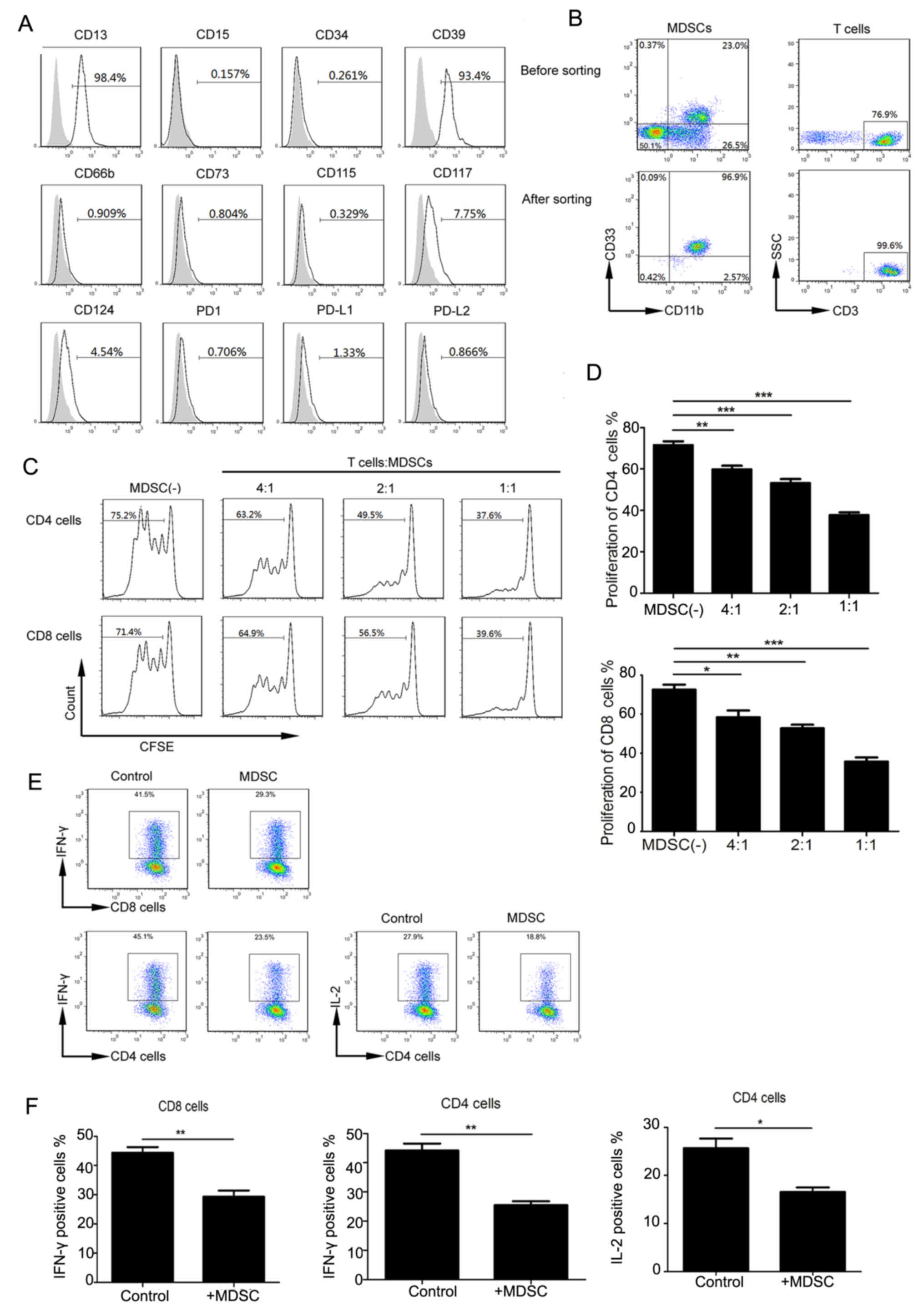
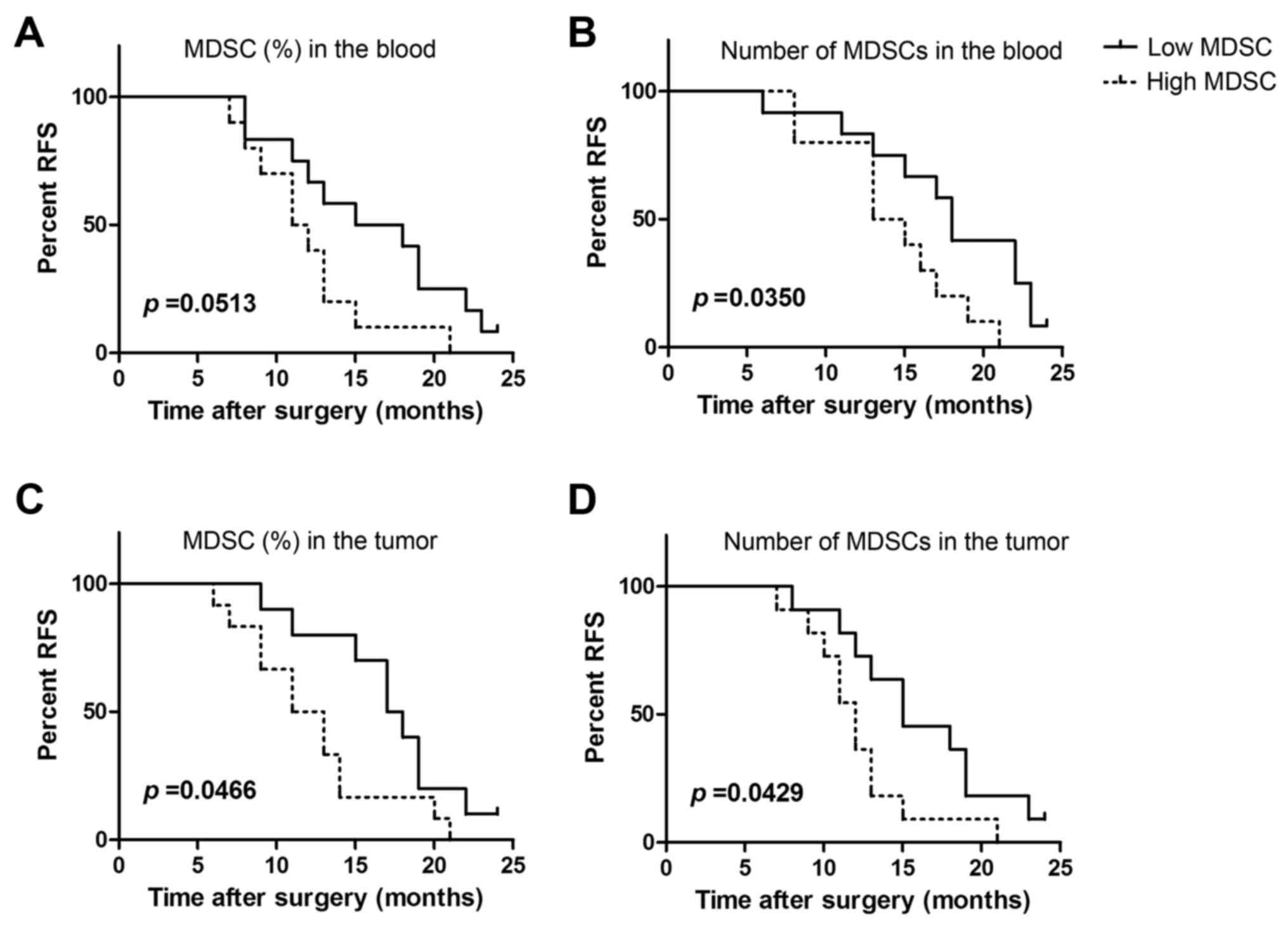
|
1
|
Ho GY, Bierman R, Beardsley L, Chang CJ
and Burk RD: Natural history of cervicovaginal papillomavirus
infection in young women. N Engl J Med. 338:423–428. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Trottier H and Franco EL: The epidemiology
of genital human papillomavirus infection. Vaccine. 24 Suppl
1:S1–S15. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Woodman CB, Collins SI and Young LS: The
natural history of cervical HPV infection: Unresolved issues. Nat
Rev Cancer. 7:11–22. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Grabowska AK and Riemer AB: The invisible
enemy-how human papillomaviruses avoid recognition and clearance by
the host immune system. Open Virol J. 6:249–256. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kanodia S, Fahey LM and Kast WM:
Mechanisms used by human papillomaviruses to escape the host immune
response. Curr Cancer Drug Targets. 7:79–89. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kobayashi A, Weinberg V, Darragh T and
Smith-McCune K: Evolving immunosuppressive microenvironment during
human cervical carcinogenesis. Mucosal Immunol. 1:412–420. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Piersma SJ: Immunosuppressive tumor
microenvironment in cervical cancer patients. Cancer Microenviron.
4:361–375. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jordanova ES, Gorter A, Ayachi O, Prins F,
Durrant LG, Kenter GG, van der Burg SH and Fleuren GJ: Human
leukocyte antigen class I, MHC class I chain-related molecule A and
CD8+/regulatory T-cell ratio: Which variable determines survival of
cervical cancer patients? Clin Cancer Res. 14:2028–2035. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
De Vos van Steenwijk PJ, Ramwadhdoebe TH,
Goedemans R, Doorduijn EM, van Ham JJ, Gorter A, van Hall T,
Kuijjer ML, van Poelgeest MI, van der Burg SH and Jordanova ES:
Tumor-infiltrating CD14-positive myeloid cells and CD8-positive
T-cells prolong survival in patients with cervical carcinoma. Int J
Cancer. 133:2884–2894. 2013.PubMed/NCBI
|
|
11
|
Gabrilovich DI, Ostrand-Rosenberg S and
Bronte V: Coordinated regulation of myeloid cells by tumours. Nat
Rev Immunol. 12:253–268. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gabrilovich DI and Nagaraj S:
Myeloid-derived suppressor cells as regulators of the immune
system. Nat Rev Immunol. 9:162–174. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Solito S, Falisi E, Diaz-Montero CM, Doni
A, Pinton L, Rosato A, Francescato S, Basso G, Zanovello P,
Onicescu G, et al: A human promyelocytic-like population is
responsible for the immune suppression mediated by myeloid-derived
suppressor cells. Blood. 118:2254–2265. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Youn JI, Nagaraj S, Collazo M and
Gabrilovich DI: Subsets of myeloid-derived suppressor cells in
tumor-bearing mice. J Immunol. 181:5791–5802. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Montero AJ, Diaz-Montero CM, Kyriakopoulos
CE, Bronte V and Mandruzzato S: Myeloid-derived suppressor cells in
cancer patients: A clinical perspective. J Immunother. 35:107–115.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Greten TF, Manns MP and Korangy F: Myeloid
derived suppressor cells in human diseases. Int Immunopharmacol.
11:802–807. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rodriguez PC, Ernstoff MS, Hernandez C,
Atkins M, Zabaleta J, Sierra R and Ochoa AC: Arginase I-producing
myeloid-derived suppressor cells in renal cell carcinoma are a
subpopulation of activated granulocytes. Cancer Res. 69:1553–1560.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Almand B, Clark JI, Nikitina E, van Beynen
J, English NR, Knight SC, Carbone DP and Gabrilovich DI: Increased
production of immature myeloid cells in cancer patients: A
mechanism of immunosuppression in cancer. J Immunol. 166:678–689.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Diaz-Montero CM, Salem ML, Nishimura MI,
Garrett-Mayer E, Cole DJ and Montero AJ: Increased circulating
myeloid-derived suppressor cells correlate with clinical cancer
stage, metastatic tumor burden and doxorubicin-cyclophosphamide
chemotherapy. Cancer Immunol Immunother. 58:49–59. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Srivastava MK, Bosch JJ, Thompson JA,
Ksander BR, Edelman MJ and Ostrand-Rosenberg S: Lung cancer
patients' CD4(+) T cells are activated in vitro by MHC II
cell-based vaccines despite the presence of myeloid-derived
suppressor cells. Cancer Immunol Immunother. 57:1493–1504. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Feng PH, Lee KY, Chang YL, Chan YF, Kuo
LW, Lin TY, Chung FT, Kuo CS, Yu CT, Lin SM, et al:
CD14(+)S100A9(+) monocytic myeloid-derived suppressor cells and
their clinical relevance in non-small cell lung cancer. Am J Respir
Crit Care Med. 186:1025–1036. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huang A, Zhang B, Wang B, Zhang F, Fan KX
and Guo YJ: Increased CD14(+)HLA-DR(−/low) myeloid-derived
suppressor cells correlate with extrathoracic metastasis and poor
response to chemotherapy in non-small cell lung cancer patients.
Cancer Immunol Immunother. 62:1439–1451. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kusmartsev S, Su Z, Heiser A, Dannull J,
Eruslanov E, Kübler H, Yancey D, Dahm P and Vieweg J: Reversal of
myeloid cell-mediated immunosuppression in patients with metastatic
renal cell carcinoma. Clin Cancer Res. 14:8270–8278. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Montero AJ, Diaz-Montero CM, Deutsch YE,
Hurley J, Koniaris LG, Rumboldt T, Yasir S, Jorda M, Garret-Mayer
E, Avisar E, et al: Phase 2 study of neoadjuvant treatment with
NOV-002 in combination with doxorubicin and cyclophosphamide
followed by docetaxel in patients with HER-2 negative clinical
stage II–IIIc breast cancer. Breast Cancer Res Treat. 132:215–223.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Raychaudhuri B, Rayman P, Ireland J, Ko J,
Rini B, Borden EC, Garcia J, Vogelbaum MA and Finke J:
Myeloid-derived suppressor cell accumulation and function in
patients with newly diagnosed glioblastoma. Neuro Oncol.
13:591–599. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang B, Wang Z, Wu L, Zhang M, Li W, Ding
J, Zhu J, Wei H and Zhao K: Circulating and tumor-infiltrating
myeloid-derived suppressor cells in patients with colorectal
carcinoma. PLoS One. 8:e571142013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bronte V, Brandau S, Chen SH, Colombo MP,
Frey AB, Greten TF, Mandruzzato S, Murray PJ, Ochoa A,
Ostrand-Rosenberg S, et al: Recommendations for myeloid-derived
suppressor cell nomenclature and characterization standards. Nat
Commun. 7:121502016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Melani C, Chiodoni C, Forni G and Colombo
MP: Myeloid cell expansion elicited by the progression of
spontaneous mammary carcinomas in c-erbB-2 transgenic BALB/c mice
suppresses immune reactivity. Blood. 102:2138–2145. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bronte V, Apolloni E, Cabrelle A, Ronca R,
Serafini P, Zamboni P, Restifo NP and Zanovello P: Identification
of a CD11b(+)/Gr-1(+)/CD31(+) myeloid progenitor capable of
activating or suppressing CD8(+) T cells. Blood. 96:3838–3846.
2000.PubMed/NCBI
|
|
30
|
Ostrand-Rosenberg S and Sinha P:
Myeloid-derived suppressor cells: Linking inflammation and cancer.
J Immunol. 182:4499–4506. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Eruslanov E, Neuberger M, Daurkin I,
Perrin GQ, Algood C, Dahm P, Rosser C, Vieweg J, Gilbert SM and
Kusmartsev S: Circulating and tumor-infiltrating myeloid cell
subsets in patients with bladder cancer. Int J Cancer.
130:1109–1119. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Corzo CA, Condamine T, Lu L, Cotter MJ,
Youn JI, Cheng P, Cho HI, Celis E, Quiceno DG, Padhya T, et al:
HIF-1α regulates function and differentiation of myeloid-derived
suppressor cells in the tumor microenvironment. J Exp Med.
207:2439–2453. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ghiringhelli F, Bruchard M, Chalmin F and
Rébé C: Production of adenosine by ectonucleotidases: A key factor
in tumor immunoescape. J Biomed Biotechnol. 2012:4737122012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Deaglio S, Dwyer KM, Gao W, Friedman D,
Usheva A, Erat A, Chen JF, Enjyoji K, Linden J, Oukka M, et al:
Adenosine generation catalyzed by CD39 and CD73 expressed on
regulatory T cells mediates immune suppression. J Exp Med.
204:1257–1265. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bergamin LS, Braganhol E, Zanin RF,
Edelweiss MI and Battastini AM: Ectonucleotidases in tumor cells
and tumor-associated immune cells: An overview. J Biomed
Biotechnol. 2012:9598482012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ryzhov S, Novitskiy SV, Goldstein AE,
Biktasova A, Blackburn MR, Biaggioni I, Dikov MM and Feoktistov I:
Adenosinergic regulation of the expansion and immunosuppressive
activity of CD11b+Gr1+ cells. J Immunol. 187:6120–6129. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zou W and Chen L: Inhibitory B7-family
molecules in the tumour microenvironment. Nat Rev Immunol.
8:467–477. 2008. View Article : Google Scholar : PubMed/NCBI
|