Angiogenesis is a biological process in which novel
capillary blood vessels grow from pre-existing vasculature
(1), providing tissues with oxygen
and nutrients. As it is correlated with numerous complicated
interactions between various biological components, such as several
cell types, soluble angiogenic factors and extracellular matrix
components, the process of angiogenesis is complex, and primarily
consists of four distinct sequential steps: i) Degradation of
basement membrane glycoproteins and other components of the
extracellular matrix surrounding the blood vessels by proteolytic
enzymes; ii) endothelial cell activation and migration; iii)
endothelial cell proliferation; and iv) endothelial cells
transforming into tube-like structures and forming capillary tubes,
and developing into novel basement membranes (2). In normal conditions, angiogenesis only
occurs during embryonic development, the female reproductive cycle
and wound repair (3). However,
aberrant angiogenesis is a key mediator and a major process in
cancer development.
Through numerous studies investigating tumor
angiogenesis, different types of regulators have been defined
(8–13). These regulators are separately
released from endothelial cells, tumor cells, stromal cells, blood
and the extracellular matrix (14–17). These
modes of tumor angiogenesis may coexist or shift from one to
another during tumor growth and proliferation (18–20).
Certain well-known pro-angiogenic regulators include vascular
endothelial growth factor (VEGF), basic fibroblast growth factor,
transforming growth factor-α and -β (TGF-α and -β), epidermal
growth factor, platelet-derived growth factor, placental-derived
growth factor and angiopoietin 1 and 2. Specific, commonly studied
anti-angiogenic regulators include angiostatin, endostatin,
tumstatin, platelet factor-4, interleukin (IL)-12, thrombospondin-1
(TSP-1), tissue inhibitors of metalloproteinases (TIMPs) and
interferon-α, -β and -γ. Various biological activities trigger this
angiogenic switch. Genetic mutations (activation of oncogenes or
loss of tumor-suppressor genes that control production of
angiogenesis regulators), metabolic stress (hypoxia, low pH or
hypoglycemia), mechanical stress (pressure generated by
proliferating cells) and the immune/inflammatory response
(immune/inflammatory cells that have infiltrated the tissue) are
important stimuli of angiogenic signaling and tend to cause tumor
formation (21,22). Among them, hypoxia is one of the
primary factors that drive tumor angiogenesis, causing increased
expression of VEGF and other angiogenesis stimulators from hypoxic
cells (23). Concurrently,
matrix-remodeling enzymes, particularly matrix metalloproteinases,
mediate a number of the changes in the microenvironment of the
tumor tissue by degrading the extracellular matrix (24). Once hypoxia induces the upregulation
of VEGF, angiogenesis is initiated with additional activation of
hypoxia-inducible factor (HIF) signaling, to provide oxygen supply
(25), which stimulates the
endothelial cells (ECs) of the preexisting vasculature to sprout
and migrate into the hypoxic tissue, led by a gradient of VEGF
(26). Subsequently, the endothelial
cells differentiate into several cell types, consisting of the tip,
stalk and tube cells (27). The tip
cells, which express delta-like 4 (DLL4), are non-proliferative
cells located at the top of the novel vessels and guide the
direction of the novel vessel in response to VEGF signals (28,29). The
stalk cells, which express Notch-1, are highly proliferative, with
the ability to elongate the sprouting vessel through proliferation
when they receive DLL4/Notch signaling (30). The tube cells are non-proliferating,
which shape the final appearance of the vessels (6). During additional vascular formation,
endothelial progenitor cells (EPCs) are involved in the
construction of the inner layer of the novel blood vessels, with
pericytes such as specialized muscle cells stabilizing the vessel
tubes by providing structural support and forming an outer layer
around the ECs (31,32). Subsequently, the ECs connect with each
other to form a continuous endothelium, which is characterized by
complex, tight junctions (32) and
create loops that allow the blood to circulate through adhesion
molecules, followed by the construction of the basement membrane.
Finally, the vessel is mature and capable of transporting oxygen
and nutrition to meet the requirements of the hypoxic tumor tissues
(33).
Gene therapy is a therapeutic technique used to
correct or alleviate the symptoms of disease by transferring the
exogenous genes into the cells of an individual, which may
supplement or alter a defective gene, or induce cell death. In
total, there are 4 major strategies exploited in gene therapy,
consisting of: Gene replacement; gene modification; gene
augmentation; and gene blockage (34). By July 2015, >2,200 clinical trials
on gene therapy had been conducted or approved worldwide (35–37). Among
these trials, >60% are associated with cancer gene therapy,
indicating that gene therapy is not limited to hereditary diseases,
but may be used for acquired diseases such as cancer, and it has
already become a promising approach in cancer therapy.
In previous years, various gene therapy strategies
for cancer have been developed, such as anti-angiogenic gene
therapy, suicide gene therapy, immunomodulatory gene therapy, siRNA
therapy, pro-apoptotic gene therapy and oncolytic gene therapy
(38,39). However, as tumorigenesis is an
intricate process that involves various signaling pathways and
different mechanisms, and often a single gene may evoke several
biological processes and activate diverse signaling pathways,
occasionally there is no explicit boundary between these
aforementioned gene therapies. For example, gene tumor protein p53
may not only elicit apoptotic activities in tumor cells (40–42), but
also has demonstrated anti-angiogenic efficacy in a number of
studies (43,44). Therefore, gene therapy exploiting the
p53 gene may be characterized as an anti-angiogenic and
pro-apoptotic therapy.
Generally, whether gene therapy may be implemented
successfully or not will depend on two conditions: i) A suitable
gene must be identified to relieve the disease symptoms; and ii)
this gene must be delivered to the right location for the gene
expression product to treat the disease without causing side
effects. As gene therapy is such a precise and delicate therapeutic
intervention at the molecular level, there remain a number of
technical difficulties to overcome, one being the ability to
develop a suitable delivery system for the gene therapy.
Constructing an efficient, safe and specific
delivery system is the fundamental basis for gene therapy. Ideal
gene delivery systems should possess several attributes: i) A
relatively broad range of insertion capacity, with high
transfection rates and a non-invasive administration method; ii) it
allows for sustained gene expression; iii) a good target-specific
selectivity for the tumor type; iv) safety-associated features,
including biocompatibility, stability and non-immunogenicity; v)
easy availability. At present, numerous different vectors have been
constructed and applied in clinical trials: Table I has listed the top 10 most used
vectors in clinical studies (35–37).
Generally, the delivery systems in gene therapy may be categorized
into two groups, namely viral and non-viral vectors systems
(45).
Viral vectors were the first studied and are the
most commonly applied gene delivery systems, as they are derived
from viruses with a natural ability to transfection (46). In order to make viral vectors more
suitable for delivering heterologous genes into targeted cells,
they are often genetically optimized for improved efficiency,
increased safety and enhanced uptake (46,47).
During previous decades, the understanding of viral vectors has
increased, concomitant with improvement in their design and
production. Based on this progress, a number of viral vectors have
been identified and explored for gene delivery, including commonly
used viral vectors, such as adenovirus, adeno-associated virus
(AAV), retrovirus, herpes simplex virus (HSV), lentivirus, and
poxvirus (45), and certain novel
developed viral vectors, such as alphavirus vectors (48). Among these, lentiviral vectors and AAV
vectors have been the subject of focus in previous years (20), and a recent patent has provided novel
methods to shield the lentiviral vectors with a thin polymer shell,
conferring the shielded virus novel binding ability with additional
characteristics, including higher thermal stability, resistance to
serum inactivation and the ability to infect cells with high
efficiency (49). Generally, compared
with traditional transfection methods, viral vectors confer a
higher transduction efficiency with long-term gene expression.
However, certain weaknesses exist in terms of the immunogenicity,
mutagenicity, toxicity and high cost of these vectors and the
limited size of the transfected gene (50). Therefore, additional studies are
required for optimal use of viral vectors in gene therapy.
In order to circumvent the limitations of viral
vectors, there has been a focus on developing non-virus-mediated
gene delivery modalities, including physical mediated methods, and
chemical and biological vectors. Physical methods primarily consist
of microinjection, microparticle bombardment, ultrasound mediated
microbubble and electroporation. Compare with viral vectors,
ultrasound-targeted microbubbles (51,52) and
gene electrotransfer plasmids (53)
have received the majority of the attention in previous years as
they are more safe and efficient in terms of gene delivery.
Commonly used chemical vectors may be classified into 2 major types
based on the nature of the synthetic material, namely cationic
polymers and cationic liposomes (45). Despite the promising prospect that
cationic liposomes presented with several studies in clinical
trials, the low transfection efficiency and side effects, including
toxicity, are the primary obstacles preventing its widespread use
(54–57). Therefore, the newly-described cationic
core, the shell nanoparticles, appears to be an alternative to
liposomes, as it offers a greater number of advantages, including
high gene transfection efficiency and the ability of the concurrent
delivery of drugs and genes to the same cells (58). Biological vectors generally refer to
bacteria and specific mammalian cells. The types of bacteria used
as vectors include attenuated strains of Bifidobacteria,
Clostridia, Listeria, Salmonella, Shigella, Yersinia and
non-pathogenic Escherichia coli (34). As for mammalian cells, hematological
cells and mesenchymal stem cells (MSCs) are usually used as
carriers of gene therapy vectors (59). Additionally, gene-transfected EPCs may
be useful as a tumor-specific drug delivery system (60). Compared with viral vectors, non-viral
vectors provide advantages, including relative safety, ability to
transfer large size genes and less toxicity. They may also be
constructed and modified by simple methods for tissue- or
cell-specific targeting (54).
However, non-viral vectors exhibit limitations of a low
transfection efficiency and poor transgene expression (61). In conclusion, all of these methods
have been investigated and each of them presents distinct
advantages and disadvantages.
For the majority of cancer therapy strategies, the
tumor vasculature has provided issues for drug delivery, as it is a
barrier that prevents drugs from reaching tumor cells. However,
tumor angiogenesis is an easily accessible target for
anti-angiogenic cancer therapy, particularly when the
anti-angiogenic drugs are administered by delivery systems with
specificity for tumor endothelial cells. Notably, compared with the
anti-angiogenic therapies directly targeting tumor cells, targeting
ECs may be more practical when compared with tumor cells, as
endothelial cells have been identified to be genetically more
stable (62). The inhibition of EC
proliferation, migration and EC apoptosis by anti-angiogenic agents
may damage the viability of numerous tumor cells, due to
destruction of ECs not only limiting the supply of oxygen,
nutrients and growth factors produced by ECs to the surrounding
tumor cells, but also leading to the lack of structural support for
tumor cells, eventually resulting in the disassembly of tumor
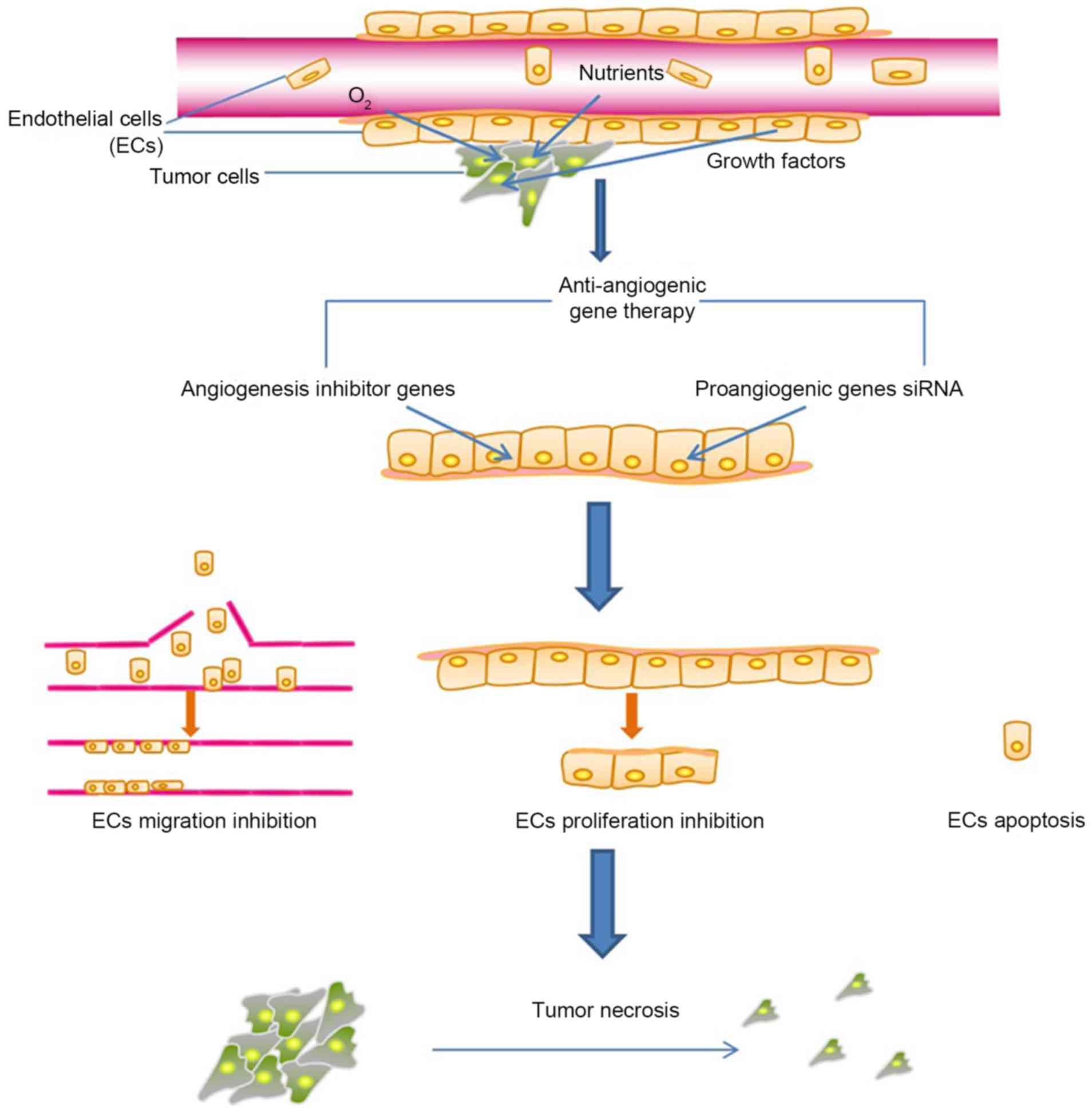
tissues (Fig. 1). In addition, the
same anti-angiogenic molecule may be efficient in various types of
cancer (63). Based on those
therapeutic advantages, efforts have been made to explore tumor
treatments that target angiogenesis. Furthermore, the comprehensive
study of various angiogenesis growth factors and inhibitors with
demonstrated therapeutic effects as administered anti-angiogenic
drugs have provided evidence for anti-angiogenesis therapy.
Table II summarizes the
anti-angiogenic drugs approved for clinical use. However, during
the long-term process of cancer treatment, the efficacy of
pharmaceutical proteins is limited due to their short half-life,
high cost and vulnerability to interference by endogenous
substances (64). Compared with
monoclonal antibodies and engineered antibodies, gene therapy has
the advantages of sustained and localized expression of the
therapeutic gene product, lower cost and fewer side effects
(65,66). Therefore, anti-angiogenesis cancer
gene therapy and combination of gene and anti-angiogenesis therapy
have become required.
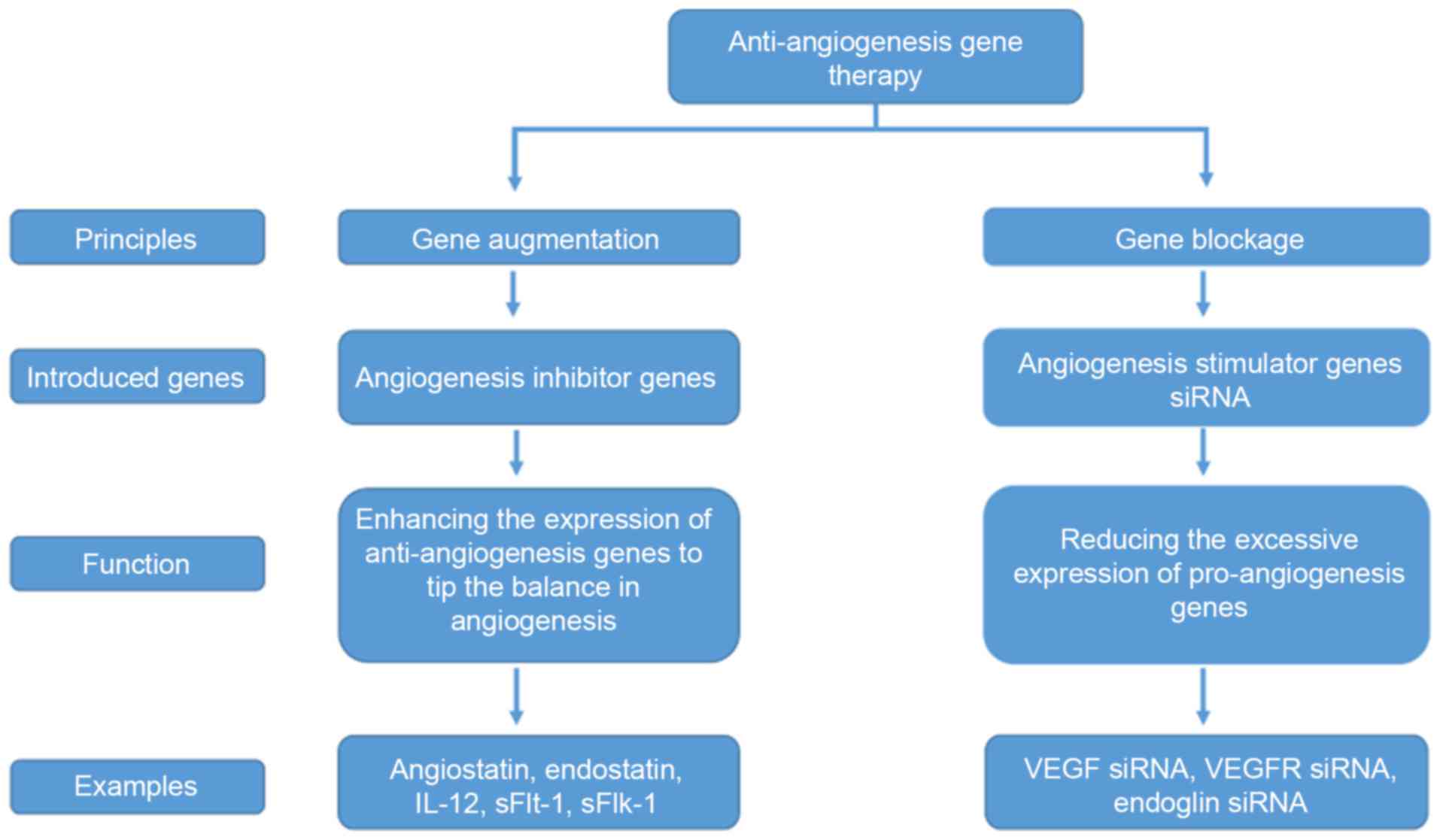
At present, anti-angiogenic cancer gene therapies
primarily adopt the following two principles: Gene augmentation;
and gene blockade. The former involves introducing exogenous
anti-angiogenic genes into targeted cells so that through their
expression tumor angiogenesis is halted, while the latter results
in the inhibition of the excessive expression of pro-angiogenic
genes in endothelial cells, and other tissue cells, of the tumor.
Therefore, the genes of interest may be divided into equivalent
categories: Anti-angiogenic genes utilized for gene augmentation;
and pro-angiogenic genes for gene blockade (Fig. 2).
With the development of biotechnology and an
improved understanding of angiogenesis mechanisms, numerous pro-
and anti-angiogenesis genes have been identified and utilized in
studies investigating cancer gene therapy (63,67). In
total, >300 angiogenesis inhibitors have been identified at
present (68); among them, >30
agents have been extensively studied in gene therapy (Table III). As various papers have already
reviewed a number of these anti-angiogenic molecules (63,64,68,69),
only the most commonly discussed inhibitors will be examined in
this paper, to avoid repetition.
IL-12, first recognized as a pro-inflammatory
cytokine with immunoregulatory functions (70,71), has
been suggested to exert an anti-angiogenesis effect in several
experiments (72–74). Due to its ability to stimulate
immunity and inhibit tumor angiogenesis, IL-12 has been identified
as one of the most potent antitumor candidates not only for cancer
immunotherapy (75), but also for
anti-angiogenic therapy (76).
Although previous evidence has indicated its anti-tumor activities
in in vitro and in vivo experiments (77), the anti-tumor effect of IL-12
evidently varies between mouse strains (78), and the mechanism that leads to the
various responses remains unclear. However, a previous study
demonstrated that the higher expression of IL-12 receptor
(IL-12RB1) by C3H/HeJ mouse splenocytes resulted in a significantly
stronger response to IL-12 compared with other mouse strains,
providing a potential explanation for the variation of IL-12
anti-tumor efficacy between different individuals (78). Although unsatisfactory side effects,
including toxicity, have been identified in several early clinical
trials using systemically delivered recombinant human IL-12
(rhIL-12) (79–81), interests in gene therapy approaches
have increased due to its potential in achieving high drug
concentrations in the local tumor environment, with low systemic
levels. Apart from several early clinical trials of gene therapy
using IL-12 in previous decades, a more recent study provided
long-term overall survival results from a phase I study of
intratumoral electroporation (EP) of plasmid (p)IL-12, which was
completed in 24 patients with malignant melanoma. This study
suggested that improved survival is correlated with systemic
disease stabilization with pIL-12 EP (82). An additional biomarker analysis study
investigating the efficacy of intratumoral electroporation of
pIL-12 from a phase 2 study in melanoma also demonstrated that
pIL-12 EP monotherapy induces tumor responses in 31% of patients,
and no severe local or systemic toxicity was observed in the
treatment (83). Concurrently,
certain gene therapies involving IL-12 use different delivery
systems to explore therapeutic methods with low systematic
toxicities, high tumorous specificities and sustained local
expression of IL-12, such as plasmid (84,85), HSV-1
(86), Semliki forest virus vector
(87), T-cells (88), a novel helper-dependent adenoviral
vector (89) and Lactococcus lactis
(90). Other strategies in previous
studies have focused on combining IL-12 with other anti-tumor
genes, including suicide genes (91),
or other therapies, such as chemotherapy (92), to explore its preclinical efficacy and
safety prior entry of these methods into clinical trials.
MDA-7, also termed IL-24, was identified through
subtraction hybridization from a human melanoma cell line (93), and has demonstrated efficacy as a
potent tumor suppressor gene in initial studies in the 1990s
(93–95). As an anti-cancer agent, MDA-7
functions through diverse modalities, including anti-angiogenesis
(96), tumor-specific apoptosis
(97) and immunotherapeutic activity
(98). Previously, a study examining
the effect of MDA-7 on Her2/Neu-induced mammary tumors concluded
that MDA-7 inhibited tumor growth of HER2+ breast cancer cells
partially through p53 apoptosis effector related to PMP-22, which
is a member of the PMP-22 family, with growth arrest and
apoptosis-inducing capacities (99).
In an additional study, the human MDA-7 gene was transfected into
the human laryngeal cancer Hep-2 cell line and human umbilical vein
endothelial cells with adenovirus vector (100), and the results demonstrated that
MDA-7 exerted anti-tumor functions in the laryngeal carcinoma cell
lines, whereas no harmful effect was observed in the healthy cells.
As for gene delivery, a study has introduced a method for
increasing the expression level of MDA-7 in osteosarcoma (OS) using
a novel oncolytic adenovirus, where an increased sensitivity of OS
to doxorubicin induced by MDA-7 was also observed (101). Finally, 3 vectors expressing MDA-7
in fusion with the arginine-glycine-aspartic acid (RGD) peptide,
which is considered to exhibit the most significant effect on the
binding specificity of integrin receptors, were constructed. With a
stronger expression potency observed and integrity validated, MDA-7
with RGD peptide appears to be a more appealing therapeutic option,
when compared with the administration of MDA-7 alone (102), indicating a future direction for
cancer gene therapy.
Angiostatin is the first of four Kringle domains of
a 38-kDa internal proteolytic fragment of plasminogen, which has
been recognized as a potent endogenous angiogenesis inhibitor, and
its anti-tumor effect has also been widely demonstrated (103). However, the primary obstacle
preventing its future application in clinical trials is that it
exhibits a limited therapeutic efficacy with a short half-life
(104). To resolve this problem,
studies have focused on elucidating efficient delivery systems, and
experiments investigating various non-viral and viral methods
delivering angiostatin gene have been conducted. At present,
angiostatin has been expressed in HSV (105,106),
vaccinia virus (107), oncolytic
measles virus (108), adenovirus
(109), adeno-associated viral
vectors (110,111) and lentivirus (112), or mediated by plasmids (113) and cationic liposomes (114). Concurrently, angiostatin is often
co-transfected with other genes for an enhanced anti-tumor
efficacy, like antisense HIF-1α (115), p53 (116), IL-12 (117), Fas gene (113), soluble form of vascular endothelial
growth factor receptor 2 sFlk1 (112) and most commonly used
endostatin-angiostatin fusion gene due to the fact that they were
identified to act synergistically when used in combination
(106–108). Previous studies (118–120)
suggested that angiostatin mimic (kringle1-5) appears more
attractive compared with angiostatin in terms of tumor suppression
and metastasis inhibition, potentially due to the synergistic
effect of the Kringle 5 domain of plasminogen.
Endostatin, a 20 kDa C-terminal cleavage fragment
from the α1 chain of type XVIII collagen, is one of the most
extensively studied endogenous angiogenesis inhibitor that was
originally identified by O'Reilly (121,122).
Endostar (YH-16), a protein drug of recombinant human endostatin,
was approved by China's State Food and Drug Administration for the
treatment of non-small cell lung cancer in 2005 (123), indicating the potential of
endostatin in cancer treatment. A gene-based endostatin approach
has also received attention, and has made its progression within a
pre-clinical context over previous decades, along with
breakthroughs in clinical trials. Previous studies primarily
focused on two categories: The joint method, combining endostatin
with other genes or with other cancer therapeutics; and the
exploration of a more suitable delivery system for endostatin to be
expressed in a more efficacious, tumor-targeted way. For example,
Huiqi et al (124) examined
the therapeutic effect of combining endostatin gene therapy with
32P colloid radiotherapy on hepatocellular carcinoma (HCC) cells,
and concluded that the combination of these two treatments
demonstrated an improved therapeutic effect on HCC compared with
either treatment alone. Kubo et al (112) also investigated a combinatorial
anti-angiogenic gene therapy with endostatin, angiostatin and
sFlk1, and an improved therapeutic efficacy was demonstrated
compared with that of a single-agent regimen, due to the ability of
three genes targeting different pathways of endothelial growth
factor signaling. An additional study identified that the
combination of human endostatin and soluble tumor necrosis factor
(TNF)-related apoptosis-inducing ligand gene transfer indicated an
enhanced tumor suppressing effect through anti-angiogenic and
pro-apoptotic mechanisms (125). As
for delivery systems, a previous study has demonstrated that
ultrasound-targeted microbubble destruction (UTMD)-mediated gene
therapy may enhance the transfection efficiency of endostatin,
indicating that the UTMD-mediated delivery system exhibits
potential as a gene therapy targeting retinal neovascularization
(126). Additionally, certain other
previous experiments concerning the efficacy of gene delivery or
the efficiency of combinatorial therapy utilizing endostatin have
all demonstrated progress in cancer treatment to a certain level
(108,127–130).
As an alternative appealing endogenous angiogenesis
inhibitor, tumstatin, a cleavage fragment of the α3 chain of type
IV collagen (131), is an exciting
candidate for cancer gene therapy, due to the fact that its
anti-angiogenic ability is 10-fold higher compared with that of
endostatin (132). By binding to
αVβ3 and α3β1 integrins (133),
tumstatin exerts its anti-angiogenic effects through diverse
modalities, including the induction of endothelial cells apoptosis,
inhibition of cell proliferation and tube formation in endothelial
cells, and a previous study has identified that tumstatin
stimulates endothelial cell apoptosis through the Fas signaling
pathway (134). The anti-angiogenic
and anti-tumorigenic effects of tumstatin have been widely
demonstrated by gene transfer experiments conducted in various
xenograft models, such as hepatocellular carcinoma (135), S180 tumor (136), lung carcinoma (137) and renal carcinoma cell (138). In previous years, efforts have been
made to develop and test diverse delivery systems in tumstatin gene
therapy. For example, lentivirus-mediated signal peptide
TNF-α-Tumstatin (45–132)-expressing mesenchymal stem cells
(SPTT-MSCs) have been used as a novel delivery approach in human
prostate cancer cells in vitro and in vivo, and
results have demonstrated significant anti-tumorigenic effects on
prostate cancer cells, indicating that SPTT-MSCs may represent a
promising solution for prostate cancer (139). In an additional experiment, gene
electrotransfer of naked plasmid DNA containing the tumstatin cDNA
has been adopted to investigate the anti-tumor effect of tumstatin
in B16F1 melanoma-bearing mice: A marked decrease in tumor growth
and an increase in mouse survival was observed, indicating that
this strategy appears appealing in terms of gene delivery and tumor
suppression (140). In addition, the
pET-15b vector generated to express a synthetic fusion protein,
VTF, which is composed of vasostatin and tumstatin with a
(Gly-Ser-Gly)2 bridge, demonstrated the suppression of B16 melanoma
growth and the potent inhibition of tumor blood vessels formation
in vivo, when compared with a single inhibitor, the fusion
proteins of different angiogenesis inhibitors targeting different
pathways exhibited improved therapeutic effects (141). Additionally, as T42, which was
derived from two active domains of tumstatin, has demonstrated
anti-tumor efficacy, a previous study constructed two adenoviral
vectors with T42 and 4xT42 peptide genes to evaluate their
anti-cancer effects on breast cancer in vitro and in
vivo; the results suggested evidence that this modality may be
a potential alternative for the treatment of breast cancer
(142).
AMEP, the disintegrin domain of human metargidin, is
a novel anticancer agent that exerts its effect by binding to α5β1
and αVβ3 integrins via its Arg-Gly-Asp (RGD) integrin binding
sequence (143–145). The antitumor and anti-angiogenic
effects of AMEP were first suggested in vitro using a
recombinant protein (143).
Subsequently, it was also demonstrated in vivo using an
AMEP-coding plasmid (146), and a
higher anti-tumor efficiency of AMEP compared with TSP-1 and
soluble fms-like tyrosine kinase-1 was observed, with a significant
decrease in tumor metastasis, suggesting that AMEP may not only
inhibit the proliferation of tumor cells but may also suppress
tumor metastasis. Following this, a phase I clinical trial study
was conducted to investigate the safety and tolerability of the
AMEP plasmid mediated by intratumoral electrotransfer into
cutaneous metastatic melanoma. Results indicated a good safety
profile and also, to a certain extent, the efficacy of AMEP plasmid
gene electrotransfer in metastatic melanoma (147). Additionally, a previous study
indicated that the anti-tumor activities of gene electrotransfer of
the AMEP plasmid in murine melanoma cells were correlated with the
integrin quantity within the melanoma cells, rather than the
expression level of AMEP; however, the anti-angiogenic effect was
only partly associated with the quantity of integrins, and appeared
to be dependent on the dose of the AMEP plasmid (148). In addition, a previous study
confirmed that the integrin quantity within melanoma cells may
serve as a biomarker for the antitumor efficacy of therapies
targeting integrins, whereas the anti-angiogenic effectiveness of
the AMEP plasmid may be predicted by the expression levels of AMEP
in the treatment of melanoma (149).
It also suggested that intratumoral delivery of the AMEP plasmid
was more effective compared with an intramuscular method. Based on
these aforementioned studies, it may be predicted that future
studies investigating the electrotransfer of the AMEP plasmid will
be more focused on particular types of cancer, in which the
overexpression of integrin is observed.
First isolated as a proteolytic digestion product of
hepatocyte growth factor (HGF) (150), NK4 is a novel anti-tumor agent
through its bifunctional activities of HGF antagonism and
anti-angiogenesis. Studies have also demonstrated that NK4 exerts
potent anti-angiogenic action via indirectly inhibiting VEGF
expression of tumor cells concomitant with direct effects on
endothelial cells (151). Although
the marked anti-angiogenic effect and anti-tumor ability of NK4 has
been confirmed in a diverse number of cancer models, such as
malignant pleural mesothelioma, melanoma, lung and pancreatic
carcinomas, and colon, biliary gastric and gall bladder cancers
(152–156), this individual anti-angiogenic agent
alone is not therapeutically sufficient, due to the fact that human
cancers are more intricate, and require treatment with multiple
targets. Therefore, subsequent studies have explored the potential
of NK4 in combination with conventional chemotherapeutic agents or
with other inhibitors targeting different signaling pathways.
Matsumoto et al (157)
identified that the anti-tumor efficacy of combining the NK4
plasmid with cisplatin to treat squamous cell carcinomas was
increased compared with NK4 gene therapy alone. An additional study
demonstrated that 5-fluorouracil enhanced the NK4-induced apoptosis
of colon cancer cells by downregulating the intracellular signaling
of the HGF/c-Met pathway (158).
Previously, studies have explored a more efficient and suitable way
to deliver NK4. Zhu et al (159) demonstrated that MSC-based NK4 gene
therapy may markedly inhibit the growth of gastric cancer
xenografts, and MSCs are a better vehicle for NK4 gene therapy
compared with lentiviral vectors. Additionally, a preliminary
clinical trial in humans has been designed to examine the safety
and possible clinical benefits of adenoviruses expressing NK4
(160).
Endoglin, a TGF-β co-receptor, is involved in the
activation of a complex signaling pathway regarding the
proliferation, migration and adhesion of endothelial cells
(161,162), particularly in tumor vasculature,
due to the fact that the expression of endoglin is markedly
increased in the endothelial cells of tumor vessels, making it a
potential predictive factor for tumor prognosis (163). As such, endoglin has been
hypothesized to serve as a promising target for cancer therapy, and
several studies using different anti-endoglin antibodies, including
monoclonal antibodies (164,165), immunotoxin-conjugated antibodies
(166) or radiolabeled antibodies
(167) have all demonstrated good
anti-angiogenic and antitumor responses. In the case of gene
therapy, silencing endoglin by RNA interference is considered to be
an alternative potential approach for endoglin targeting, and one
study group have conducted a series of experiments to explore the
potential of this approach. Dolinsek et al (168) first investigated the therapeutic
effectiveness of small interfering RNA (siRNA) molecules against
endoglin in vitro and in vivo, and the results
indicated that siRNA molecules targeting endoglin exhibited good
anti-angiogenic and antitumor efficacy on endothelial cells in
vitro, and on tumors in vivo. However, as the effect of
siRNA against endoglin exhibited a short half-life a plasmid DNA
encoding shRNA against endoglin was constructed and delivered into
murine endothelial cells in vitro and tumors in vivo
using gene electrotransfer to determine its antitumor and
vascular-targeted effects (169).
Furthermore, in order to specifically silence endoglin within the
tumor vasculature, the same study group also prepared a plasmid
that silenced endoglin with a tissue-specific promoter (hTERT)
(170), which was
endothelin-1-dependent and was involved in migration of endothelial
cells (171). The results of the
study indicated that this plasmid may achieve higher levels of
specificity and safety with the same efficacy as a plasmid with a
constitutive promoter. An additional previous study demonstrated
that endothelial and melanoma cells expressed high levels of
endoglin, and that subsequent to endoglin silencing with gene
electrotransfer, cell viability was specifically decreased; whereas
in tumor cells with low expression of endoglin, only a non-specific
decrease in cell viability was observed following electrotransfer
(172), providing novel
possibilities for melanoma treatment with targeted gene therapy
approach.
The previous 4 decades have witnessed the
feasibility of Folkman's theory in cancer treatment.
Anti-angiogenesis therapy, which used to be described as a novel
and potential method waiting to be verified of its efficacy in
treatment of various diseases, particularly cancer, now represents
one of the most significant and promising treatment modalities in
clinical oncology. With numerous efforts exploring the various
possibilities in utilizing anti-angiogenesis therapy, gene therapy
has become an attractive alternative to conventional protein drugs
due to its ability to achieve prolonged and localized gene
expression, without the issues of high cost and complex processes
of production associated with protein drugs.
With increased interest in angiogenesis during the
previous two decades, studies examining gene-based anti-angiogenic
approaches have made progress in the following three aspects.
Firstly, there has been continuous identification of targets for
anti-angiogenic gene therapy due to the additional understanding of
tumor angiogenesis. Secondly, further improving the efficacy of
existing gene delivery systems, with detailed optimization,
including the use of tissue-specific promoters or peptides
specifically targeted to tumor ECs, and exploring novel methods to
better facilitate gene transfer, particularly in the field of
non-viral delivery method, such as ultrasound and gene
electrotransfer. Thirdly, constantly improving anti-tumor efficacy
by combining anti-angiogenic genes with other genes, including
different angiogenesis or suicide genes, or genes that neutralize
anti-angiogenic resistance such as antisense HIF-1α. As an
increasing number of studies have identified that using the
anti-angiogenic gene approach as a monotherapy is not sufficient
for tumor eradication, and that certain angiogenesis inhibitors may
make tumor cells more susceptible towards chemotherapy and
radiotherapy, subsequent studies have investigated anti-angiogenic
gene therapy in combination with chemotherapy or radiotherapy.
Although a number of individual angiogenesis
inhibitors have demonstrated the ability to suppress tumor
progression and metastasis in a variety of cancer models, the
efficacy of tumor regression varies between different types of
cancer when using the same angiogenesis inhibitor, indicating that
a future direction for anti-angiogenic gene therapy is to identify
prognostic biomarkers to assist in determining the most efficient
angiogenesis inhibitor gene for each type of cancer, which will
largely rely on an improved understanding of the biological
mechanisms of tumor angiogenesis. In addition, as anti-angiogenic
gene therapy has demonstrated more potent effectiveness in small
tumors compared with large ones, future application of
anti-angiogenic gene therapy may be more involved in preventing and
treating early-stage cancers. Notably, the methods of gene delivery
and the limited therapeutic effect of monotherapy are major
obstacles to anti-angiogenic gene therapy; therefore, efforts to
develop more efficient gene delivery methods, and to explore
additional possibilities in combination therapy, are required.
Furthermore, a better understanding of the mechanisms of action and
a better selection of the clinical trial patient population should
also be performed by future studies.
Not applicable.
The present study was supported by the National
Nature Science Foundation of China (grant no. 31470967).
Not applicable.
HH gave guidance on the conception and design of the
article. TL and GK contributed their ideas on this topic and were
involved in planning the structure of this review. GK and TW
participated in the collection and organization. TL was a major
contributor in writing the review. HH made critical modifications
to important knowledge content within the manuscript. GK was a
major contributor in revision of the manuscript. All authors read
and approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Hanahan D and Folkman J: Patterns and
emerging mechanisms of the angiogenic switch during tumorigenesis.
Cell. 86:353–364. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fan TP, Jaggar R and Bicknell R:
Controlling the vasculature: Angiogenesis, anti-angiogenesis and
vascular targeting of gene therapy. Trends Pharmacol Sci. 16:57–66.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Folkman J and Shing Y: Angiogenesis. J
Biol Chem. 267:10931–10934. 1992.PubMed/NCBI
|
|
4
|
Folkman J: Tumor angiogenesis: Therapeutic
implications. N Engl J Med. 285:1182–1186. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Folkman J: Anti-angiogenesis: New concept
for therapy of solid tumors. Ann Surg. 175:409–416. 1972.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dimova I, Popivanov G and Djonov V:
Angiogenesis in cancer-general pathways and their therapeutic
implications. J BUON. 19:15–21. 2014.PubMed/NCBI
|
|
7
|
Ribatti D, Nico B, Crivellato E, Roccaro
AM and Vacca A: The history of the angiogenic switch concept.
Leukemia. 21:44–52. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Welti J, Loges S, Dimmeler S and Carmeliet
P: Recent molecular discoveries in angiogenesis and antiangiogenic
therapies in cancer. J Clin Invest. 123:3190–3200. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ribatti D and Djonov V: Intussusceptive
microvascular growth in tumors. Cancer Lett. 316:126–131. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Donnem T, Hu J, Ferguson M, Adighibe O,
Snell C, Harris AL, Gatter KC and Pezzella F: Vessel co-option in
primary human tumors and metastases: An obstacle to effective
anti-angiogenic treatment? Cancer Med. 2:427–436. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
de la Puente P, Muz B, Azab F and Azab AK:
Cell trafficking of endothelial progenitor cells in tumor
progression. Clin Cancer Res. 19:3360–3368. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Moschetta M, Mishima Y, Sahin I, Manier S,
Glavey S, Vacca A, Roccaro AM and Ghobrial IM: Role of endothelial
progenitor cells in cancer progression. Biochim Biophys Acta.
1846:26–39. 2014.PubMed/NCBI
|
|
13
|
Seftor RE, Hess AR, Seftor EA, Kirschmann
DA, Hardy KM, Margaryan NV and Hendrix MJ: Tumor cell vasculogenic
mimicry: From controversy to therapeutic promise. Am J Pathol.
181:1115–1125. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ferrara N and Adamis AP: Ten years of
anti-vascular endothelial growth factor therapy. Nat Rev Drug
Discov. 15:385–403. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mihicprobst D, Ikenberg K, Tinguely M,
Schraml P, Behnke S, Seifert B, Civenni G, Sommer L, Moch H and
Dummer R: Tumor cell plasticity and angiogenesis in human
melanomas. PLoS One. 7:e335712012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liekens S, Schols D and Hatse S:
CXCL12-CXCR4 axis in angiogenesis, metastasis and stem cell
mobilization. Curr Pharm Des. 16:3903–3920. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Eelen G, de Zeeuw P, Simons M and
Carmeliet P: Endothelial cell metabolism in normal and diseased
vasculature. Circ Res. 116:1231–1244. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bridgeman VL, Vermeulen PB, Foo S, Bilecz
A, Daley F, Kostaras E, Nathan MR, Wan E, Frentzas S, Schweiger T,
et al: Vessel co-option is common in human lung metastases and
mediates resistance to anti-angiogenic therapy in preclinical lung
metastasis models. J Pathol. 241:362–374. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hlushchuk R, Riesterer O, Baum O, Wood J,
Gruber G, Pruschy M and Djonov V: Tumor recovery by angiogenic
switch from sprouting to intussusceptive angiogenesis after
treatment with PTK787/ZK222584 or ionizing radiation. Am J Pathol.
173:1173–1185. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Frentzas S, Simoneau E, Bridgeman VL,
Vermeulen PB, Foo S, Kostaras E, Nathan M, Wotherspoon A, Gao ZH,
Shi Y, et al: Vessel co-option mediates resistance to
anti-angiogenic therapy in liver metastases. Nat Med. 22:1294–1302.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kerbel RS: Tumor angiogenesis: Past,
present and the near future. Carcinogenesis. 21:505–515. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Carmeliet P: Developmental biology.
Controlling the cellular brakes. Nature. 401:657–658. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dor Y, Porat R and Keshet E: Vascular
endothelial growth factor and vascular adjustments to perturbations
in oxygen homeostasis. Am J Physiol Cell Physiol. 280:C1367–C1374.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Littlepage LE, Sternlicht MD, Rougier N,
Phillips J, Gallo E, Yu Y, Williams K, Brenot A, Gordon JI and Werb
Z: Matrix metalloproteinases contribute distinct roles in
neuroendocrine prostate carcinogenesis, metastasis, and
angiogenesis progression. Cancer Res. 70:2224–2234. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Carmeliet P, Dor Y, Herbert JM, Fukumura
D, Brusselmans K, Dewerchin M, Neeman M, Bono F, Abramovitch R,
Maxwell P, et al: Role of HIF-1alpha in hypoxia-mediated apoptosis,
cell proliferation and tumour angiogenesis. Nature. 394:485–490.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Maracle CX and Tas SW: Inhibitors of
angiogenesis: Ready for prime time? Best Pract Res Clin Rheumatol.
28:637–649. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Blancas AA, Wong LE, Glaser DE and
McCloskey KE: Specialized tip/stalk-like and phalanx-like
endothelial cells from embryonic stem cells. Stem Cells Dev.
22:1398–1407. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jakobsson L, Franco CA, Bentley K, Collins
RT, Ponsioen B, Aspalter IM, Rosewell I, Busse M, Thurston G,
Medvinsky A, et al: Endothelial cells dynamically compete for the
tip cell position during angiogenic sprouting. Nat Cell Biol.
12:943–953. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lobov IB, Renard RA, Papadopoulos N, Gale
NW, Thurston G, Yancopoulos GD and Wiegand SJ: Delta-like ligand 4
(Dll4) is induced by VEGF as a negative regulator of angiogenic
sprouting. Proc Natl Acad Sci USA. 104:3219–3224. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu Z, Fan F, Wang A, Zheng S and Lu Y:
Dll4-Notch signaling in regulation of tumor angiogenesis. J Cancer
Res Clin Oncol. 140:525–536. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pandya NM, Dhalla NS and Santani DD:
Angiogenesis-a new target for future therapy. Vasc Pharmacol.
44:265–274. 2006. View Article : Google Scholar
|
|
32
|
Bergers G and Song S: The role of
pericytes in blood-vessel formation and maintenance. Neuro Oncol.
7:452–464. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Izzedine H, Ederhy S, Goldwasser F, Soria
JC, Milano G, Cohen A, Khayat D and Spano JP: Management of
hypertension in angiogenesis inhibitor-treated patients. Ann Oncol.
20:807–815. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu SX, Xia ZS and Zhong YQ: Genetic
therapy in pancreatic cancer. World J Gastroenterol.
20:13343–13368. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Edelstein ML, Abedi MR, Wixon J and
Edelstein RM: Gene therapy clinical trials worldwide 1989–2004-an
overview. J Gene Med. 6:597–602. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Edelstein ML, Abedi MR and Wixon J: Gene
therapy clinical trials worldwide to 2007-an update. J Gene Med.
9:833–842. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ginn SL, Alexander IE, Edelstein ML, Abedi
MR and Wixon J: Gene therapy clinical trials worldwide to 2012-an
update. J Gene Med. 15:65–77. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ortiz R, Melguizo C, Prados J, Álvarez PJ,
Caba O, Rodríguez-Serrano F, Hita F and Aránega A: New gene therapy
strategies for cancer treatment: A review of recent patents. Recent
Pat Anticancer Drug Discov. 7:297–312. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cao S, Cripps A and Wei MQ: New strategies
for cancer gene therapy: progress and opportunities. Clin Exp
Pharmacol Physiol. 37:108–114. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tseng SJ, Liao ZX, Kao SH, Zeng YF, Huang
KY, Li HJ, Yang CL, Deng YF, Huang CF, Yang SC, et al: Highly
specific in vivo gene delivery for p53-mediated apoptosis
and genetic photodynamic therapies of tumour. Nat Commun.
6:64562015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gogiraju R, Steinbrecher JH, Lehnart SE,
Kessel M, Dobbelstein M and Schaefer K: Importance of tumor
suppressor gene p53-mediated endothelial cell apoptosis for cardiac
angiogenesis and hypertrophy. Eur Heart J. 34 Suppl 1:S16162013.
View Article : Google Scholar
|
|
42
|
Tazawa H, Kagawa S and Fujiwara T:
Advances in adenovirus-mediated p53 cancer gene therapy. Expert
Opin Biol Ther. 13:1569–1583. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Prabha S, Sharma B and Labhasetwar V:
Inhibition of tumor angiogenesis and growth by
nanoparticle-mediated p53 gene therapy in mice. Cancer Gene Ther.
19:530–537. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Teodoro JG, Evans SK and Green MR:
Inhibition of tumor angiogenesis by p53: A new role for the
guardian of the genome. J Mol Med (Berl). 85:1175–1186. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhang C, Wang QT, Liu H, Zhang ZZ and
Huang WL: Advancement and prospects of tumor gene therapy. Chin J
Cancer. 30:182–188. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
El-Aneed A: An overview of current
delivery systems in cancer gene therapy. J Control Release.
94:1–14. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ramsey JD, Vu HN and Pack DW: A top-down
approach for construction of hybrid polymer-virus gene delivery
vectors. J Control Release. 144:39–45. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lundstrom K: Alphavirus vectors as tools
in neuroscience and gene therapy. Virus Res. 216:16–25. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
LU Y, Yan M, Chen IS and Liang M: Viral
vector nanocapsule for targeting gene therapy and its preparation.
Journal. 2015.
|
|
50
|
Touchefeu Y, Harrington KJ, Galmiche JP
and Vassaux G: Review article: Gene therapy, recent developments
and future prospects in gastrointestinal oncology. Aliment
Pharmacol Ther. 32:953–968. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Liao ZK, Tsai KC, Wang HT, Tseng SH, Deng
WP, Chen WS and Hwang LH: Sonoporation-mediated anti-angiogenic
gene transfer into muscle effectively regresses distant orthotopic
tumors. Cancer Gene Ther. 19:171–180. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ren J, Zhang P, Tian J, Zhou Z, Liu X,
Wang D and Wang Z: A targeted ultrasound contrast agent carrying
gene and cell-penetrating peptide: Preparation and gene
transfection in vitro. Colloids Surf B Biointerfaces.
121:362–370. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Yarmush ML, Golberg A, Serša G, Kotnik T
and Miklavčič D: Electroporation-based technologies for medicine:
principles, applications, and challenges. Annu Rev Biomed Eng.
16:295–320. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wang W, Li W, Ma N and Steinhoff G:
Non-viral gene delivery methods. Curr Pharm Biotechnol. 14:46–60.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Audouy SA, de Leij LF, Hoekstra D and
Molema G: In vivo characteristics of cationic liposomes as delivery
vectors for gene therapy. Pharm Res. 19:1599–1605. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Hortobagyi GN, Ueno NT, Xia W, Zhang S,
Wolf JK, Putnam JB, Weiden PL, Willey JS, Carey M, Branham DL, et
al: Cationic liposome-mediated E1A gene transfer to human breast
and ovarian cancer cells and its biologic effects: A phase i
clinical trial. J Clin Oncol. 19:3422–3433. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Wakabayashi T, Natsume A, Mizuno M, Fujii
M, Shimato S and Yoshida J: A clinical trial of cationic liposomes
containing interferon-b gene for patients with malignant glioma.
Int Conf Brain Tumor Res Ther. 225. 2009.
|
|
58
|
Wang Y, Gao S, Ye WH, Yoon HS and Yang YY:
Co-delivery of drugs and DNA from cationic core-shell nanoparticles
self-assembled from a biodegradable copolymer. Nat Mater.
5:791–796. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Power AT and Bell JC: Cell-based delivery
of oncolytic viruses: A new strategic alliance for a biological
strike against cancer. Mol Ther. 15:660–665. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Muta M, Matsumoto G, Hiruma K, Nakashima E
and Toi M: Study of cancer gene therapy using IL-12-secreting
endothelial progenitor cells in a rat solid tumor model. Oncol Rep.
10:1765–1769. 2003.PubMed/NCBI
|
|
61
|
Yin H, Kanasty RL, Eltoukhy AA, Vegas AJ,
Dorkin JR and Anderson DG: Non-viral vectors for gene-based
therapy. Nat Rev Genet. 15:541–555. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Kim WJ, Yockman JW, Lee M, Jeong JH, Kim
YH and Kim SW: Soluble Flt-1 gene delivery using PEI-g-PEG-RGD
conjugate for anti-angiogenesis. J Control Release. 106:224–234.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Persano L, Crescenzi M and Indraccolo S:
Anti-angiogenic gene therapy of cancer: Current status and future
prospects. Mol Aspects Med. 28:87–114. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Albini A, Tosetti F, Li VW, Noonan DM and
Li WW: Cancer prevention by targeting angiogenesis. Nat Rev Clin
Oncol. 9:498–509. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Morrison C: $1-million price tag set for
Glybera gene therapy. Nat Biotechnol. 33:217–218. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Rodriguez D and Miessner P: Production of
AAV vectors for gene therapy: A cost-effectiveness and risk
assessment (unpublished PhD thesis). Department of Chemical
Engineering and the MIT Sloan School of Management. 2016.
|
|
67
|
Chen HH, Kuliszewski MA, Rudenko D and
Leong-Poi H: Pre-clinical evaluation of pro-angiogenic gene therapy
by ultrasound-targeted microbubble destruction of vascular
endothelial growth factor minicircle dna in an model of severe
peripheral arterial disease in watanabe heritable hyperlipidemic
rabbits. Can J Cardiol. 31 Suppl:S2822015. View Article : Google Scholar
|
|
68
|
Feng X: Angiogenesis and Antiangiogenesis
Therapies: Spear and Shield of Pharmacotherapy. J Pharma Care
Health Sys. 1:e1102014.
|
|
69
|
Ichihara E, Kiura K and Tanimoto M:
Targeting angiogenesis in cancer therapy. Acta Med Okayama.
65:353–362. 2011.PubMed/NCBI
|
|
70
|
Trinchieri G: Interleukin-12: A cytokine
produced by antigen-presenting cells with immunoregulatory
functions in the generation of T-helper cells type 1 and cytotoxic
lymphocytes. Blood. 84:4008–4027. 1994.PubMed/NCBI
|
|
71
|
Trinchieri G: Interleukin-12: A
proinflammatory cytokine with immunoregulatory functions that
bridge innate resistance and antigen-specific adaptive immunity.
Annu Rev Immunol. 13:251–276. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Duda DG, Sunamura M, Lozonschi L, Kodama
T, Egawa S, Matsumoto G, Shimamura H, Shibuya K, Takeda K and
Matsuno S: Direct in vitro evidence and in vivo
analysis of the antiangiogenesis effects of interleukin 12. Cancer
Res. 60:1111–1116. 2000.PubMed/NCBI
|
|
73
|
Dias S, Boyd R and Balkwill F: IL-12
regulates VEGF and MMPs in a murine breast cancer model. Int J
Cancer. 78:361–365. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Voest EE, Kenyon BM, O'Reilly MS, Truitt
G, D'Amato RJ and Folkman J: Inhibition of angiogenesis in
vivo by interleukin 12. J Natl Cancer Inst. 87:581–586. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Akiyama Y, Maruyama K, Watanabe M and
Yamaguchi K: Retroviral-mediated IL-12 gene transduction into human
CD34+ cell-derived dendritic cells. Int J Oncol. 21:509–514.
2002.PubMed/NCBI
|
|
76
|
Sunamura M, Sun L, Lozonschi L, Duda DG,
Kodama T, Matsumoto G, Shimamura H, Takeda K, Kobari M, Hamada H
and Matsuno S: The antiangiogenesis effect of interleukin 12 during
early growth of human pancreatic cancer in SCID mice. Pancreas.
20:227–233. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Li Q, Zhihua W, Xiumin Y, et al: The
effect of il-12 on the proliferation in vitro and anti-tumor
effects of cik cells in vivo and in vitro. J Pract
Oncol. 21:212–215. 2007.(In Chinese).
|
|
78
|
Nguyen K, Koppolu B, Smith G,
Ravindranathan S and Zaharoff D: Interleukin-12 elicits various
responses of splenocytes from different mouse strains. J Immunol.
194 1 Suppl:(S49): 82015.
|
|
79
|
Portielje JE, Kruit WH, Schuler M, Beck J,
Lamers CH, Stoter G, Huber C, de Boer-Dennert M, Rakhit A, Bolhuis
RL and Aulitzky WE: Phase I study of subcutaneously administered
recombinant human interleukin 12 in patients with advanced renal
cell cancer. Clin Cancer Res. 5:3983–3989. 1999.PubMed/NCBI
|
|
80
|
Gollob JA, Mier JW, Veenstra K, McDermott
DF, Clancy D, Clancy M and Atkins MB: Phase I trial of twice-weekly
intravenous interleukin 12 in patients with metastatic renal cell
cancer or malignant melanoma: Ability to maintain IFN-gamma
induction is associated with clinical response. Clin Cancer Res.
6:1678–1692. 2000.PubMed/NCBI
|
|
81
|
Hurteau JA, Blessing JA, DeCesare SL and
Creasman WT: Evaluation of recombinant human interleukin-12 in
patients with recurrent or refractory ovarian cancer: A gynecologic
oncology group study. Gynecol Oncol. 82:7–10. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Daud A, Takamura KT, Diep T, Heller R and
Pierce RH: Long-term overall survival from a phase I trial using
intratumoral plasmid interleukin-12 with electroporation in
patients with melanoma. J Transl Med. 13 Suppl 1:O32015. View Article : Google Scholar
|
|
83
|
Daud A, Algazi A, Ashworth M, Buljan M,
Takamura KT, Diep T, Pierce RH and Bhatia S: Intratumoral
electroporation of plasmid interleukin-12: Efficacy and biomarker
analyses from a phase 2 study in melanoma (OMS-I100). J Transl Med.
13 Suppl 1:O112015. View Article : Google Scholar
|
|
84
|
Cutrera J, King G, Jones P, Kicenuik K,
Gumpel E, Xia X and Li S: Safety and Efficacy of Tumor-Targeted
Interleukin 12 Gene Therapy in Treated and Non-Treated, Metastatic
Lesions. Curr Gene Ther. 15:44–55. 2014. View Article : Google Scholar
|
|
85
|
Lampreht U, Kamensek U, Stimac M, et al:
Gene electrotransfer of canine interleukin 12 into canine melanoma
cell lines. J Membr Biol. 248:909–917. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Markert JM, Cody JJ, Parker JN, Coleman
JM, Price KH, Kern ER, Quenelle DC, Lakeman AD, Schoeb TR, Palmer
CA, et al: Preclinical evaluation of a genetically engineered
herpes simplex virus expressing interleukin-12. J Virol.
86:5304–5313. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Kramer MG, Masner M, Casales E, Moreno M,
Smerdou C and Chabalgoity JA: Neoadjuvant administration of Semliki
Forest virus expressing interleukin-12 combined with attenuated
Salmonella eradicates breast cancer metastasis and achieves
long-term survival in immunocompetent mice. BMC Cancer. 15:6202015.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Koneru M, O'Cearbhaill R, Pendharkar S,
Spriggs DR and Brentjens RJ: A phase I clinical trial of adoptive T
cell therapy using IL-12 secreting MUC-16(ecto) directed chimeric
antigen receptors for recurrent ovarian cance. J Transl Med.
13:1022015. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Poutou J, Bunuales M, Gonzalez-Aparicio M,
Garcia-Aragoncillo E, Quetglas JI, Casado R, Bravo-Perez C,
Alzuguren P and Hernandez-Alcoceba R: Safety and antitumor effect
of oncolytic and helper-dependent adenoviruses expressing
interleukin-12 variants in a hamster pancreatic cancer model. Gene
Ther. 22:696–706. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Li Y, Li X, Liu H, Zhuang S, Yang J and
Zhang F: Intranasal immunization with recombinant Lactococci
carrying human papillomavirus E7 protein and mouse interleukin-12
DNA induces E7-specific antitumor effects in C57BL/6 mice. Oncol
Lett. 7:576–582. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Freytag SO, Zhang Y and Siddiqui F:
Preclinical toxicology of oncolytic adenovirus-mediated cytotoxic
and interleukin-12 gene therapy for prostate cancer. Mol Ther
Oncolytics. 2:pii: 15006. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Cutrera J, King G, Jones P, Kicenuik K,
Gumpel E, Xia X and Li S: Safe and effective treatment of
spontaneous neoplasms with interleukin 12 electro-chemo-gene
therapy. J Cell Mol Med. 19:664–675. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Jiang H, Lin JJ, Su Z, Goldstein N and
Fisher P: Subtraction hybridization identifies a novel melanoma
differentiation associated gene, mda-7, modulated during human
melanoma differentiation, growth and progression. Oncogene.
11:2477–2486. 1995.PubMed/NCBI
|
|
94
|
Jiang H, Su ZZ, Lin JJ, Goldstein NI,
Young CS and Fisher PB: The melanoma differentiation associated
gene mda-7 suppresses cancer cell growth. Proc Natl Acad Sci USA.
93:9160–9165. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Su ZZ, Madireddi MT, Lin JJ, Young CS,
Kitada S, Reed JC, Goldstein NI and Fisher PB: The cancer growth
suppressor gene mda-7 selectively induces apoptosis in human breast
cancer cells and inhibits tumor growth in nude mice. Proc Natl Acad
Sci USA. 95:14400–14405. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Saeki T, Mhashilkar A, Swanson X, Zou-Yang
XH, Sieger K, Kawabe S, Branch CD, Zumstein L, Meyn RE, Roth JA, et
al: Inhibition of human lung cancer growth following
adenovirus-mediated mda-7 gene expression in vivo. Oncogene.
21:4558–4566. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Sauane M, Gopalkrishnan RV, Lebedeva I,
Mei MX, Sarkar D, Su ZZ, Kang DC, Dent P, Pestka S and Fisher PB:
Mda-7/IL-24 induces apoptosis of diverse cancer cell lines through
JAK/STAT-independent pathways. J Cell Physiol. 196:334–345. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Menezes ME, Shen XN, Das SK, Emdad L, Guo
C, Yuan F, Li YJ, Archer MC, Zacksenhaus E, Windle JJ, et al:
MDA-7/IL-24 functions as a tumor suppressor gene in vivo in
transgenic mouse models of breast cancer. Oncotarget.
6:36928–36942. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Li YJ, Liu G, Xia L, Xiao X, Liu JC,
Menezes ME, Das SK, Emdad L, Sarkar D, Fisher PB, et al:
Suppression of Her2/Neu mammary tumor development in mda-7/IL-24
transgenic mice. Oncotarget. 6:36943–36954. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Chen X, Liu D, Wang J, Su Q, Zhou P, Liu
J, Luan M and Xu X: Suppression effect of recombinant adenovirus
vector containing hIL-24 on Hep-2 laryngeal carcinoma cells. Oncol
Lett. 7:771–777. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Liu Z, Xu L, Yuan H, Zhang Y, Zhang X and
Zhao D: Oncolytic adenovirus-mediated mda-7/IL-24 expression
suppresses osteosarcoma growth and enhances sensitivity to
doxorubicin. Mol Med Rep. 12:6358–6364. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Khodadad M, Hosseini SY, Shenavar F,
Erfani N, Bina S, Ahmadian S, Fattahi MR and Hajhosseini R:
Construction of expressing vectors including melanoma
differentiation-associated gene-7 (mda-7) fused with the RGD
sequences for better tumor targeting. Iran J Basic Med Sci.
18:780–787. 2015.PubMed/NCBI
|
|
103
|
O'Reilly MS, Holmgren L, Shing Y, Chen C,
Rosenthal RA, Moses M, Lane WS, Cao Y, Sage EH and Folkman J:
Angiostatin: A novel angiogenesis inhibitor that mediates the
suppression of metastases by a Lewis lung carcinoma. Cell.
79:315–328. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Wahl ML, Moser TL and Pizzo SV:
Angiostatin and anti-angiogenic therapy in human disease. Recent
Prog Horm Res. 59:73–104. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Zhang G, Jin G, Nie X, Mi R, Zhu G, Jia W
and Liu F: Enhanced antitumor efficacy of an oncolytic herpes
simplex virus expressing an endostatin-angiostatin fusion gene in
human glioblastoma stem cell xenografts. PLoS One. 9:e958722014.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Zhu G, Su W, Jin G, Xu F, Hao S, Guan F,
Jia W and Liu F: Glioma stem cells targeted by oncolytic virus
carrying endostatin-angiostatin fusion gene and the expression of
its exogenous gene in vitro. Brain Res. 1390:59–69. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Tysome JR, Wang P, Alusi G, Briat A,
Gangeswaran R, Wang J, Bhakta V, Fodor I, Lemoine NR and Wang Y:
Lister vaccine strain of vaccinia virus armed with the
endostatin-angiostatin fusion gene: An oncolytic virus superior to
dl1520 (ONYX-015) for human head and neck cancer. Hum Gene Ther.
22:1101–1108. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Hutzen B, Bid HK, Houghton PJ, Pierson CR,
Powell K, Bratasz A, Raffel C and Studebaker AW: Treatment of
medulloblastoma with oncolytic measles viruses expressing the
angiogenesis inhibitors endostatin and angiostatin. BMC Cancer.
14:2062014. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Li X, Liu YH, Lee SJ, Gardner TA, Jeng MH
and Kao C: Prostate-restricted replicative adenovirus expressing
human endostatin-angiostatin fusion gene exhibiting dramatic
antitumor efficacy. Clin Cancer Res. 14:291–299. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Ma HI, Lin SZ, Chiang YH, Li J, Chen SL,
Tsao YP and Xiao X: Intratumoral gene therapy of malignant brain
tumor in a rat model with angiostatin delivered by adeno-associated
viral (AAV) vector. Gene Ther. 9:2–11. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Li R, Chen H and Ren CS: Growth inhibition
of breast cancer in rat by AAV mediated angiostatin gene. Chin J
Cancer Res. 19:108–112. 2007. View Article : Google Scholar
|
|
112
|
Kubo S, Takagi-Kimura M and Kasahara N:
Combinatorial anti-angiogenic gene therapy in a human malignant
mesothelioma model. Oncol Rep. 34:633–638. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Tan JF, Lu Q, Zhang XW and Tan M: Effect
of co-transfection of angiostatin and Fas gene on growth of
transplanted tumor in nude mice. China J Mod Med. 8:0132008.(In
Chinese).
|
|
114
|
Kim HS, Jeong HY, Lee YK, Kim KS and Park
YS: Synergistic antitumoral effect of IL-12 gene cotransfected with
antiangiogenic genes for Angiostatin, Endostatin, and Saxatilin.
Oncol Res Featuring Preclinical Clin Cancer Ther. 21:209–216.
2013.
|
|
115
|
Sun X, Vale M, Jiang X, Gupta R and
Krissansen G: Antisense HIF-1alpha prevents acquired tumor
resistance to angiostatin gene therapy. Cancer Gene Ther.
17:532–540. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Chen XJ, Zhu YY, Hu ZT, Zhang HH, Weng SM
and Zhuang HZ: Effect of co-transfection of p53 and angiostatin
gene on the apoptosis of gastric cancer SG7901 cells. Tumor.
7:577–580. 2010.
|
|
117
|
Schmitz V, Tirado-Ledo L, Raskopf E, Rabe
C, Wernert N, Wang L, Prieto J, Qian C, Sauerbruch T and Caselmann
WH: Effective antitumour mono- and combination therapy by gene
delivery of angiostatin-like molecule and interleukin-12 in a
murine hepatoma model. Int J Colorectal Dis. 20:494–501. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Kuo CH, Chang BI, Lee FT, Chen PK, Lee JS,
Shi GY and Wu HL: Development of recombinant adeno-associated virus
serotype 2/8 carrying kringle domains of human plasminogen for
sustained expression and cancer therapy. Hum Gene Ther. 26:603–613.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Chu Y, Liu H, Lou G, Zhang Q and Wu C:
Human placenta mesenchymal stem cells expressing exogenous
kringle1-5 protein by fiber-modified adenovirus suppress
angiogenesis. Cancer Gene Ther. 21:200–208. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Schmitz V, Sauerbruch T and Raskopf E:
Anti-tumoural effects of PlgK1-5 are directly linked to reduced
ICAM expression, resulting in hepatoma cell apoptosis. Int J
Colorectal Dis. 27:1029–1038. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
O'Reilly MS, Boehm T, Shing Y, Fukai N,
Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR and Folkman J:
Endostatin: An endogenous inhibitor of angiogenesis and tumor
growth. Cell. 88:277–285. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Sasaki T, Fukai N, Mann K, Göhring W,
Olsen BR and Timpl R: Structure, function and tissue forms of the
C-terminal globular domain of collagen XVIII containing the
angiogenesis inhibitor endostatin. EMBO J. 17:4249–4256. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Rong B, Yang S, Li W, Zhang W and Ming Z:
Systematic review and meta-analysis of Endostar (rh-endostatin)
combined with chemotherapy versus chemotherapy alone for treating
advanced non-small cell lung cancer. World J Surg Oncol.
10:1702012. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Huiqi G, Jing Z, Peng F, Yong L and
Baozhong S: In vivo study of the effect of combining endostatin
gene therapy with 32P-colloid on hepatocarcinoma and its
functioning mechanism. J BUON. 20:1042–1047. 2015.PubMed/NCBI
|
|
125
|
Yan F, Zheng Y and Huang L:
Adenovirus-mediated combined anti-angiogenic and pro-apoptotic gene
therapy enhances antitumor efficacy in hepatocellular carcinoma.
Oncol Lett. 5:348–354. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Xu Y, Xie Z, Zhou Y, Zhou X, Li P, Wang Z
and Zhang Q: Experimental endostatin-GFP gene transfection into
human retinal vascular endothelial cells using ultrasound-targeted
cationic microbubble destruction. Mol Vis. 21:930–938.
2015.PubMed/NCBI
|
|
127
|
Li XP, Zhang HL, Wang HJ, Li YX, Li M, Lu
L, Wan Y, Zhou BL, Liu Y, Pan Y, et al: Ad-endostatin treatment
combined with low-dose irradiation in a murine lung cancer model.
Oncol Rep. 32:650–658. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Liu RY, Zhou L, Zhang YL, Huang BJ, Ke ML,
Chen JM, Li LX, Fu X, Wu JX and Huang W: An oncolytic adenovirus
enhances antiangiogenic and antitumoral effects of a
replication-deficient adenovirus encoding endostatin by rescuing
its selective replication in nasopharyngeal carcinoma cells.
Biochem Biophys Res Commun. 442:171–176. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Li L, Zhang Y, Zhou L, Ke ML, Chen JM, Fu
X, Ye CL, Wu JX, Liu RY and Huang W: Antitumor efficacy of a
recombinant adenovirus encoding endostatin combined with an
E1B55KD-deficient adenovirus in gastric cancer cells. J Transl Med.
11:2572013. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Zhou Y, Gu H, Xu Y, Li F, Kuang S, Wang Z,
Zhou X, Ma H, Li P, Zheng Y, et al: Targeted antiangiogenesis gene
therapy using targeted cationic microbubbles conjugated with CD105
antibody compared with untargeted cationic and neutral
microbubbles. Theranostics. 5:399–417. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Maeshima Y, Colorado PC, Torre A, Holthaus
KA, Grunkemeyer JA, Ericksen MB, Hopfer H, Xiao Y, Stillman IE and
Kalluri R: Distinct antitumor properties of a type IV collagen
domain derived from basement membrane. J Biol Chem.
275:21340–21348. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Yang YP, Xu CX, Hou GS, Xin JX, Wang W and
Liu XX: Effects of eukaryotic expression plasmid encoding human
tumstatin gene on endothelial cells in vitro. Chin Med J
(Engl). 123:2269–2273. 2010.PubMed/NCBI
|
|
133
|
Borza CM, Pozzi A, Borza DB, Pedchenko V,
Hellmark T, Hudson BG and Zent R: Integrin alpha3beta1, a novel
receptor for alpha3(IV) noncollagenous domain and a trans-dominant
Inhibitor for integrin alphavbeta3. J Biol Chem. 281:20932–20939.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Hwang-Bo J, Park JH and Chung IS:
Tumstatin induces apoptosis mediated by Fas signaling pathway in
oral squamous cell carcinoma SCC-VII cells. Oncol Lett.
10:1016–1022. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Goto T, Ishikawa H, Matsumoto K, Nishimura
D, Kusaba M, Taura N, Shibata H, Miyaaki H, Ichikawa T, Hamasaki K,
et al: Tum-1, a tumstatin fragment, gene delivery into
hepatocellular carcinoma suppresses tumor growth through inhibiting
angiogenesis. Int J Oncol. 33:33–40. 2008.PubMed/NCBI
|
|
136
|
You Y, Xue X, Li M, Qin X, Zhang C, Wang
W, Giang C, Wu S, Liu Y, Zhu W, et al: Inhibition effect of
pcDNA-tum-5 on the growth of S180 tumor. Cytotechnology. 56:97–104.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Caudroy S, Cucherousset J, Lorenzato M,
Zahm JM, Martinella-Catusse C, Polette M and Birembaut P:
Implication of tumstatin in tumor progression of human
bronchopulmonary carcinomas. Hum Pathol. 35:1218–1222. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Yang YP, Xu CX, Hou GS, Xin JX, Wang W and
Liu XX: Effects of eukaryotic expression plasmid encoding human
tumstatin gene on endothelial cells in vitro. Chin Med J
(Engl). 123:2269–2273. 2010.PubMed/NCBI
|
|
139
|
Zhang X, Xu W, Qian H, Zhu W and Zhang R:
Mesenchymal stem cells modified to express lentivirus TNF-α
Tumstatin(45–132) inhibit the growth of prostate cancer. J Cell Mol
Med. 15:433–444. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Thevenard J, Ramont L, Mir LM,
Dupont-Deshorgue A, Maquart FX, Monboisse JC and Brassart-Pasco S:
A new anti-tumor strategy based on in vivo tumstatin
overexpression after plasmid electrotransfer in muscle. Biochem
Biophys Res Commun. 432:549–552. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Gu Q, Sun C, Luo J, Zhang T and Wang L:
Inhibition of angiogenesis by a synthetic fusion protein VTF
derived from vasostatin and tumstatin. Anticancer Drugs.
25:1044–1051. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Zhang X, Qi DD, Zhang TT, Chen QX, Wang
GZ, Sui GY, Hao XW, Sun S, Song X and Chen YL: Antitumor activity
of adenoviral vector containing T42 and 4xT42 peptide gene through
inducing apoptosis of tumor cells and suppressing angiogenesis. Mol
Med Rep. 11:2083–2091. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Trochon-Joseph V, Martel-Renoir D, Mir LM,
Thomaïdis A, Opolon P, Connault E, Li H, Grenet C, Fauvel-Lafève F,
Soria J, et al: Evidence of antiangiogenic and antimetastatic
activities of the recombinant disintegrin domain of metargidin.
Cancer Res. 64:2062–2069. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Nath D, Slocombe PM, Stephens PE, Warn A,
Hutchinson GR, Yamada KM, Docherty AJ and Murphy G: Interaction of
metargidin (ADAM-15) with alphavbeta3 and alpha5beta1 integrins on
different haemopoietic cells. J Cell Sci. 112:579–587.
1999.PubMed/NCBI
|
|
145
|
Danhier F, Le Breton A and Préat V:
RGD-based strategies to target alpha(v) beta(3) integrin in cancer
therapy and diagnosis. Mol Pharm. 9:2961–2973. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Daugimont L, Vandermeulen G, Defresne F,
Bouzin C, Mir LM, Bouquet C, Feron O and Préat V: Antitumoral and
antimetastatic effect of antiangiogenic plasmids in B16 melanoma:
Higher efficiency of the recombinant disintegrin domain of ADAM 15.
Eur J Pharm Biopharm. 78:314–319. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Spanggaard I, Snoj M, Cavalcanti A,
Bouquet C, Sersa G, Robert C, Cemazar M, Dam E, Vasseur B, Attali
P, et al: Gene electrotransfer of plasmid antiangiogenic metargidin
peptide (AMEP) in disseminated melanoma: Safety and efficacy
results of a phase I first-in-man study. Hum Gene Ther Clin Dev.
24:99–107. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Bosnjak M, Prosen L, Dolinsek T, Blagus T,
Markelc B, Cemazar M, Bouquet C and Sersa G: Biological properties
of melanoma and endothelial cells after plasmid AMEP gene
electrotransfer depend on integrin quantity on cells. J Membr Biol.
246:803–819. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Bosnjak M, Dolinsek T, Cemazar M, Kranjc
S, Blagus T, Markelc B, Stimac M, Zavrsnik J, Kamensek U, Heller L,
et al: Gene electrotransfer of plasmid AMEP, an integrin-targeted
therapy, has antitumor and antiangiogenic action in murine B16
melanoma. Gene Ther. 22:578–590. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Date K, Matsumoto K, Shimura H, Tanaka M
and Nakamura T: HGF/NK4 is a specific antagonist for pleiotrophic
actions of hepatocyte growth factor. FEBS Lett. 420:1–6. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Kubota T, Matsumura A, Taiyoh H, Izumiya
Y, Fujiwara H, Okamoto K, Ichikawa D, Shiozaki A, Komatsu S,
Nakanishi M, et al: Interruption of the HGF paracrine loop by NK4,
an HGF antagonist, reduces VEGF expression of CT26 cells. Oncol
Rep. 30:567–572. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Kishi Y, Kuba K and Nakamura T, Wen J,
Suzuki Y, Mizuno S, Nukiwa T, Matsumoto K and Nakamura T: Systemic
NK4 gene therapy inhibits tumor growth and metastasis of melanoma
and lung carcinoma in syngeneic mouse tumor models. Cancer Sci.
100:1351–1358. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Ogura Y, Mizumoto K, Nagai E, Murakami M,
Inadome N, Saimura M, Matsumoto K, Nakamura T, Maemondo M, Nukiwa T
and Tanaka M: Peritumoral injection of adenovirus vector expressing
NK4 combined with gemcitabine treatment suppresses growth and
metastasis of human pancreatic cancer cells implanted
orthotopically in nude mice and prolongs survival. Cancer Gene
Ther. 13:520–529. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Nakamura T, Sakai K, Nakamura T and
Matsumoto K: Anti-cancer approach with NK4: Bivalent action and
mechanisms. Anticancer Agents Med Chem. 10:36–46. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Matsumoto K and Nakamura T: Mechanisms and
significance of bifunctional NK4 in cancer treatment. Biochem
Biophys Res Commun. 333:316–327. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Suzuki Y, Sakai K, Ueki J, Xu Q, Nakamura
T, Shimada H, Nakamura T and Matsumoto K: Inhibition of Met/HGF
receptor and angiogenesis by NK4 leads to suppression of tumor
growth and migration in malignant pleural mesothelioma. Int J
Cancer. 127:1948–1957. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Matsumoto G, Omi Y, Lee U, Kubota E and
Tabata Y: NK4 gene therapy combined with cisplatin inhibits tumour
growth and metastasis of squamous cell carcinoma. Anticancer Res.
31:105–111. 2011.PubMed/NCBI
|
|
158
|
Taiyoh H, Kubota T, Fujiwara H, Matsumura
A, Murayama Y, Okamoto K, Ichikawa D, Ochiai T, Nakamura T,
Matsumoto K, et al: NK4 gene expression enhances
5-fluorouracil-induced apoptosis of murine colon cancer cells.
Anticancer Res. 31:2217–2224. 2011.PubMed/NCBI
|
|
159
|
Zhu Y, Cheng M, Yang Z, Zeng CY, Chen J,
Xie Y, Luo SW, Zhang KH, Zhou SF and Lu NH: Mesenchymal stem
cell-based NK4 gene therapy in nude mice bearing gastric cancer
xenografts. Drug Des Devel Ther. 8:2449–2462. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Tada Y, Hiroshima K, Shimada H, Morishita
N, Shirakawa T, Matsumoto K, Shingyoji M, Sekine I, Tatsumi K and
Tagawa M: A clinical protocol to inhibit the HGF/c-Met pathway for
malignant mesothelioma with an intrapleural injection of
adenoviruses expressing the NK4 gene. Springerplus. 4:3582015.
View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Lebrin F, Goumans MJ, Jonker L, Carvalho
RL, Valdimarsdottir G, Thorikay M, Mummery C, Arthur HM and ten
Dijke P: Endoglin promotes endothelial cell proliferation and
TGF-beta/ALK1 signal transduction. EMBO J. 23:4018–4028. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Ten Dijke P, Goumans MJ and Pardali E:
Endoglin in angiogenesis and vascular diseases. Angiogenesis.
11:79–89. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Nassiri F, Cusimano MD, Scheithauer BW,
Rotondo F, Fazio A, Yousef GM, Syro LV, Kovacs K and Lloyd RV:
Endoglin (CD105): A review of its role in angiogenesis and tumor
diagnosis, progression and therapy. Anticancer Res. 31:2283–2290.
2011.PubMed/NCBI
|
|
164
|
Tsujie M, Tsujie T, Toi H, Uneda S,
Shiozaki K, Tsai H and Seon BK: Anti-tumor activity of an
anti-endoglin monoclonal antibody is enhanced in immunocompetent
mice. Int J Cancer. 122:2266–2273. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Uneda S, Toi H, Tsujie T, Tsujie M, Harada
N, Tsai H and Seon BK: Anti-endoglin monoclonal antibodies are
effective for suppressing metastasis and the primary tumors by
targeting tumor vasculature. Int J Cancer. 125:1446–1453. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Muñoz R, Arias Y, Ferreras JM, Jiménez P,
Langa C, Rojo MA, Gayoso MJ, Córdoba-Díaz D, Bernabéu C and Girbés
T: In vitro and in vivo effects of an anti-mouse endoglin
(CD105)-immunotoxin on the early stages of mouse B16MEL4A5 melanoma
tumours. Cancer Immunol Immunother. 62:541–551. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Tabata M, Kondo M, Haruta Y and Seon BK:
Antiangiogenic radioimmunotherapy of human solid tumors in SCID
mice using (125)I-labeled anti-endoglin monoclonal antibodies. Int
J Cancer. 82:737–742. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Dolinsek T, Markelc B, Sersa G, Coer A,
Stimac M, Lavrencak J, Brozic A, Kranjc S and Cemazar M: Multiple
delivery of siRNA against endoglin into murine mammary
adenocarcinoma prevents angiogenesis and delays tumor growth. PLoS
One. 8:e587232013. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Dolinsek T, Markelc B, Bosnjak M, Blagus
T, Prosen L, Kranjc S, Stimac M, Lampreht U, Sersa G and Cemazar M:
Endoglin silencing has significant antitumor effect on murine
mammary adenocarcinoma mediated by vascular targeted effect. Curr
Gene Ther. 15:228–244. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Xu Y, Hou J, Liu Z, Yu H, Sun W, Xiong J,
Liao Z, Zhou F, Xie C and Zhou Y: Gene therapy with tumor-specific
promoter mediated suicide gene plus IL-12 gene enhanced tumor
inhibition and prolonged host survival in a murine model of Lewis
lung carcinoma. J Transl Med. 9:392011. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Stimac M, Dolinsek T, Lampreht U, Cemazar
M and Sersa G: Gene electrotransfer of plasmid with tissue specific
promoter encoding shRNA against endoglin exerts antitumor efficacy
against murine TS/A tumors by vascular targeted effects. PLoS One.
10:e01249132015. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Dolinsek T, Sersa G and Cemazar M:
Melanoma cell viability is reduced after endoglin silencing with
gene electrotransfer. Biol Med Food Environ Technol. 325–328.
2016.
|
|
173
|
Pujade-Lauraine E, Hilpert F, Weber B, et
al: Bevacizumab combined with chemotherapy for platinum-resistant
recurrent ovarian cancer: the AURELIA open-label randomized phase
III trial. J Clin Oncol. 32:1302–1308. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Heinemann V, von Weikersthal LF, Decker T,
Kiani A, Vehling-Kaiser U, Al-Batran SE, Heintges T, Lerchenmüller
C, Kahl C, Seipelt G, et al: FOLFIRI plus cetuximab versus FOLFIRI
plus bevacizumab as first-line treatment for patients with
metastatic colorectal cancer (FIRE-3): A randomised, open-label,
phase 3 trial. Lancet Oncol. 15:1065–1075. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Rini BI, Bellmunt J, Clancy J, Wang K,
Niethammer AG, Hariharan S and Escudier B: Randomized phase III
trial of temsirolimus and bevacizumab versus interferon alfa and
bevacizumab in metastatic renal cell carcinoma: INTORACT trial. J
Clin Oncol. 32:752–759. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Bear HD, Tang G, Rastogi P, Geyer CE Jr,
Liu Q, Robidoux A, Baez-Diaz L, Brufsky AM, Mehta RS, Fehrenbacher
L, et al: Neoadjuvant plus adjuvant bevacizumab in early breast
cancer (NSABP B-40 [NRG Oncology]): Secondary outcomes of a phase
3, randomised controlled trial. Lancet Oncol. 16:1037–1048. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Van Cutsem E, Tabernero J, Lakomy R,
Prenen H, Prausová J, Macarulla T, Ruff P, van Hazel GA, Moiseyenko
V, Ferry D, et al: Addition of aflibercept to fluorouracil,
leucovorin, and irinotecan improves survival in a phase III
randomized trial in patients with metastatic colorectal cancer
previously treated with an oxaliplatin-based regimen. J Clin Oncol.
30:3499–3450. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Tannock IF, Fizazi K, Ivanov S, Karlsson
CT, Fléchon A, Skoneczna I, Orlandi F, Gravis G, Matveev V, Bavbek
S, et al: Aflibercept versus placebo in combination with docetaxel
and prednisone for treatment of men with metastatic
castration-resistant prostate cancer (VENICE): A phase 3,
double-blind randomised trial. Lancet Oncol. 14:760–768. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Ramlau R, Gorbunova V, Ciuleanu TE,
Novello S, Ozguroglu M, Goksel T, Baldotto C, Bennouna J, Shepherd
FA, Le-Guennec S, et al: Aflibercept and docetaxel versus docetaxel
alone after platinum failure in patients with advanced or
metastatic non-small-cell lung cancer: A randomized, controlled
phase III trial. J Clin Oncol. 30:3640–3647. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Allen JW, Moon J, Redman M, Gadgeel SM,
Kelly K, Mack PC, Saba HM, Mohamed MK, Jahanzeb M and Gandara DR:
Southwest oncology group S0802: A randomized, phase II trial of
weekly topotecan with and without ziv-aflibercept in patients with
platinum-treated small-cell lung cancer. J Clin Oncol.
32:2463–2470. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Siu LL, Shapiro JD, Jonker DJ, Karapetis
CS, Zalcberg JR, Simes J, Couture F, Moore MJ, Price TJ, Siddiqui
J, et al: Phase III randomized, placebo-controlled study of
cetuximab plus brivanib alaninate versus cetuximab plus placebo in
patients with metastatic, chemotherapy-refractory, wild-type K-RAS
colorectal carcinoma: The NCIC clinical trials group and AGITG CO.
20 trial. J Clin Oncol. 31:2477–2484. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Lordick F, Kang YK, Chung HC, Salman P, Oh
SC, Bodoky G, Kurteva G, Volovat C, Moiseyenko VM, Gorbunova V, et
al: Capecitabine and cisplatin with or without cetuximab for
patients with previously untreated advanced gastric cancer
(EXPAND): A randomised, open-label phase 3 trial. Lancet Oncol.
14:490–499. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Hitre E, Budai B, Takácsi-Nagy Z,
Rubovszky G, Tóth E, Remenár É, Polgár C and Láng I: Cetuximab and
platinum-based chemoradio- or chemotherapy of patients with
epidermal growth factor receptor expressing adenoid cystic
carcinoma: A phase II trial. Br J Cancer. 109:1117–1122. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Massarelli E, Haddad RI, Lee JJ, Garden
AS, Blumenschein GR, William WN, Tisshler RB, Glisson BS, Gold KA,
Johnson FM, et al: Randomized phase II trial of weekly paclitaxel,
carboplatin, cetuximab (PCC) versus cetuximab, docetaxel,
cisplatin, and fluorouracil (C-TPF) in previously untreated
patients with locally advanced head and neck squamous cell
carcinoma. J Clin Oncol. 32:TPS61022014.
|
|
185
|
Wang J, Sun Y and Qin S: Endostar Phase IV
Study Group: Results of phase IV clinical trial of combining
endostar with chemotherapy for treatment of advanced non-small cell
lung cancer (NSCLC). J Clin Oncol. 28:75982010. View Article : Google Scholar
|
|
186
|
Cui C, Mao L, Chi Z, Si L, Sheng X, Kong
Y, Li S, Lian B, Gu K, Tao M, et al: A phase II, randomized,
double-blind, placebo-controlled multicenter trial of Endostar in
patients with metastatic melanoma. Mol Ther. 21:1456–1463. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Jin T, Li B and Chen XZ: AA phase II trial
of Endostar combined with gemcitabine and cisplatin chemotherapy in
patients with metastatic nasopharyngeal carcinoma (NCT01612286).
Oncol Res. 21:317–323. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Chen Z, Guo W, Cao J, Lv F, Zhang W, Qiu
L, Li W, Ji D, Zhang S, Xia Z, et al: Endostar in combination with
modified FOLFOX6 as an initial therapy in advanced colorectal
cancer patients: A phase I clinical trial. Cancer Chemother
Pharmacol. 75:547–557. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Zhu AX, Rosmorduc O, Evans TJ, Ross PJ,
Santoro A, Carrilho FJ, Bruix J, Qin S, Thuluvath PJ, Llovet JM, et
al: SEARCH: A phase III, randomized, double-blind,
placebo-controlled trial of sorafenib plus erlotinib in patients
with advanced hepatocellular carcinoma. J Clin Oncol. 33:559–566.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Brose MS, Nutting CM, Jarzab B, Elisei R,
Siena S, Bastholt L, de la Fouchardiere C, Pacini F, Paschke R,
Shong YK, et al: Sorafenib in radioactive iodine-refractory,
locally advanced or metastatic differentiated thyroid cancer: A
randomised, double-blind, phase 3 trial trial. Lancet. 384:319–328.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Bruix J, Takayama T, Mazzaferro V, Chau
GY, Yang J, Kudo M, Cai J, Poon RT, Han KH, Tak WY, et al: STORM: A
phase III randomized, double-blind, placebo-controlled trial of
adjuvant sorafenib after resection or ablation to prevent
recurrence of hepatocellular carcinoma (HCC). J Clin Oncol.
32:40062014.
|
|
192
|
Schwandt A, von Gruenigen VE, Wenham RM,
Frasure H, Eaton S, Fusco N, Fu P, Wright JJ, Dowlati A and
Waggoner S: Randomized phase II trial of sorafenib alone or in
combination with carboplatin/paclitaxel in women with recurrent
platinum sensitive epithelial ovarian, peritoneal, or fallopian
tube cancer. Invest New Drugs. 32:729–738. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Motzer RJ, Hutson TE, Tomczak P, Tomczak
P, Michaelson M, Bukowski RM, Rixe O, Oudard S, Kim ST, Baum CM and
Figlin RA: Phase III randomized trial of sunitinib malate (SU11248)
versus interferon-alfa (IFN-{alpha}) as first-line systemic therapy
for patients with metastatic renal cell carcinoma (mRCC). J Clin
Oncol. 24:LBA32006.
|
|
194
|
Socinski MA, Novello S, Brahmer JR, Rosell
R, Sanchez JM, Belani CP, Govindan R, Atkins JN, Gillenwater HH,
Pallares C, et al: Multicenter, phase II trial of sunitinib in
previously treated, advanced non-small-cell lung cance. J Clin
Oncol. 26:650–656. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
195
|
Cheng A, Kang Y, Lin D, Park J, Kudo M,
Qin S, Omata M, Lowenthal SWP, Lanzalone S, Yang L, et al: Phase
III trial of sunitinib (Su) versus sorafenib (So) in advanced
hepatocellular carcinoma (HCC). J Clin Oncol. 29:40002011.
View Article : Google Scholar
|
|
196
|
Michaelson MD, Oudard S, Ou YC, Sengeløv
L, Saad F, Houede N, Ostler P, Stenzl A, Daugaard G, Jones R, et
al: Randomized, placebo-controlled, phase III trial of sunitinib
plus prednisone versus prednisone alone in progressive, metastatic,
castration-resistant prostate cancer. J Clin Oncol. 32:76–82. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
197
|
Kantarjian HM, Shah NP, Cortes JE,
Baccarani M, Agarwal MB, Undurraga MS, Wang J, Ipiña JJ, Kim DW,
Ogura M, et al: Dasatinib or imatinib in newly diagnosed
chronic-phase chronic myeloid leukemia: 2-year follow-up from a
randomized phase 3 trial (DASISION). Blood. 119:1123–1129. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
198
|
Kluger HM, Dudek AZ, McCann C, Ritacco J,
Southard N, Jilaveanu LB, Molinaro A and Sznol M: A phase 2 trial
of dasatinib in advanced melanoma. Cancer. 117:2202–2208. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
199
|
Wong SJ, Karrison T, Hayes DN, Kies MS,
Cullen KJ, Tanvetyanon T, Argiris A, Takebe N, Lim D, Saba NF, et
al: Phase II trial of dasatinib for recurrent or metastatic c-KIT
expressing adenoid cystic carcinoma and for nonadenoid cystic
malignant salivary tumors. Ann Oncol. 27:318–123. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
200
|
Herbst RS, Giaccone G, Schiller JH, Natale
RB, Miller V, Manegold C, Scagliotti G, Rosell R, Oliff I, Reeves
JA, et al: Gefitinib in combination with paclitaxel and carboplatin
in advanced non-small-cell lung cancer: a phase III trial-INTACT 2.
J Clin Oncol. 22:785–794. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
201
|
Argiris A, Ghebremichael M, Gilbert J, Lee
JW, Sachidanandam K, Kolesar JM, Burtness B and Forastiere AA:
Phase III randomized, placebo-controlled trial of docetaxel with or
without gefitinib in recurrent or metastatic head and neck cancer:
An eastern cooperative oncology group trial. J Clin Oncol.
31:1405–1414. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Dutton SJ, Ferry DR, Blazeby JM, Abbas H,
Dahle-Smith A, Mansoor W, Thompson J, Harrison M, Chatterjee A,
Falk S, et al: Gefitinib for oesophageal cancer progressing after
chemotherapy (COG): A phase 3, multicentre, double-blind,
placebo-controlled randomised tria. Lancet Oncol. 15:894–904. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
203
|
Moore MJ, Goldstein D, Hamm J, Figer A,
Hecht JR, Gallinger S, Au HJ, Murawa P, Walde D, Wolff RA, et al:
Erlotinib plus gemcitabine compared with gemcitabine alone in
patients with advanced pancreatic cancer: A phase III trial of the
national cancer institute of canada clinical trials group. J Clin
Oncol. 25:1960–1966. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
204
|
Rosell R, Carcereny E, Gervais R,
Vergnenegre A, Massuti B, Felip E, Palmero R, Garcia-Gomez R,
Pallares C, Sanchez JM, et al: Erlotinib versus standard
chemotherapy as first-line treatment for European patients with
advanced EGFR mutation-positive non-small-cell lung cancer
(EURTAC): A multicentre, open-label, randomised phase 3 trial.
Lancet Oncol. 13:239–246. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
205
|
Sternberg CN, Davis ID, Mardiak J,
Szczylik C, Lee E, Wagstaff J, Barrios CH, Salman P, Gladkov OA,
Kavina A, et al: Pazopanib in locally advanced or metastatic renal
cell carcinoma: results of a randomized phase III trial. J Clin
Oncol. 28:1061–1068. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
206
|
van der Graaf WT, Blay JY, Chawla SP, Kim
DW, Bui-Nguyen B, Casali PG, Schöffski P, Aglietta M, Staddon AP,
Beppu Y, et al: Pazopanib for metastatic soft-tissue sarcoma
(PALETTE): A randomised, double-blind, placebo-controlled phase 3
trial. Lancet. 379:1879–1886. 2012. View Article : Google Scholar : PubMed/NCBI
|