, Daha
MR and Rieben R: Activation of complement pathways in
xenotransplantation: an in vitro study. Transpl Immunol. 9:271–280.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Link CJ Jr, Seregina T, Atchison R, Hall
A, Muldoon R and Levy JP: Eliciting hyperacute xenograft response
to treat human cancer: alpha (1,3) galactosyltransferase gene
therapy. Anticancer Res. 18:2301–2308. 1998.PubMed/NCBI
|
|
13
|
Aubert M, Crotte C, Bernard JP, Lombardo
D, Sadoulet MO and Mas E: Decrease of human pancreatic cancer cell
tumorigenicity by alpha1,3galactosyltransferase gene transfer. Int
J Cancer. 107:910–918. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xing L, Xia GH, Fei J, Huang F and Guo LH:
Adenovirus-mediated expression of pig alpha (1, 3)
galactosyltransferase reconstructs Gal alpha (1, 3) gal epitope on
the surface of human tumor cells. Cell Res. 11:116–124. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Deriy L, Chen ZC, Gao GP and Galili U:
Expression of alpha-gal epitopes on HeLa cells transduced with
adenovirus containing alpha1,3galactosyltransferase cDNA.
Glycobiology. 12:135–144. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yoshimura N, Sawada T, Furusawa M and
Fuchinoue S: Expression of xenoantigen transformed human cancer
cells to be susceptible to antibody-mediated cell killing. Cancer
Lett. 164:155–160. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Takeuchi Y, Porter CD, Strahan KM, Preece
AF, Gustafsson K, Cosset FL, Weiss RA and Collins MK: Sensitization
of cells and retroviruses to human serum by (alpha 1–3)
galactosyltransferase. Nature. 379:85–88. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Unfer RC, Hellrung D and Link CJ Jr:
Immunity to the alpha (1,3)galactosyl epitope provides protection
in mice challenged with colon cancer cells expressing alpha
(1,3)galactosyl-transferase: A novel suicide gene for cancer gene
therapy. Cancer Res. 63:987–993. 2003.PubMed/NCBI
|
|
19
|
Liu M, Zhu SM, Zheng H, Wang Y, Wang Z,
Yang YJ, Wu YX, Zeng YZ and Wang YP: Cloning of splicing variants
of alpha1,3-galactosyltransferase cDNA of Chinese Banna Minipig
inbred line and its expression in human cells. Sichuan Da Xue Xue
Bao Yi Xue Ban. 43:145–150. 2012.PubMed/NCBI
|
|
20
|
Qin F, Zhu SM, Zheng H, Wang Z, Wang Y,
Zuo Y, Chen J, Sun WL and Wang YP: Establishment and identification
of human lung adenocarcinoma cell line stably expressing the
alpha1, 3-galaetosyltransferase gene from pig. Sichuan Da Xue Xue
Bao Yi Xue Ban. 41:194–198. 2010.PubMed/NCBI
|
|
21
|
Bajic G, Degn SE, Thiel S and Andersen GR:
Complement activation, regulation and molecular basis for
complement-related diseases. EMBO J. 34:2735–2757. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jurianz K, Ziegler S, Donin N, Reiter Y,
Fishelson Z and Kirschfink M: K562 erythroleukemic cells are
equipped with multiple mechanisms of resistance to lysis by
complement. Int J Cancer. 93:848–854. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Loberg RD, Day LL, Dunn R, Kalikin LM and
Pienta KJ: Inhibition of decay-accelerating factor (CD55)
attenuates prostate cancer growth and survival in vivo. Neoplasia.
8:69–78. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ziller F, Macor P, Bulla R, Sblattero D,
Marzari R and Tedesco F: Controlling complement resistance in
cancer by using human monoclonal antibodies that neutralize
complement-regulatory proteins CD55 and CD59. Eur J Immunol.
35:2175–2183. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Treon SP, Mitsiades C, Mitsiades N, Young
G, Doss D, Schlossman R and Anderson KC: Tumor cell expression of
CD59 is associated with resistance to CD20 Serotherapy in patients
with B-cell malignancies. J Immunother (1991). 24:263–271. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu M, Yang YJ, Zheng H, Zhong XR, Wang Y,
Wang Z, Wang YG and Wang YP: Membrane-bound complement regulatory
proteins are prognostic factors of operable breast cancer treated
with adjuvant trastuzumab: A retrospective study. Oncol Rep.
32:2619–2627. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang Y, Yang YJ, Wang Z, Liao J, Liu M,
Zhong XR, Zheng H and Wang YP: CD55 and CD59 expression protects
HER2-overexpressing breast cancer cells from trastuzumab-induced
complement-dependent cytotoxicity. Oncol Lett. 14:2961–2969. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhu S, Wang Y, Zheng H, Cheng J, Lu Y,
Zeng Y, Wang Y and Wang Z: Cloning of Chinese Banna minipig
inbred-line alpha1,3-galactosyltransferase gene and construction of
its recombinant eukaryotic expression vector. Sheng Wu Yi Xue Gong
Cheng Xue Za Zhi. 26:360–365. 2009.PubMed/NCBI
|
|
29
|
Jäger U, Takeuchi Y and Porter C:
Induction of complement attack on human cells by Gal (alpha1,3)Gal
xenoantigen expression as a gene therapy approach to cancer. Gene
Ther. 6:1073–1083. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hendriks D, Choi G, de Bruyn M, Wiersma VR
and Bremer E: Antibody-based cancer therapy: successful agents and
novel approaches. Int Rev Cell Mol Biol. 331:289–383. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shuptrine CW, Surana R and Weiner LM:
Monoclonal antibodies for the treatment of cancer. Semin Cancer
Biol. 22:3–13. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hakulinen J, Junnikkala S, Sorsa T and
Meri S: Complement inhibitor membrane cofactor protein (MCP; CD46)
is constitutively shed from cancer cell membranes in vesicles and
converted by a metalloproteinase to a functionally active soluble
form. Eur J Immunol. 34:2620–2629. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bellone S, Roque D, Cocco E, Gasparrini S,
Bortolomai I, Buza N, Abu-Khalaf M, Silasi DA, Ratner E, Azodi M,
et al: Downregulation of membrane complement inhibitors CD55 and
CD59 by siRNA sensitises uterine serous carcinoma overexpressing
Her2/neu to complement and antibody-dependent cell cytotoxicity in
vitro: Implications for trastuzumab-based immunotherapy. Br J
Cancer. 106:1543–1550. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kesselring R, Thiel A, Pries R,
Fichtner-Feigl S, Brunner S, Seidel P, Bruchhage KL and Wollenberg
B: The complement receptors CD46, CD55 and CD59 are regulated by
the tumour microenvironment of head and neck cancer to facilitate
escape of complement attack. Eur J Cancer. 50:2152–2161. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Devarapu SK, Mamidi S, Plöger F, Dill O,
Blixt O, Kirschfink M and Schwartz-Albiez R: Cytotoxic activity
against human neuroblastoma and melanoma cells mediated by IgM
antibodies derived from peripheral blood of healthy donors. Int J
Cancer. 138:2963–2973. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fishelson Z, Donin N, Zell S, Schultz S
and Kirschfink M: Obstacles to cancer immunotherapy: expression of
membrane complement regulatory proteins (mCRPs) in tumors. Mol
Immunol. 40:109–123. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gorter A and Meri S: Immune evasion of
tumor cells using membrane-bound complement regulatory proteins.
Immunol Today. 20:576–582. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mamidi S, Cinci M, Hasmann M, Fehring V
and Kirschfink M: Lipoplex mediated silencing of membrane
regulators (CD46, CD55 and CD59) enhances complement-dependent
anti-tumor activity of trastuzumab and pertuzumab. Mol Oncol.
7:580–594. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Loberg RD, Wojno KJ, Day LL and Pienta KJ:
Analysis of membrane-bound complement regulatory proteins in
prostate cancer. Urology. 66:1321–1326. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
McMorrow IM, Comrack CA, Nazarey PP, Sachs
DH and DerSimonian H: Relationship between ABO blood group and
levels of Gal alpha,3Galactose-reactive human immunoglobulin G.
Transplantation. 64:546–549. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang L, Anaraki F, Henion TR and Galili U:
Variations in activity of the human natural anti-Gal antibody in
young and elderly populations. J Gerontol A Biol Sci Med Sci.
50:M227–M233. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Koopmans J, de Haan A, Bruin E, van der
Gun I, van Dijk H, Rozing J, de Leij L and Staal M: Individual
human serum differs in the amount of antibodies with affinity for
pig fetal ventral mesencephalic cells and the ability to lyse these
cells by complement activation. Cell Transplant. 13:631–637. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
White MJ, Boyd JM, Horswill AR and Nauseef
WM: Phosphatidylinositol-specific phospholipase C contributes to
survival of Staphylococcus aureus USA300 in human blood and
neutrophils. Infect Immun. 82:1559–1571. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Cummerson JA, Flanagan BF, Spiller DG and
Johnson PM: The complement regulatory proteins CD55 (decay
accelerating factor) and CD59 are expressed on the inner acrosomal
membrane of human spermatozoa as well as CD46 (membrane cofactor
protein). Immunology. 118:333–342. 2006. View Article : Google Scholar : PubMed/NCBI
|