Introduction
Esophageal carcinoma, which including squamous cell
carcinoma (SCC) and adenocarcinoma, is a common serious malignancy
worldwide; it ranks eighth in incidence and fourth in mortality of
all cancer types owing to its extremely aggressive nature and the
poor survival rate of patients (1–3). The
genesis of esophageal carcinoma is a gradual process that combining
variety of carcinogenic factors and multiple stages, which includes
various physical and chemical factors, the abnormal activation of
signaling pathways, disequilibrium of apoptosis regulation and
anti-apoptotic signaling. All of these changes result in the loss
of normal regulatory mechanisms of cells, culminating in
over-proliferation, which leads to the occurrence of cancer
(4).
Bufalin is a cardiotonic steroid and a component of
the traditional Chinese medicine, Chansu, which is obtained from
the skin and parotid venom gland of toads (5). Owing to the similarity in the chemical
structure between bufalin and digoxin, bufalin is expected to have
a digoxin-like function (5,6). Bufalin has been shown to induce
apoptosis in leukemia and solid tumor cells (5–10). In
recent years, studies have revealed that bufalin could effectively
inhibit the proliferation of tumor cells, and also assessed its
possible molecular mechanism (6–8). The
results of previous research also demonstrated that bufalin could
inhibit the activation of ERK pathway, accordingly inhibit the
proliferation and migration of esophageal carcinoma cells (11); however, the mechanism of inducing
apoptosis has remained unclear.
Data has revealed that the RAC
serine/threonine-protein kinase (Akt)/mechanistic target of
rapamycin (mTOR)/p70 S6 kinase (p70S6K) pathway broadly exists in
every cell of organism (12,13). This pathway is often irregularly
activated in the genesis and development of tumor (12–14).
Aberration of upstream signaling causes the phosphorylation of
p70S6K, inhibits apoptosis, increases cell proliferation, produces
abnormal translation of protein and induces tumor formation.
Inhibition of mTOR can prevent the phosphorylation of p70S6K,
inhibit the translation of protein, prevent cell cycle progression
and induce cell apoptosis (15). On
the basis of previous studies (15),
the present study transfected human esophageal carcinoma ECA109
cells with wild-type mTOR (wtmTOR) and the changes to p70S6K,
phosphorylated (p)-p70S6K, Bcl-2-associated death promoter (BAD)
and cellular inhibitor of apoptosis-1 (cIAP-1) protein were
detected. The present study investigated the biochemical mechanisms
of apoptosis by bufalin in human esophageal carcinoma cells. The
current study assessed the influence of bufalin on ECA109 cell
apoptosis, and attempted to reveal the function of gene
intervention on development of tumor, and provide novel guidance
for the clinical treatment of esophageal carcinoma.
Materials and methods
Cell lines
The human ESCC ECA109 cell line was purchased from
Academia Sinica (Shanghai, China). All cells were cultured in
Roswell Park Memorial Institute (RPMI)-1640 (Sijiqing, Beijing,
China) enriched medium containing 1% penicillin/streptomycin and
10% fetal bovine serum (FBS; Sijiqing, Beijing, China). Cell
culture plates were maintained in humidified incubators at 37°C in
a 5% CO2 incubator.
Reagents and antibodies
Bufalin was purchased from Sigma-Aldrich; Merck KGaA
(Darmstadt, Germany) and was dissolved in ethyl alcohol to make a
0.01 mol/l stock solution, which was kept at −20°C and diluted in
phosphate buffer saline (PBS) when used. A plasmid containing
wtmTOR was produced by Prof. Hao Jun (Department of Pathology,
Hebei Medical University). Rabbit anti-human polychonal antibodies
p70S6K, phosphorylated (p)-p70S6K, cIAP-1, BAD and β-actin were
purchased from Epitomics; Abcam (Cambridge, UK). The reagents of
propidium iodide (PI) and RNAse for flow cytometry analysis were
purchased from Sigma-Aldrich; Merck KGaA.
Transfection experiments
The transfection of wtmTOR plasmid (3 µg) and empty
vector (3 µg) into ECA109 cells was performed using Lipofectamine
2000 (Life Technologies; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) and incubated for 24 h. The wtmTOR plasmid was kindly
provided by Professor Haojun (Department of Pathology, Hebei
Medical University, Shijiazhuang, China). The empty vector
(pEGFP-C1) was purchased from Invitrogen: Thermo Fisher Scientific,
Inc. The cells were divided into four groups: The control group
(untransfected group), the empty vector-transfected group, the
wtmTOR transfected group, and the group treated with add bufalin
(60 nmol/l) after 24 h transfection.
Protein extraction and western blot
analysis
Cells were harvested and lysed with lysis buffer (20
mM sucrose, 1 mM EDTA, 20 µM Tris-Cl, pH 7.2, 1 mM DTT, 10 mM KCl,
1.5 mM MgCl2 and 5 µg/ml aprotinin) for 30 min. Protein
concentration was measured using a Bio-Rad protein assay (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) according to the
manufacturer's instructions. The total proteins (80 µg/lane) were
separated on 10% SDS-PAGE gel and blotted onto a nitrocellulose
membrane. The membrane was incubated with blocking buffer and then
was incubated overnight with anti-p70S6K antibody (T2921; 1:5,000;
Epitomics: Abcam anti-p-p70S6K antibody (ab2571; 1:2,000;
Epitomics: Abcam), anti-cIAP-1 antibody (3302-1; 1:2,000;
Epitomics: Abcam), anti-BAD antibody (1541-1; 1:2,000; Epitomics:
Abcam) and β-actin antibody (ab8226; 1:500; Epitomics: Abcam)
primary antibodies at 4°C. The membranes were washed three times
with PBST and incubated for 1 h with a horseradish
peroxidase-conjugated secondary antibody (goat anti-rabbit IgG-HRP;
SAB3700852; 1:5,000; Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany). Blots were then developed with Super signal West Femto
Maximum Sensitivity substrate (Pierce; Thermo Fisher Scientific,
Inc.) on a FujiFilm LAS-3000 detection system (Fujifilm
Corporation, Tokyo, Japan).
Giemsa staining
ECA109 cells were treated with 60 nmol/l bufalin for
48 h, and the cells were collected and put on the slides. The
slides were then rinsed with sterile water and stained with freshly
prepared Giemsa stain solution (Giemsa: phosphate=1:9; BDH
Chemicals; Merck KGaA) for 5 min at room temperature. Following
three washes in sterile water, the cells were examined for
morphological changes using a light microscope at ×400,
magnification.
Flow cytometry analysis
ECA109 cells were collected for PI staining.
Briefly, the cells were fixed in 70% ethyl alcohol at 4°C
overnight, then washed with PBS and incubated with RNAse (10 µg/ml)
at 37°C for 30 min. Next the cells were incubated with PI (final
concentration, 10 µg/ml) for 30 min in the dark. After incubating
at 4°C for 30 min, flow cytometry was performed. MultiCycle AV
software (Version 295, Beckman Coulter, Miami, FL, USA) was used to
analyze the cell cycle and Expo32 ADC software (Version 1.2,
Beckman Coulter, Miami, FL, USA) was used to analyze the apoptosis
rate.
Statistical analysis
All data are presented as the mean ± standard
deviation. Significant differences among the groups were determined
by one-way ANOVA followed by Newman-Keuls method of post-hoc
comparison. All results presented were obtained from at least three
independent experiments. The statistical analyses were performed
using SPSS 13.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Transfection with wtmTOR plasmid
influences the expression of p70S6K and p-p70S6K protein in ECA109
cells by western blot analysis
The wtmTOR plasmid was amplified successfully and
transfected into esophageal carcinoma ECA109 cells. The expression
of p70S6K and the activation of p70S6K at 0, 12, 24, 30, 36, 42 and
48 h were examined by western blot analysis. The expression of
p70S6K was not significantly different at 0, 12, 24, 30, 36, 42 or
48 h (0.599±0.011, 0.594±0.013, 0.606±0.012, 0.608±0.010,
0.592±0.017, 0.599±0.021, 0.600±0.036, respectively; P>0.05).
Therefore, p-p70S6K levels increased along with the time (at 0, 12
and 24 h, levels were 0.389±0.013, 0.411±0.019 and 0.609±0.016,
respectively), and then decreased at 30, 36, 42 and 48 h following
transfection (0.573±0.015, 0.394±0.013, 0.383±0.006 and
0.262±0.018, respectively; P<0.05). The expression of p-p70S6K
was the highest at 24 h. The difference was statistically
significant. We selected 24 h as the optimal transfection time
(Fig. 1).
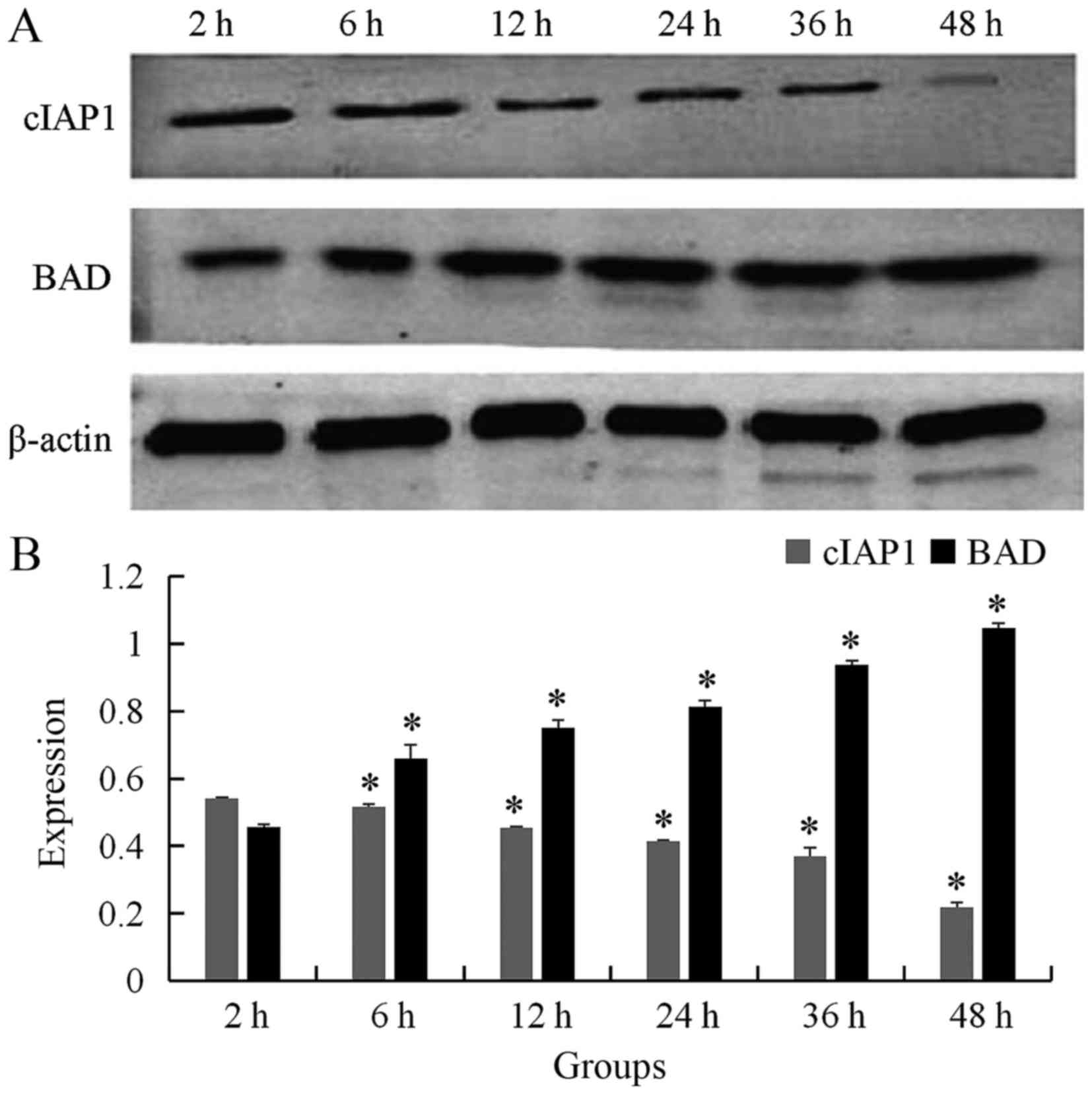
Examination of cIAP-1 and BAD
expression by western blot analysis following bufalin
treatment
To confirm that bufalin induced ECA109 cell
apoptosis, western blot analysis was performed to assess the
expression of cIAP-1 and BAD following incubation with 60 nmol/l
bufalin. The result showed that the expression of cIAP-1 was
gradually decreased as time progressed for 2, 6, 12, 24, 36, and 48
h (0.542±0.003, 0.517±0.007, 0.455±0.002, 0.414±0.004, 0.369±0.026,
0.218±0.015, respectively; P<0.05), whereas the expression of
BAD was gradually increased following addition of bufalin for 2, 6,
12, 24, 36, and 48 h (0.456±0.009, 0.659±0.042, 0.750±0.023,
0.813±0.019, 0.937±0.013 and 1.047±0.013, respectively; P<0.05)
(Fig. 2). The difference was
statistically significant.
Detection of p70S6K, p-p70S6K, cIAP-1
and BAD levels by western blot in different groups
The experiment was divided into four groups: The
control group, empty vector transfected group, wtmTOR transfected
group, and the bufalin/wtmTOR transfected group. Western blot
analysis revealed that the level of p70S6K was not significant
different in the control, compared with empty vector-transfected,
wtmTOR-transfected, and the bufalin/wtmTOR-transfected groups
(0.901±0.045, 0.914±0.023, 0.900±0.020, 0.898±0.022, respectively;
P>0.05) (Fig. 3A). However,
p-p70S6K was significantly higher in wtmTOR-transfected group
compared with the control and empty vector groups, and then reduced
following addition of bufalin for 2 h (0.761±0.085, 0.766±0.068,
0.952±0.059, 0.762±0.019; P<0.05; Fig.
3A).
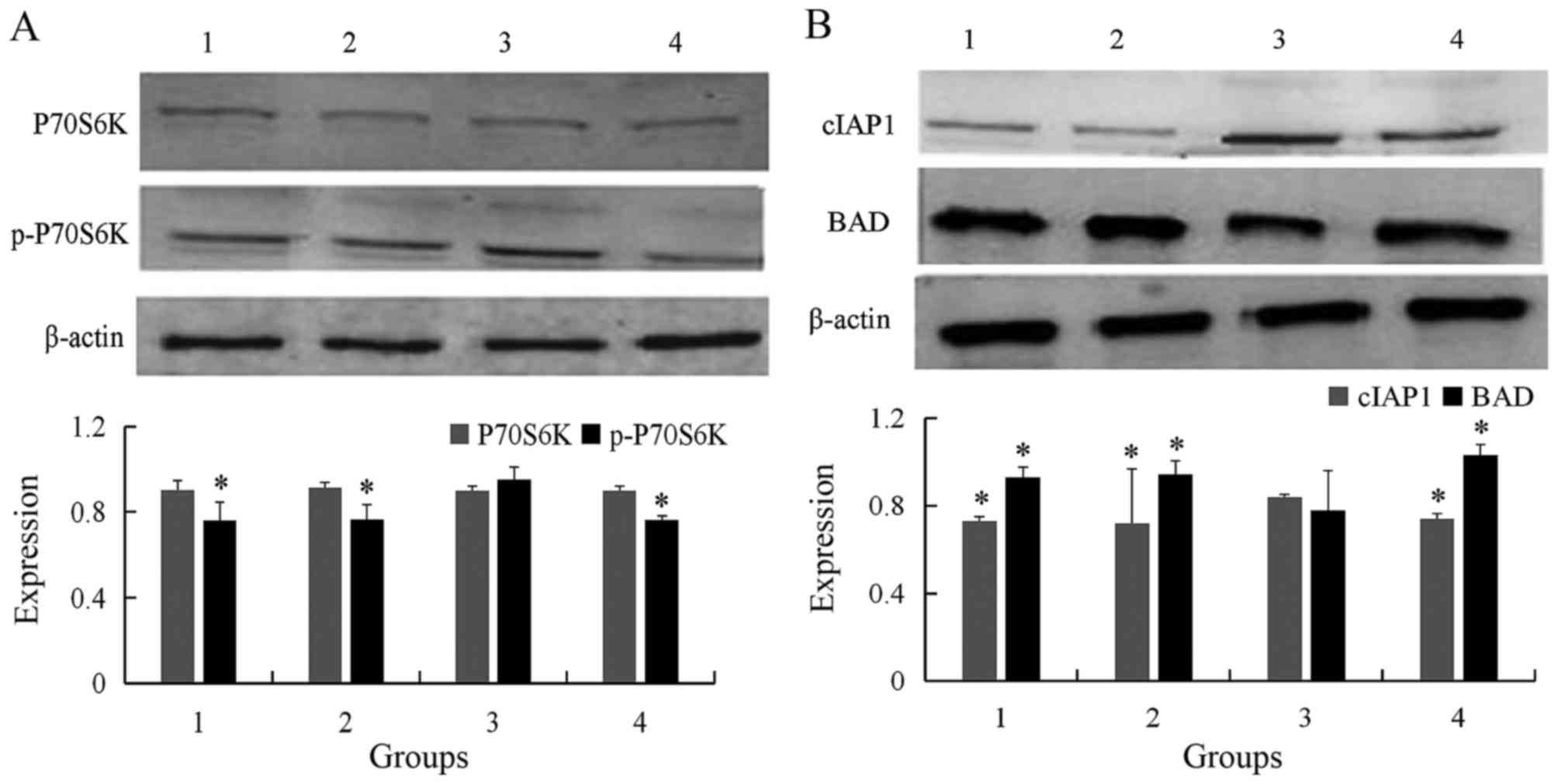 | Figure 3.Western blot analysis of protein
expression in the four different cell groups. (A) Expression of
p70S6K and p-p70S6K in different groups were detected by western
blot. The level of p-p70S6K was significantly higher in wtmTOR
plasmid transfected group comparing with control and empty vector
group, and then reduced following addition of bufalin 2 h. (B)
Expression cIAP-1 and BAD in different groups were detected by
western blot analysis. The level of cIAP-1 was significantly higher
in wtmTOR plasmid transfected group comparing with control and
empty vector group, and then decreased following addition of
bufalin for 24 h. Contrarily, the expression of BAD was
significantly lower in wtmTOR plasmid transfected group. *P<0.05
vs. wtmTOR-transfected group. Group 1, control; group 2, empty
vector-transfected; group 3, wtmTOR-transfected; group 4, 60 nmol/l
bufalin. p-p70S6K, phosphorylated p70 S6 kinase; wtmTOR, wild-type
mechanistic target of rapamycin; BAD, Bcl-2-associated death
promoter. |
The level of cIAP-1 was significantly higher in
wtmTOR-transfected group compared with in control group, empty
vector group, and addition of bufalin for 24 h (0.721±0.019,
0.731±0.248, 0.840±0.010 and 0.742±0.021, respectively; P<0.05).
On the contrary, the expression of BAD was significantly lower in
wtmTOR-transfected group than in the control and empty vector
group, and then increased following addition of bufalin for 24 h
(0.929±0.046, 0.944±0.060, 0.779±0.182 and 1.029±0.049,
respectively; P<0.05; Fig.
3B).
Morphological analysis of ECA109 cells
by Giemsa staining
To confirm that bufalin induced the morphology of
apoptosis in ECA109 cells, ECA109 cells was treated with 60 nmol/l
bufalin for 48 h and then Giemsa staining was performed. The
apoptotic morphology was evident in bufalin-treated cells under a
microscope at ×400 magnification, including cytoplasmic shrinkage,
nuclear condensation and the formation of apoptotic bodies
(Fig. 4). However, no apoptotic
morphology was observed in the control-treated cells.
Changes to ECA109 cells apoptotic rate
and cell cycle detected by flow cytometry
Flow cytometry results showed that the apoptotic
rate was gradually increased following addition of bufalin at
concentrations of 0, 20, 40, 60, 80 and 100 nmol/l for 24 h
(3.01±0.317, 3.67±0.306, 6.74±0.198, 7.59±0.340, 18.22±0.651 and
28.60±1.737%, respectively; P<0.05) (Fig. 5). The cell cycle of ECA109 was
arrested at G2/M phase, and the cell percentage of
G2/M is increased from 9.24±1.919 to 70.5±2.934%
following addition of bufalin from 0 nmol/l to 100 nmol/l; this
difference was statistically significant (Fig. 6).
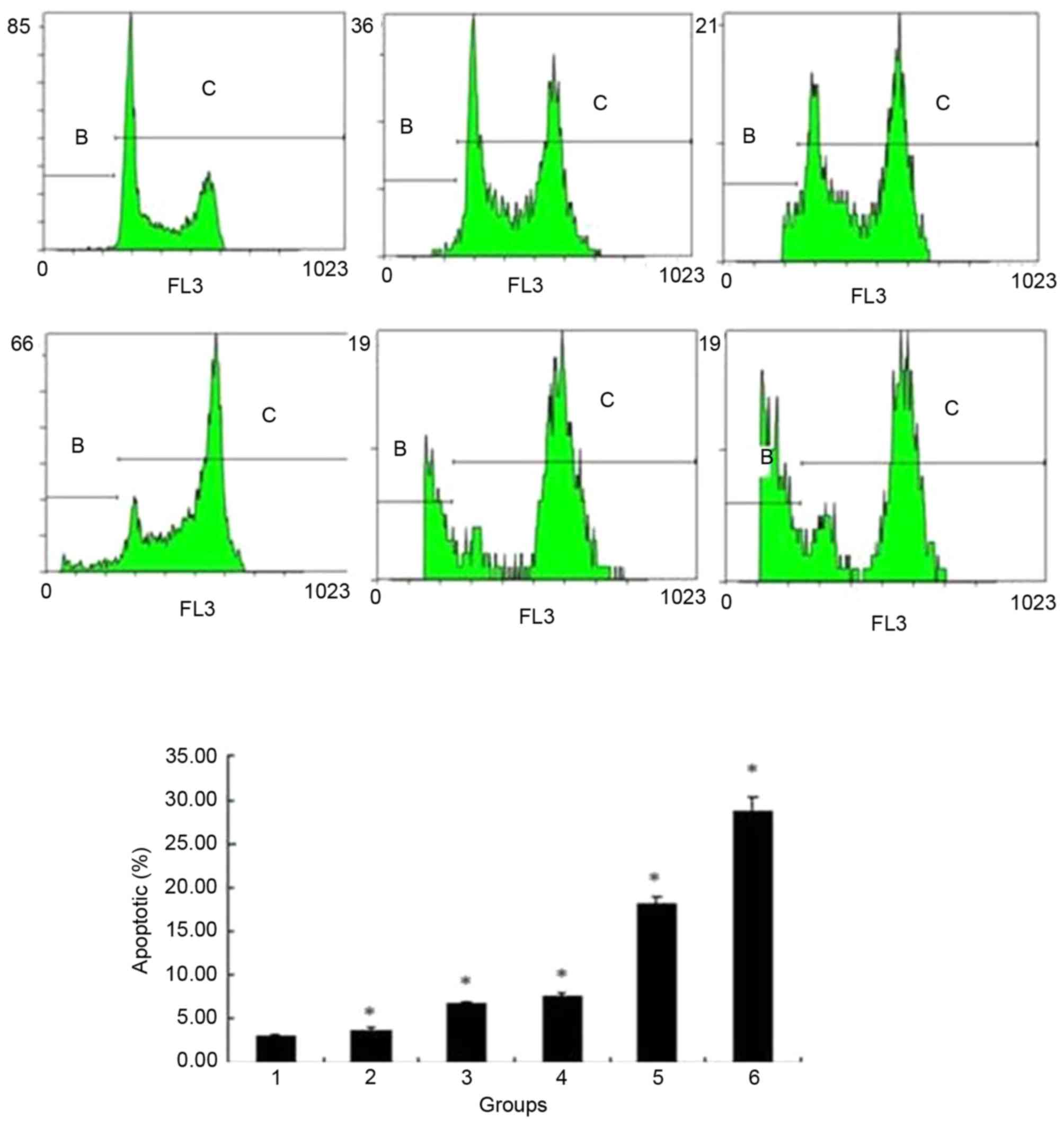 | Figure 5.Flow cytometry analysis of the
apoptotic rate of ECA109 cells treated with different
concentrations of bufalin. The apoptotic rate gradually increased
following addition of 0, 20, 40, 60, 80 or 100 nmol/l bufalin for
24 h. Group 1, 0 nmol/l; group 2, 20 nmol/l; group 3, 40 nmol/l;
group 4, 60 nmol/l; group 5, 80 nmol/l; group 6, 100 nmol/l.
*P<0.05 vs. control. |
The apoptotic rate of the cells in the different
groups were examined, the results of which revealed that the
apoptotic rate was significantly lower in wtmTOR-transfected group
compared with in the control group, empty vector groups, and
addition of bufalin for 24 h group (5.60±0.411, 5.46±0.341,
4.19±0.210, 10.12±0.325%, respectively; all P<0.05; Fig. 7).
Discussion
Esophageal carcinoma is a high-incidence malignancy
in China, which also has a high morbidity and mortality rate in the
rest of the world (16). The genesis
and development of this disease is a complicated process, including
the accumulation and interaction of several factors, phases and
multiple gene variations. Patients are primarily treated with
surgery combining with radiotherapy, chemotherapy or immunotherapy;
however, the 5-year survival rate remains low and the prognosis of
patients is poor (2,5). The genesis and development of tumor is
closely associated with abnormities to signaling pathways (14,17). The
apoptosis signaling pathway activated by bufalin has attracted
considerable attention (7–9); however, there is little research on
esophageal carcinoma and the exact mechanism by which this pathway
is activated is unclear.
Apoptosis is a cell defense mechanism to eliminate
malignant cells and has a notable role in preventing tumor
development. In fact, a number of anticancer drugs function
primarily to induce apoptosis through regulating
apoptosis-associated signaling (18,19).
Bufalin is a member of a class of toxic steroids purified from the
traditional Chinese medicine Chansu. Previous studies have revealed
that bufalin also exhibited antitumor effects; it induced the
differentiation and apoptosis of leukemia HL-60 cells, and induce
the apoptosis of gastric cancer cells, colon cancer cells and
breast cancer cells (20–22). Bufalin could therefore induce cell
cycle arrest and/or apoptosis as concentration increased. The cell
cycle is divided into G0/G1, S, and
G2/M phases, which refers to the presynthetic phase,
synthesis period, and post-synthetic phase of DNA, respectively
(23). The cell cycle is a useful
index for judging the status of cell proliferation. At present,
there are several reports concerning bufalin-induced cell cycle
retardation. A prior study revealed that bufalin mainly induced
G2/M phase arrest in leukemia ML1 cells (24). Another study reported that bufalin
could induce G0/G1 phase arrest in
endometriosis matrix cell (25).
Together, these results demonstrated that the influence of bufalin
on cell cycle varied in different cells. However, the present study
assessed whether bufalin could induce apoptosis in esophageal
carcinoma cells and arrest the cell cycle. In the current study,
flow cytometry analysis demonstrated that as bufalin concentration
increased (0, 20, 40, 60, 80, 100 nmol/l) the apoptotic rate of
ECA109 increased from 3.01±0.317% (bufalin, 0 nmol/l) to
28.60±1.737% (bufalin, 100 nmol/l) at 24 h of treatment with
bufalin. The percentage of cells in G2/M phase was
70.5±2.934% compared with that in control group, which was
9.24±1.919%. The expression of cIAP-1 protein, which is a member of
the IAP family, gradually decreased as the time of bufalin
incubation increased effecting on esophageal carcinoma cells
ECA109. The expression of the apoptosis-promoting gene BAD, which
belongs to the Bcl-2 family, gradually increased as the time of
incubation increased. These results revealed that bufalin might
induce cell cycle arrest at G2/M phase, and affect
apoptosis of ECA109 cells on in a time- and dose-dependent
manner.
A variety of signaling pathways are abnormally
activated in the development of tumor; one such pathway, the
PI3K/Akt/mTOR pathway, has been demonstrated to serve an essential
role in the genesis of esophageal carcinoma. The PI3K/Akt/mTOR
pathway is a notable signal transduction pathway that mediates
tumor cell apoptosis. Activation of this pathway could inhibit
apoptosis, increase cell cycle progression, and accordingly improve
the survival and proliferation of tumor cells (12,15,26). mTOR
contributes to the genesis and development of a variety of
malignancies, including breast cancer (27) and lung cancer (28). mTOR accelerates the translation and
expression of protein, alters the cell cycle distribution, affects
apoptosis and participates in adjusting multiple types of
physiological and pathological changes in the organism, mainly
through phosphorylating the main signal factors p70S6K and
Eukaryotic translation initiation factor 4E-binding protein 1
(4E-BP1) downstream (13,29). Activated mTOR can positively regulate
the translation of S6K1, which phosphorylates p70S6 downstream, and
promotes protein synthesis; this process promotes the synthesis of
protein (15,29). 4E-BP1 is another notable regulation
pathway downstream of mTOR; it combines with the cap-binding
protein in its dephosphorylated state, inhibiting the origin of
translation. Activated mTOR can phosphorylate 4E-BP1, and
eukaryotic translation initiation factor 4E is released and form
compound with other translation original factors, initiating
translation and accelerating the expression of proteins associated
with growth and differentiation (30). Therefore, the PI3K/Akt/mTOR pathway is
considered to be an essential signaling pathway in protein
synthesis that participates in the regulation of cellular
proliferation, differentiation and apoptosis.
The present study successfully transfected the
active mTOR plasmid wtmTOR into the ECA109 cell line. Previous
studies revealed that the consisting activated mTOR signaling
pathway could promote the transformation of normal cells to tumor
cells (9,31). If the tumor cells could be reverted to
the non-transformation form in vitro following inhibition of
the mTOR signal transduction pathway, this reversion could have a
role in inhibiting tumor development. Accordingly, the present
study used bufalin in different treatment groups and assessed the
changes in activation of the mTOR signaling pathway and the
apoptosis-associated proteins cIAP-1 and BAD. The levels of
p-p70S6K level in the wtmTOR-transfected group increased as the
transfection period increased, and reached the maximum value at 24
h, and then gradually decreased; however, the expression of p70S6K
did not evidently change. For different treatment groups, the level
of p70S6K activated (that is, p-p70S6K) in wtmTOR-transfected group
was evidently higher than in the control, empty vector-transfected
and bufalin-treated group. The transfection of plasmid makes the
activation of p70S6K increase, whereas bufalin could inhibit this
process. The expression of cIAP-1 was higher in the
wtmTOR-transfected group than the others, despite the lower
expression of BAD than in the other three groups, which revealed
that transfection with this plasmid inhibited cell apoptosis,
whereas bufalin promotes cell apoptosis. The results of the present
study indicated that bufalin could inhibit the development of
esophageal carcinoma by inhibiting the mTOR pathway and inducing
cell apoptosis.
In summary, the results of the present study
indicate that bufalin induces cell apoptosis through inhibiting the
activation of the mTOR/p70S6K signaling pathway. These results
indicated that bufalin could be used for clinical treatment and
provided an experimental basis and future direction for the
treatment of esophageal carcinoma. Bufalin may represent a novel
antitumor drug, meaning that its feasibility and clinical utility
degree should be thoroughly investigated.
Acknowledgements
Not applicable.
Funding
The present study was supported by a grant from
National Nature Science Foundation of P.R. China (grant no.
81303271).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YD performed cell culture and was a major
contributor in writing the manuscript. WL performed the flow
cytometry analysis. XW was mainly responsible for the analysis of
flow cytometry and revision of the paper. LZ performed western
blotting experiments and analysis. MZ performed Giemsa staining. HD
performed transfection experiments. YL was responsible for the
analysis of data and the revision of the paper. All authors read
and approved the final manuscript.
Ethics approval and consent to publish
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cai EH, Gao YX, Wei ZZ, Chen WY, Yu P and
Li K: Serum miR-21 expression in human esophageal squamous cell
carcinomas. Asian Pac J Cancer Prev. 3:1563–1567. 2012. View Article : Google Scholar
|
|
2
|
Mao WM, Zheng WH and Ling ZQ:
Epidemiologic risk factors for esophageal cancer development. Asian
Pac J Cancer Prev. 12:2461–2466. 2011.PubMed/NCBI
|
|
3
|
Enziger PC and Mayer RJ: Esophageal
cancer. N Engl J Med. 349:2241–2252. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li B, Li J, Xu WW, Guan XY, Qin YR, Zhang
LY, Law S, Tsao SW and Cheung AL: Suppression of esophageal tumor
growth and chemoresistance by directly targeting the PI3K/AKT
pathway. Oncotarget. 5:11576–11587. 2014.PubMed/NCBI
|
|
5
|
Watabe M, Kawazoe N, Masuda Y, Nakajo S
and Nakaya K: Bcl-2 protein inhibits bufalin-induced apoptosis
through inhibition of mitogen-activated protein kinase activation
in human leukemia U937 cells. Cancer Res. 57:3097–3100.
1997.PubMed/NCBI
|
|
6
|
Yeh JY, Huang WJ, Kan SF and Wang PS:
Effects of bufalin and cinobufagin on the proliferation of androgen
dependent and independent prostate cancer cells. Prostate.
54:112–124. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhu Z, Li E and Liu Y, Gao Y, Sun H, Wang
Y, Wang Z, Liu X, Wang Q and Liu Y: Bufalin induces the apoptosis
of acute promyelocytic leukemia cells via the downregulation of
survivin expression. Acta Heamatol. 128:144–150. 2012. View Article : Google Scholar
|
|
8
|
Zhu Z, Sun H, Ma G, Wang Z, Li E and Liu Y
and Liu Y: Bufalin induces lung cancer cell apoptosis via the
inhibition of PI3K/Akt pathway. Int J Mol Sci. 13:2025–2035. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li D, Qu X, Hou K, Zhang Y, Dong Q, Teng
Y, Zhang J and Liu Y: PI3K/Akt is involved in bufalin-induced
apoptosis in gastric cancer cells. Anticancer Drugs. 20:59–64.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hong SH and Choi YH: Bufalin induces
apoptosis through activation of both the intrinsic and extrinsic
pathways in human bladder cancer cells. Oncol Rep. 27:114–120.
2012.PubMed/NCBI
|
|
11
|
Chen YN, Deng HY and Zhang P: Bufalin
Inhibits the Proliferation of human esophageal carcinoma TE13 cells
through down-regulation of ERK. Asian J Pharm Nurs Med Sci.
2:90–98. 2014.
|
|
12
|
Ponnurangam S, Standing D, Rangarajan P
and Subramaniam D: Tandutinib inhibits the Akt/mTOR signaling
pathway to inhibit colon cancer growth. Mol Cancer Ther.
12:598–609. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Meng Q, Xia C, Fang J, Rojanasakul Y and
Jiang BH: Role of PI3K and AKT specific isoforms in ovarian cancer
cell migration, invasion and proliferation through the p70S6K1
pathway. Cell Signal. 18:2262–2271. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ji J and Zheng PS: Activation of mTOR
signaling pathway contributes to survival of cervical cancer cells.
Gynecol Oncol. 117:103–108. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu Y, Wang X, Jia Y and Liu Y: Effects of
bufalin on the mTOR/p70S6K pathway and apoptosis in esophageal
squamous cell carcinoma in nude mice. Int J Mol Med. 40:357–366.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang Y: Epidemiology of esophageal
cancer. World J Gastroenterol. 14:5598–5606. 2013. View Article : Google Scholar
|
|
17
|
Engels K, Knauer S K, Metzler D, Simf C,
Struschka O, Bier C, Mann W, Kovács AF and Stauber RH: Dynamic
intracellular survivin in oral squamous cell carcinoma: Underlying
molecular mechanism and potential as an early prognostic marker. J
Pathol. 211:532–540. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li W, Wang J, Jiang HR, Xu XL, Zhang J,
Liu ML and Zhai LY: Combined effects of cyclooxygenase-1 and
cyclooxygenase-2 selective inhibitors on ovarian carcinoma in vivo.
Int J Mol Sci. 12:668–681. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Khoo BY, Chua SL and Balaram P: Apoptotic
effects of chrysin in human cancer cell lines. Int J Mol Sci.
11:2188–2199. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen A, Yu J, Zhang L, Sun Y, Zhang Y, Guo
H, Zhou Y, Mitchelson K and Cheng J: Microarray and biochemical
analysis of bufalin-induced apoptosis of HL-60 cells. Biotechnol
Lett. 31:487–494. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Takai N, Ueda T, Nishida M, Nasu K and
Narahara H: Bufalin induces growth inhibition, cell cycle arrest
and apoptosis in human endometrial and ovarian cancer cells. Int J
Mol Med. 21:637–643. 2008.PubMed/NCBI
|
|
22
|
Xie CM, Chan WY, Yu S, Zhao J and Cheng
CH: Bufalin induces autophagy-mediated cell death in human colon
cancer cells through reactive oxygen species generation and JNK
activation. Free Radic Biol Med. 51:1365–1375. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ding L, Huang Y, Dai M, Zhao X, Du Q, Dong
F, Wang L, Huo R, Zhang W, Xu X and Tong D: Transmissible
gastroenteritis virus infection induces cell cycle arrest at S and
G2/M phases via p53-dependent pathway. 178:241–251. 2013.
|
|
24
|
Jing Y, Watabe M, Hashimoto S, Nakajo S
and Nakaya K: Cell cycle arrest and protein kinase modulating
effect of bufalin on human leukemia ML1 cells. Anticancer Res.
14:1193–11198. 1994.PubMed/NCBI
|
|
25
|
Nasu K, Nishida M, Ueda T, Takai N, Bing
S, Narahara H and Miyakawa I: Bufalin induces apoptosis and the
G0/G1 cell cycle arrest of endometriotic stromal cells: A promising
agent for the treatment of endometriosis. Mol Hum Reprod.
11:817–823. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nishioka C, Ikezoe T, Yang J, Koeffler HP
and Yokoyama A: Blockade of mTOR Signaling potentiates the ability
of histone deacetylase inhibitor to induce growth arre stand
differentiation of acute myelogenous leukemia cells. Leukemia.
22:2159–2168. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lin HJ, Hsieh FC, Song H and Lin J:
Elevated phosphorylation and activation of PDK-1/AKT pathway in
human breast cancer. Br J Cancer. 93:1372–1381. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tsurutani J, West KA, Sayyah J, Gills JJ
and Dennis PA: Inhibition of the phosphatidylinositol
3-kinase/Akt/mammalian target of rapamycin pathway but not the
WTMTOR/p70S6K pathway attenuates laminin-mediated small cell lung
cancer cellular survival and resistance to imatinib mesylate or
chemotherapy. Cancer Res. 65:8423–8432. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fenton TR, Gwalter J, Cramer R and Gout
IT: S6K1 is acetylated at lysine 516 in response to growth factor
stimulation. Biochem Biophys Res Commun. 398:400–405. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Darb-Esfahani S, Faggad A, Noske A,
Weichert W, Buckendahl AC, Müller B, Budczies J, Röske A, Dietel M
and Denkert C: Phospho-mTOR and phospho-4EBP1 in endometrial
adenocarcinoma: association with stage and grade in vivo and link
with response to rapamycin treatment in vitro. Cancer Res Clin
Oncol. 135:933–941. 2009. View Article : Google Scholar
|
|
31
|
Sutherland S: Several therapies may
prevent or reduce the severity of oral mueositis associated with
cancer treatment. Evid Based Dent. 7:104–105. 2006. View Article : Google Scholar : PubMed/NCBI
|