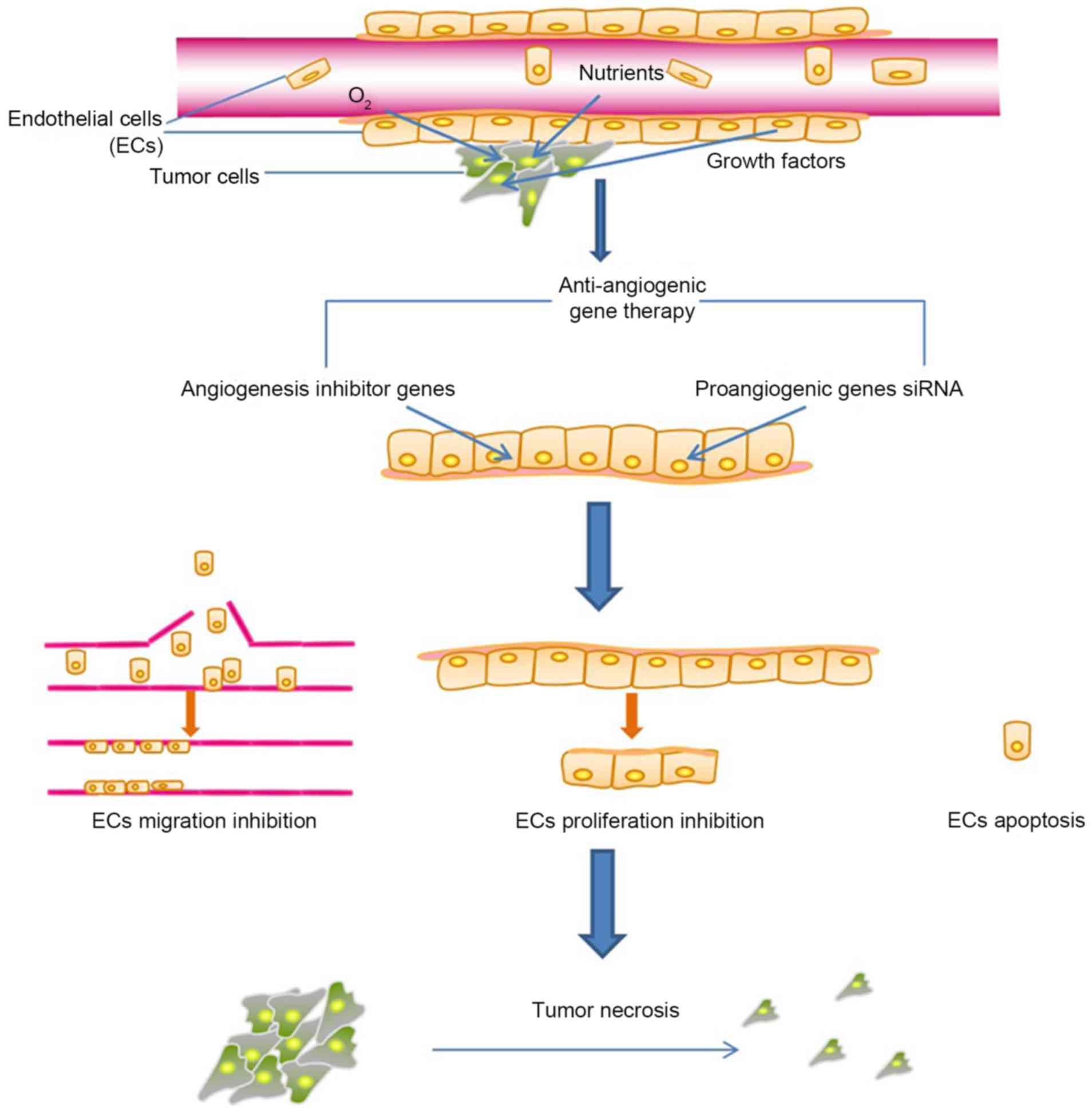
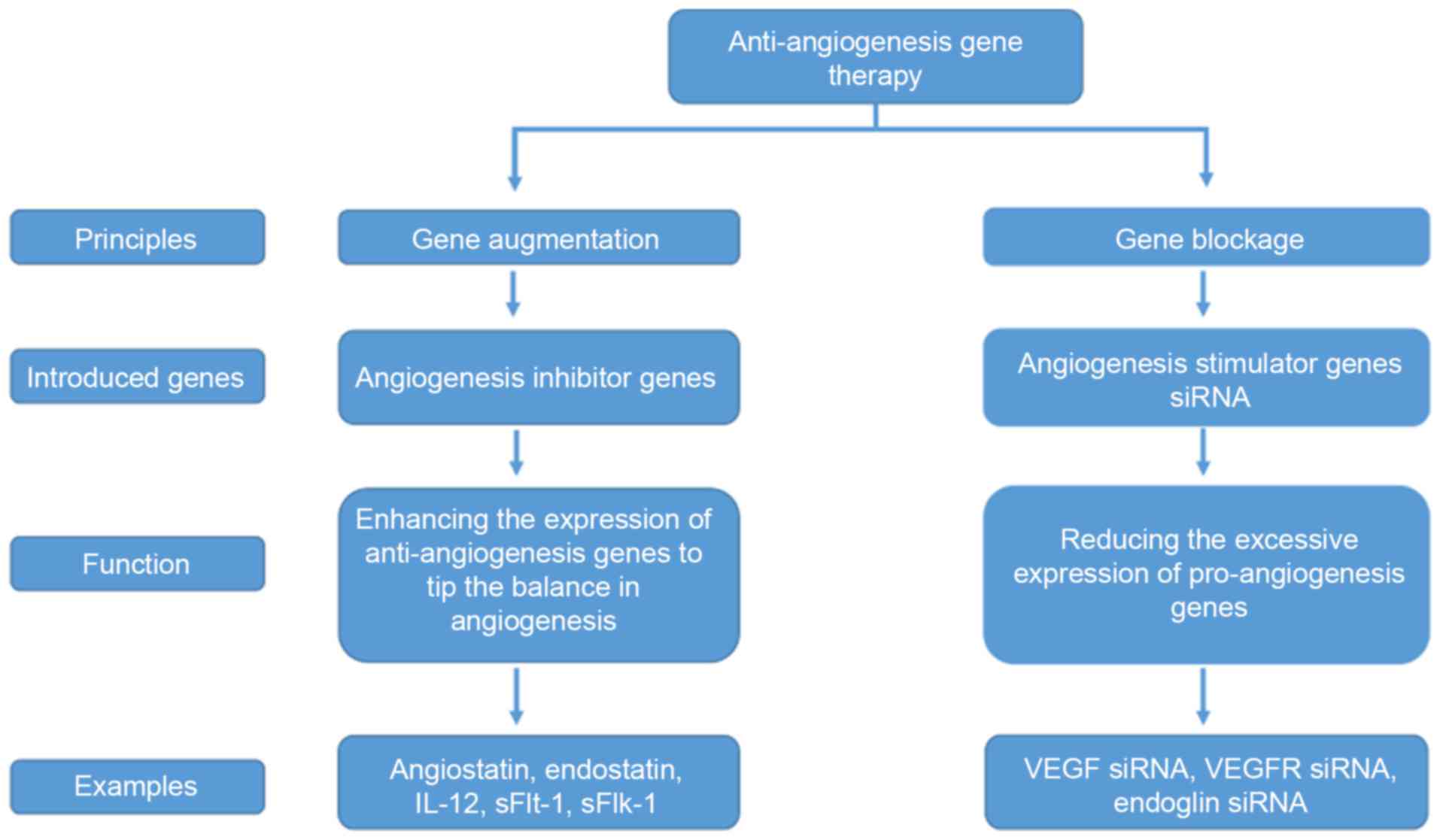
|
1
|
Hanahan D and Folkman J: Patterns and
emerging mechanisms of the angiogenic switch during tumorigenesis.
Cell. 86:353–364. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fan TP, Jaggar R and Bicknell R:
Controlling the vasculature: Angiogenesis, anti-angiogenesis and
vascular targeting of gene therapy. Trends Pharmacol Sci. 16:57–66.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Folkman J and Shing Y: Angiogenesis. J
Biol Chem. 267:10931–10934. 1992.PubMed/NCBI
|
|
4
|
Folkman J: Tumor angiogenesis: Therapeutic
implications. N Engl J Med. 285:1182–1186. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Folkman J: Anti-angiogenesis: New concept
for therapy of solid tumors. Ann Surg. 175:409–416. 1972.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dimova I, Popivanov G and Djonov V:
Angiogenesis in cancer-general pathways and their therapeutic
implications. J BUON. 19:15–21. 2014.PubMed/NCBI
|
|
7
|
Ribatti D, Nico B, Crivellato E, Roccaro
AM and Vacca A: The history of the angiogenic switch concept.
Leukemia. 21:44–52. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Welti J, Loges S, Dimmeler S and Carmeliet
P: Recent molecular discoveries in angiogenesis and antiangiogenic
therapies in cancer. J Clin Invest. 123:3190–3200. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ribatti D and Djonov V: Intussusceptive
microvascular growth in tumors. Cancer Lett. 316:126–131. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Donnem T, Hu J, Ferguson M, Adighibe O,
Snell C, Harris AL, Gatter KC and Pezzella F: Vessel co-option in
primary human tumors and metastases: An obstacle to effective
anti-angiogenic treatment? Cancer Med. 2:427–436. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
de la Puente P, Muz B, Azab F and Azab AK:
Cell trafficking of endothelial progenitor cells in tumor
progression. Clin Cancer Res. 19:3360–3368. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Moschetta M, Mishima Y, Sahin I, Manier S,
Glavey S, Vacca A, Roccaro AM and Ghobrial IM: Role of endothelial
progenitor cells in cancer progression. Biochim Biophys Acta.
1846:26–39. 2014.PubMed/NCBI
|
|
13
|
Seftor RE, Hess AR, Seftor EA, Kirschmann
DA, Hardy KM, Margaryan NV and Hendrix MJ: Tumor cell vasculogenic
mimicry: From controversy to therapeutic promise. Am J Pathol.
181:1115–1125. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ferrara N and Adamis AP: Ten years of
anti-vascular endothelial growth factor therapy. Nat Rev Drug
Discov. 15:385–403. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mihicprobst D, Ikenberg K, Tinguely M,
Schraml P, Behnke S, Seifert B, Civenni G, Sommer L, Moch H and
Dummer R: Tumor cell plasticity and angiogenesis in human
melanomas. PLoS One. 7:e335712012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liekens S, Schols D and Hatse S:
CXCL12-CXCR4 axis in angiogenesis, metastasis and stem cell
mobilization. Curr Pharm Des. 16:3903–3920. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Eelen G, de Zeeuw P, Simons M and
Carmeliet P: Endothelial cell metabolism in normal and diseased
vasculature. Circ Res. 116:1231–1244. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bridgeman VL, Vermeulen PB, Foo S, Bilecz
A, Daley F, Kostaras E, Nathan MR, Wan E, Frentzas S, Schweiger T,
et al: Vessel co-option is common in human lung metastases and
mediates resistance to anti-angiogenic therapy in preclinical lung
metastasis models. J Pathol. 241:362–374. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hlushchuk R, Riesterer O, Baum O, Wood J,
Gruber G, Pruschy M and Djonov V: Tumor recovery by angiogenic
switch from sprouting to intussusceptive angiogenesis after
treatment with PTK787/ZK222584 or ionizing radiation. Am J Pathol.
173:1173–1185. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Frentzas S, Simoneau E, Bridgeman VL,
Vermeulen PB, Foo S, Kostaras E, Nathan M, Wotherspoon A, Gao ZH,
Shi Y, et al: Vessel co-option mediates resistance to
anti-angiogenic therapy in liver metastases. Nat Med. 22:1294–1302.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kerbel RS: Tumor angiogenesis: Past,
present and the near future. Carcinogenesis. 21:505–515. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Carmeliet P: Developmental biology.
Controlling the cellular brakes. Nature. 401:657–658. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dor Y, Porat R and Keshet E: Vascular
endothelial growth factor and vascular adjustments to perturbations
in oxygen homeostasis. Am J Physiol Cell Physiol. 280:C1367–C1374.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Littlepage LE, Sternlicht MD, Rougier N,
Phillips J, Gallo E, Yu Y, Williams K, Brenot A, Gordon JI and Werb
Z: Matrix metalloproteinases contribute distinct roles in
neuroendocrine prostate carcinogenesis, metastasis, and
angiogenesis progression. Cancer Res. 70:2224–2234. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Carmeliet P, Dor Y, Herbert JM, Fukumura
D, Brusselmans K, Dewerchin M, Neeman M, Bono F, Abramovitch R,
Maxwell P, et al: Role of HIF-1alpha in hypoxia-mediated apoptosis,
cell proliferation and tumour angiogenesis. Nature. 394:485–490.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Maracle CX and Tas SW: Inhibitors of
angiogenesis: Ready for prime time? Best Pract Res Clin Rheumatol.
28:637–649. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Blancas AA, Wong LE, Glaser DE and
McCloskey KE: Specialized tip/stalk-like and phalanx-like
endothelial cells from embryonic stem cells. Stem Cells Dev.
22:1398–1407. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jakobsson L, Franco CA, Bentley K, Collins
RT, Ponsioen B, Aspalter IM, Rosewell I, Busse M, Thurston G,
Medvinsky A, et al: Endothelial cells dynamically compete for the
tip cell position during angiogenic sprouting. Nat Cell Biol.
12:943–953. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lobov IB, Renard RA, Papadopoulos N, Gale
NW, Thurston G, Yancopoulos GD and Wiegand SJ: Delta-like ligand 4
(Dll4) is induced by VEGF as a negative regulator of angiogenic
sprouting. Proc Natl Acad Sci USA. 104:3219–3224. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu Z, Fan F, Wang A, Zheng S and Lu Y:
Dll4-Notch signaling in regulation of tumor angiogenesis. J Cancer
Res Clin Oncol. 140:525–536. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pandya NM, Dhalla NS and Santani DD:
Angiogenesis-a new target for future therapy. Vasc Pharmacol.
44:265–274. 2006. View Article : Google Scholar
|
|
32
|
Bergers G and Song S: The role of
pericytes in blood-vessel formation and maintenance. Neuro Oncol.
7:452–464. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Izzedine H, Ederhy S, Goldwasser F, Soria
JC, Milano G, Cohen A, Khayat D and Spano JP: Management of
hypertension in angiogenesis inhibitor-treated patients. Ann Oncol.
20:807–815. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu SX, Xia ZS and Zhong YQ: Genetic
therapy in pancreatic cancer. World J Gastroenterol.
20:13343–13368. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Edelstein ML, Abedi MR, Wixon J and
Edelstein RM: Gene therapy clinical trials worldwide 1989–2004-an
overview. J Gene Med. 6:597–602. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Edelstein ML, Abedi MR and Wixon J: Gene
therapy clinical trials worldwide to 2007-an update. J Gene Med.
9:833–842. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ginn SL, Alexander IE, Edelstein ML, Abedi
MR and Wixon J: Gene therapy clinical trials worldwide to 2012-an
update. J Gene Med. 15:65–77. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ortiz R, Melguizo C, Prados J, Álvarez PJ,
Caba O, Rodríguez-Serrano F, Hita F and Aránega A: New gene therapy
strategies for cancer treatment: A review of recent patents. Recent
Pat Anticancer Drug Discov. 7:297–312. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cao S, Cripps A and Wei MQ: New strategies
for cancer gene therapy: progress and opportunities. Clin Exp
Pharmacol Physiol. 37:108–114. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tseng SJ, Liao ZX, Kao SH, Zeng YF, Huang
KY, Li HJ, Yang CL, Deng YF, Huang CF, Yang SC, et al: Highly
specific in vivo gene delivery for p53-mediated apoptosis
and genetic photodynamic therapies of tumour. Nat Commun.
6:64562015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gogiraju R, Steinbrecher JH, Lehnart SE,
Kessel M, Dobbelstein M and Schaefer K: Importance of tumor
suppressor gene p53-mediated endothelial cell apoptosis for cardiac
angiogenesis and hypertrophy. Eur Heart J. 34 Suppl 1:S16162013.
View Article : Google Scholar
|
|
42
|
Tazawa H, Kagawa S and Fujiwara T:
Advances in adenovirus-mediated p53 cancer gene therapy. Expert
Opin Biol Ther. 13:1569–1583. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Prabha S, Sharma B and Labhasetwar V:
Inhibition of tumor angiogenesis and growth by
nanoparticle-mediated p53 gene therapy in mice. Cancer Gene Ther.
19:530–537. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Teodoro JG, Evans SK and Green MR:
Inhibition of tumor angiogenesis by p53: A new role for the
guardian of the genome. J Mol Med (Berl). 85:1175–1186. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhang C, Wang QT, Liu H, Zhang ZZ and
Huang WL: Advancement and prospects of tumor gene therapy. Chin J
Cancer. 30:182–188. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
El-Aneed A: An overview of current
delivery systems in cancer gene therapy. J Control Release.
94:1–14. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ramsey JD, Vu HN and Pack DW: A top-down
approach for construction of hybrid polymer-virus gene delivery
vectors. J Control Release. 144:39–45. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lundstrom K: Alphavirus vectors as tools
in neuroscience and gene therapy. Virus Res. 216:16–25. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
LU Y, Yan M, Chen IS and Liang M: Viral
vector nanocapsule for targeting gene therapy and its preparation.
Journal. 2015.
|
|
50
|
Touchefeu Y, Harrington KJ, Galmiche JP
and Vassaux G: Review article: Gene therapy, recent developments
and future prospects in gastrointestinal oncology. Aliment
Pharmacol Ther. 32:953–968. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Liao ZK, Tsai KC, Wang HT, Tseng SH, Deng
WP, Chen WS and Hwang LH: Sonoporation-mediated anti-angiogenic
gene transfer into muscle effectively regresses distant orthotopic
tumors. Cancer Gene Ther. 19:171–180. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ren J, Zhang P, Tian J, Zhou Z, Liu X,
Wang D and Wang Z: A targeted ultrasound contrast agent carrying
gene and cell-penetrating peptide: Preparation and gene
transfection in vitro. Colloids Surf B Biointerfaces.
121:362–370. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Yarmush ML, Golberg A, Serša G, Kotnik T
and Miklavčič D: Electroporation-based technologies for medicine:
principles, applications, and challenges. Annu Rev Biomed Eng.
16:295–320. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wang W, Li W, Ma N and Steinhoff G:
Non-viral gene delivery methods. Curr Pharm Biotechnol. 14:46–60.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Audouy SA, de Leij LF, Hoekstra D and
Molema G: In vivo characteristics of cationic liposomes as delivery
vectors for gene therapy. Pharm Res. 19:1599–1605. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Hortobagyi GN, Ueno NT, Xia W, Zhang S,
Wolf JK, Putnam JB, Weiden PL, Willey JS, Carey M, Branham DL, et
al: Cationic liposome-mediated E1A gene transfer to human breast
and ovarian cancer cells and its biologic effects: A phase i
clinical trial. J Clin Oncol. 19:3422–3433. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Wakabayashi T, Natsume A, Mizuno M, Fujii
M, Shimato S and Yoshida J: A clinical trial of cationic liposomes
containing interferon-b gene for patients with malignant glioma.
Int Conf Brain Tumor Res Ther. 225. 2009.
|
|
58
|
Wang Y, Gao S, Ye WH, Yoon HS and Yang YY:
Co-delivery of drugs and DNA from cationic core-shell nanoparticles
self-assembled from a biodegradable copolymer. Nat Mater.
5:791–796. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Power AT and Bell JC: Cell-based delivery
of oncolytic viruses: A new strategic alliance for a biological
strike against cancer. Mol Ther. 15:660–665. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Muta M, Matsumoto G, Hiruma K, Nakashima E
and Toi M: Study of cancer gene therapy using IL-12-secreting
endothelial progenitor cells in a rat solid tumor model. Oncol Rep.
10:1765–1769. 2003.PubMed/NCBI
|
|
61
|
Yin H, Kanasty RL, Eltoukhy AA, Vegas AJ,
Dorkin JR and Anderson DG: Non-viral vectors for gene-based
therapy. Nat Rev Genet. 15:541–555. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Kim WJ, Yockman JW, Lee M, Jeong JH, Kim
YH and Kim SW: Soluble Flt-1 gene delivery using PEI-g-PEG-RGD
conjugate for anti-angiogenesis. J Control Release. 106:224–234.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Persano L, Crescenzi M and Indraccolo S:
Anti-angiogenic gene therapy of cancer: Current status and future
prospects. Mol Aspects Med. 28:87–114. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Albini A, Tosetti F, Li VW, Noonan DM and
Li WW: Cancer prevention by targeting angiogenesis. Nat Rev Clin
Oncol. 9:498–509. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Morrison C: $1-million price tag set for
Glybera gene therapy. Nat Biotechnol. 33:217–218. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Rodriguez D and Miessner P: Production of
AAV vectors for gene therapy: A cost-effectiveness and risk
assessment (unpublished PhD thesis). Department of Chemical
Engineering and the MIT Sloan School of Management. 2016.
|
|
67
|
Chen HH, Kuliszewski MA, Rudenko D and
Leong-Poi H: Pre-clinical evaluation of pro-angiogenic gene therapy
by ultrasound-targeted microbubble destruction of vascular
endothelial growth factor minicircle dna in an model of severe
peripheral arterial disease in watanabe heritable hyperlipidemic
rabbits. Can J Cardiol. 31 Suppl:S2822015. View Article : Google Scholar
|
|
68
|
Feng X: Angiogenesis and Antiangiogenesis
Therapies: Spear and Shield of Pharmacotherapy. J Pharma Care
Health Sys. 1:e1102014.
|
|
69
|
Ichihara E, Kiura K and Tanimoto M:
Targeting angiogenesis in cancer therapy. Acta Med Okayama.
65:353–362. 2011.PubMed/NCBI
|
|
70
|
Trinchieri G: Interleukin-12: A cytokine
produced by antigen-presenting cells with immunoregulatory
functions in the generation of T-helper cells type 1 and cytotoxic
lymphocytes. Blood. 84:4008–4027. 1994.PubMed/NCBI
|
|
71
|
Trinchieri G: Interleukin-12: A
proinflammatory cytokine with immunoregulatory functions that
bridge innate resistance and antigen-specific adaptive immunity.
Annu Rev Immunol. 13:251–276. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Duda DG, Sunamura M, Lozonschi L, Kodama
T, Egawa S, Matsumoto G, Shimamura H, Shibuya K, Takeda K and
Matsuno S: Direct in vitro evidence and in vivo
analysis of the antiangiogenesis effects of interleukin 12. Cancer
Res. 60:1111–1116. 2000.PubMed/NCBI
|
|
73
|
Dias S, Boyd R and Balkwill F: IL-12
regulates VEGF and MMPs in a murine breast cancer model. Int J
Cancer. 78:361–365. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Voest EE, Kenyon BM, O'Reilly MS, Truitt
G, D'Amato RJ and Folkman J: Inhibition of angiogenesis in
vivo by interleukin 12. J Natl Cancer Inst. 87:581–586. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Akiyama Y, Maruyama K, Watanabe M and
Yamaguchi K: Retroviral-mediated IL-12 gene transduction into human
CD34+ cell-derived dendritic cells. Int J Oncol. 21:509–514.
2002.PubMed/NCBI
|
|
76
|
Sunamura M, Sun L, Lozonschi L, Duda DG,
Kodama T, Matsumoto G, Shimamura H, Takeda K, Kobari M, Hamada H
and Matsuno S: The antiangiogenesis effect of interleukin 12 during
early growth of human pancreatic cancer in SCID mice. Pancreas.
20:227–233. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Li Q, Zhihua W, Xiumin Y, et al: The
effect of il-12 on the proliferation in vitro and anti-tumor
effects of cik cells in vivo and in vitro. J Pract
Oncol. 21:212–215. 2007.(In Chinese).
|
|
78
|
Nguyen K, Koppolu B, Smith G,
Ravindranathan S and Zaharoff D: Interleukin-12 elicits various
responses of splenocytes from different mouse strains. J Immunol.
194 1 Suppl:(S49): 82015.
|
|
79
|
Portielje JE, Kruit WH, Schuler M, Beck J,
Lamers CH, Stoter G, Huber C, de Boer-Dennert M, Rakhit A, Bolhuis
RL and Aulitzky WE: Phase I study of subcutaneously administered
recombinant human interleukin 12 in patients with advanced renal
cell cancer. Clin Cancer Res. 5:3983–3989. 1999.PubMed/NCBI
|
|
80
|
Gollob JA, Mier JW, Veenstra K, McDermott
DF, Clancy D, Clancy M and Atkins MB: Phase I trial of twice-weekly
intravenous interleukin 12 in patients with metastatic renal cell
cancer or malignant melanoma: Ability to maintain IFN-gamma
induction is associated with clinical response. Clin Cancer Res.
6:1678–1692. 2000.PubMed/NCBI
|
|
81
|
Hurteau JA, Blessing JA, DeCesare SL and
Creasman WT: Evaluation of recombinant human interleukin-12 in
patients with recurrent or refractory ovarian cancer: A gynecologic
oncology group study. Gynecol Oncol. 82:7–10. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Daud A, Takamura KT, Diep T, Heller R and
Pierce RH: Long-term overall survival from a phase I trial using
intratumoral plasmid interleukin-12 with electroporation in
patients with melanoma. J Transl Med. 13 Suppl 1:O32015. View Article : Google Scholar
|
|
83
|
Daud A, Algazi A, Ashworth M, Buljan M,
Takamura KT, Diep T, Pierce RH and Bhatia S: Intratumoral
electroporation of plasmid interleukin-12: Efficacy and biomarker
analyses from a phase 2 study in melanoma (OMS-I100). J Transl Med.
13 Suppl 1:O112015. View Article : Google Scholar
|
|
84
|
Cutrera J, King G, Jones P, Kicenuik K,
Gumpel E, Xia X and Li S: Safety and Efficacy of Tumor-Targeted
Interleukin 12 Gene Therapy in Treated and Non-Treated, Metastatic
Lesions. Curr Gene Ther. 15:44–55. 2014. View Article : Google Scholar
|
|
85
|
Lampreht U, Kamensek U, Stimac M, et al:
Gene electrotransfer of canine interleukin 12 into canine melanoma
cell lines. J Membr Biol. 248:909–917. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Markert JM, Cody JJ, Parker JN, Coleman
JM, Price KH, Kern ER, Quenelle DC, Lakeman AD, Schoeb TR, Palmer
CA, et al: Preclinical evaluation of a genetically engineered
herpes simplex virus expressing interleukin-12. J Virol.
86:5304–5313. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Kramer MG, Masner M, Casales E, Moreno M,
Smerdou C and Chabalgoity JA: Neoadjuvant administration of Semliki
Forest virus expressing interleukin-12 combined with attenuated
Salmonella eradicates breast cancer metastasis and achieves
long-term survival in immunocompetent mice. BMC Cancer. 15:6202015.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Koneru M, O'Cearbhaill R, Pendharkar S,
Spriggs DR and Brentjens RJ: A phase I clinical trial of adoptive T
cell therapy using IL-12 secreting MUC-16(ecto) directed chimeric
antigen receptors for recurrent ovarian cance. J Transl Med.
13:1022015. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Poutou J, Bunuales M, Gonzalez-Aparicio M,
Garcia-Aragoncillo E, Quetglas JI, Casado R, Bravo-Perez C,
Alzuguren P and Hernandez-Alcoceba R: Safety and antitumor effect
of oncolytic and helper-dependent adenoviruses expressing
interleukin-12 variants in a hamster pancreatic cancer model. Gene
Ther. 22:696–706. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Li Y, Li X, Liu H, Zhuang S, Yang J and
Zhang F: Intranasal immunization with recombinant Lactococci
carrying human papillomavirus E7 protein and mouse interleukin-12
DNA induces E7-specific antitumor effects in C57BL/6 mice. Oncol
Lett. 7:576–582. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Freytag SO, Zhang Y and Siddiqui F:
Preclinical toxicology of oncolytic adenovirus-mediated cytotoxic
and interleukin-12 gene therapy for prostate cancer. Mol Ther
Oncolytics. 2:pii: 15006. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Cutrera J, King G, Jones P, Kicenuik K,
Gumpel E, Xia X and Li S: Safe and effective treatment of
spontaneous neoplasms with interleukin 12 electro-chemo-gene
therapy. J Cell Mol Med. 19:664–675. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Jiang H, Lin JJ, Su Z, Goldstein N and
Fisher P: Subtraction hybridization identifies a novel melanoma
differentiation associated gene, mda-7, modulated during human
melanoma differentiation, growth and progression. Oncogene.
11:2477–2486. 1995.PubMed/NCBI
|
|
94
|
Jiang H, Su ZZ, Lin JJ, Goldstein NI,
Young CS and Fisher PB: The melanoma differentiation associated
gene mda-7 suppresses cancer cell growth. Proc Natl Acad Sci USA.
93:9160–9165. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Su ZZ, Madireddi MT, Lin JJ, Young CS,
Kitada S, Reed JC, Goldstein NI and Fisher PB: The cancer growth
suppressor gene mda-7 selectively induces apoptosis in human breast
cancer cells and inhibits tumor growth in nude mice. Proc Natl Acad
Sci USA. 95:14400–14405. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Saeki T, Mhashilkar A, Swanson X, Zou-Yang
XH, Sieger K, Kawabe S, Branch CD, Zumstein L, Meyn RE, Roth JA, et
al: Inhibition of human lung cancer growth following
adenovirus-mediated mda-7 gene expression in vivo. Oncogene.
21:4558–4566. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Sauane M, Gopalkrishnan RV, Lebedeva I,
Mei MX, Sarkar D, Su ZZ, Kang DC, Dent P, Pestka S and Fisher PB:
Mda-7/IL-24 induces apoptosis of diverse cancer cell lines through
JAK/STAT-independent pathways. J Cell Physiol. 196:334–345. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Menezes ME, Shen XN, Das SK, Emdad L, Guo
C, Yuan F, Li YJ, Archer MC, Zacksenhaus E, Windle JJ, et al:
MDA-7/IL-24 functions as a tumor suppressor gene in vivo in
transgenic mouse models of breast cancer. Oncotarget.
6:36928–36942. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Li YJ, Liu G, Xia L, Xiao X, Liu JC,
Menezes ME, Das SK, Emdad L, Sarkar D, Fisher PB, et al:
Suppression of Her2/Neu mammary tumor development in mda-7/IL-24
transgenic mice. Oncotarget. 6:36943–36954. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Chen X, Liu D, Wang J, Su Q, Zhou P, Liu
J, Luan M and Xu X: Suppression effect of recombinant adenovirus
vector containing hIL-24 on Hep-2 laryngeal carcinoma cells. Oncol
Lett. 7:771–777. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Liu Z, Xu L, Yuan H, Zhang Y, Zhang X and
Zhao D: Oncolytic adenovirus-mediated mda-7/IL-24 expression
suppresses osteosarcoma growth and enhances sensitivity to
doxorubicin. Mol Med Rep. 12:6358–6364. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Khodadad M, Hosseini SY, Shenavar F,
Erfani N, Bina S, Ahmadian S, Fattahi MR and Hajhosseini R:
Construction of expressing vectors including melanoma
differentiation-associated gene-7 (mda-7) fused with the RGD
sequences for better tumor targeting. Iran J Basic Med Sci.
18:780–787. 2015.PubMed/NCBI
|
|
103
|
O'Reilly MS, Holmgren L, Shing Y, Chen C,
Rosenthal RA, Moses M, Lane WS, Cao Y, Sage EH and Folkman J:
Angiostatin: A novel angiogenesis inhibitor that mediates the
suppression of metastases by a Lewis lung carcinoma. Cell.
79:315–328. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Wahl ML, Moser TL and Pizzo SV:
Angiostatin and anti-angiogenic therapy in human disease. Recent
Prog Horm Res. 59:73–104. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Zhang G, Jin G, Nie X, Mi R, Zhu G, Jia W
and Liu F: Enhanced antitumor efficacy of an oncolytic herpes
simplex virus expressing an endostatin-angiostatin fusion gene in
human glioblastoma stem cell xenografts. PLoS One. 9:e958722014.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Zhu G, Su W, Jin G, Xu F, Hao S, Guan F,
Jia W and Liu F: Glioma stem cells targeted by oncolytic virus
carrying endostatin-angiostatin fusion gene and the expression of
its exogenous gene in vitro. Brain Res. 1390:59–69. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Tysome JR, Wang P, Alusi G, Briat A,
Gangeswaran R, Wang J, Bhakta V, Fodor I, Lemoine NR and Wang Y:
Lister vaccine strain of vaccinia virus armed with the
endostatin-angiostatin fusion gene: An oncolytic virus superior to
dl1520 (ONYX-015) for human head and neck cancer. Hum Gene Ther.
22:1101–1108. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Hutzen B, Bid HK, Houghton PJ, Pierson CR,
Powell K, Bratasz A, Raffel C and Studebaker AW: Treatment of
medulloblastoma with oncolytic measles viruses expressing the
angiogenesis inhibitors endostatin and angiostatin. BMC Cancer.
14:2062014. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Li X, Liu YH, Lee SJ, Gardner TA, Jeng MH
and Kao C: Prostate-restricted replicative adenovirus expressing
human endostatin-angiostatin fusion gene exhibiting dramatic
antitumor efficacy. Clin Cancer Res. 14:291–299. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Ma HI, Lin SZ, Chiang YH, Li J, Chen SL,
Tsao YP and Xiao X: Intratumoral gene therapy of malignant brain
tumor in a rat model with angiostatin delivered by adeno-associated
viral (AAV) vector. Gene Ther. 9:2–11. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Li R, Chen H and Ren CS: Growth inhibition
of breast cancer in rat by AAV mediated angiostatin gene. Chin J
Cancer Res. 19:108–112. 2007. View Article : Google Scholar
|
|
112
|
Kubo S, Takagi-Kimura M and Kasahara N:
Combinatorial anti-angiogenic gene therapy in a human malignant
mesothelioma model. Oncol Rep. 34:633–638. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Tan JF, Lu Q, Zhang XW and Tan M: Effect
of co-transfection of angiostatin and Fas gene on growth of
transplanted tumor in nude mice. China J Mod Med. 8:0132008.(In
Chinese).
|
|
114
|
Kim HS, Jeong HY, Lee YK, Kim KS and Park
YS: Synergistic antitumoral effect of IL-12 gene cotransfected with
antiangiogenic genes for Angiostatin, Endostatin, and Saxatilin.
Oncol Res Featuring Preclinical Clin Cancer Ther. 21:209–216.
2013.
|
|
115
|
Sun X, Vale M, Jiang X, Gupta R and
Krissansen G: Antisense HIF-1alpha prevents acquired tumor
resistance to angiostatin gene therapy. Cancer Gene Ther.
17:532–540. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Chen XJ, Zhu YY, Hu ZT, Zhang HH, Weng SM
and Zhuang HZ: Effect of co-transfection of p53 and angiostatin
gene on the apoptosis of gastric cancer SG7901 cells. Tumor.
7:577–580. 2010.
|
|
117
|
Schmitz V, Tirado-Ledo L, Raskopf E, Rabe
C, Wernert N, Wang L, Prieto J, Qian C, Sauerbruch T and Caselmann
WH: Effective antitumour mono- and combination therapy by gene
delivery of angiostatin-like molecule and interleukin-12 in a
murine hepatoma model. Int J Colorectal Dis. 20:494–501. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Kuo CH, Chang BI, Lee FT, Chen PK, Lee JS,
Shi GY and Wu HL: Development of recombinant adeno-associated virus
serotype 2/8 carrying kringle domains of human plasminogen for
sustained expression and cancer therapy. Hum Gene Ther. 26:603–613.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Chu Y, Liu H, Lou G, Zhang Q and Wu C:
Human placenta mesenchymal stem cells expressing exogenous
kringle1-5 protein by fiber-modified adenovirus suppress
angiogenesis. Cancer Gene Ther. 21:200–208. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Schmitz V, Sauerbruch T and Raskopf E:
Anti-tumoural effects of PlgK1-5 are directly linked to reduced
ICAM expression, resulting in hepatoma cell apoptosis. Int J
Colorectal Dis. 27:1029–1038. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
O'Reilly MS, Boehm T, Shing Y, Fukai N,
Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR and Folkman J:
Endostatin: An endogenous inhibitor of angiogenesis and tumor
growth. Cell. 88:277–285. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Sasaki T, Fukai N, Mann K, Göhring W,
Olsen BR and Timpl R: Structure, function and tissue forms of the
C-terminal globular domain of collagen XVIII containing the
angiogenesis inhibitor endostatin. EMBO J. 17:4249–4256. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Rong B, Yang S, Li W, Zhang W and Ming Z:
Systematic review and meta-analysis of Endostar (rh-endostatin)
combined with chemotherapy versus chemotherapy alone for treating
advanced non-small cell lung cancer. World J Surg Oncol.
10:1702012. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Huiqi G, Jing Z, Peng F, Yong L and
Baozhong S: In vivo study of the effect of combining endostatin
gene therapy with 32P-colloid on hepatocarcinoma and its
functioning mechanism. J BUON. 20:1042–1047. 2015.PubMed/NCBI
|
|
125
|
Yan F, Zheng Y and Huang L:
Adenovirus-mediated combined anti-angiogenic and pro-apoptotic gene
therapy enhances antitumor efficacy in hepatocellular carcinoma.
Oncol Lett. 5:348–354. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Xu Y, Xie Z, Zhou Y, Zhou X, Li P, Wang Z
and Zhang Q: Experimental endostatin-GFP gene transfection into
human retinal vascular endothelial cells using ultrasound-targeted
cationic microbubble destruction. Mol Vis. 21:930–938.
2015.PubMed/NCBI
|
|
127
|
Li XP, Zhang HL, Wang HJ, Li YX, Li M, Lu
L, Wan Y, Zhou BL, Liu Y, Pan Y, et al: Ad-endostatin treatment
combined with low-dose irradiation in a murine lung cancer model.
Oncol Rep. 32:650–658. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Liu RY, Zhou L, Zhang YL, Huang BJ, Ke ML,
Chen JM, Li LX, Fu X, Wu JX and Huang W: An oncolytic adenovirus
enhances antiangiogenic and antitumoral effects of a
replication-deficient adenovirus encoding endostatin by rescuing
its selective replication in nasopharyngeal carcinoma cells.
Biochem Biophys Res Commun. 442:171–176. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Li L, Zhang Y, Zhou L, Ke ML, Chen JM, Fu
X, Ye CL, Wu JX, Liu RY and Huang W: Antitumor efficacy of a
recombinant adenovirus encoding endostatin combined with an
E1B55KD-deficient adenovirus in gastric cancer cells. J Transl Med.
11:2572013. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Zhou Y, Gu H, Xu Y, Li F, Kuang S, Wang Z,
Zhou X, Ma H, Li P, Zheng Y, et al: Targeted antiangiogenesis gene
therapy using targeted cationic microbubbles conjugated with CD105
antibody compared with untargeted cationic and neutral
microbubbles. Theranostics. 5:399–417. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Maeshima Y, Colorado PC, Torre A, Holthaus
KA, Grunkemeyer JA, Ericksen MB, Hopfer H, Xiao Y, Stillman IE and
Kalluri R: Distinct antitumor properties of a type IV collagen
domain derived from basement membrane. J Biol Chem.
275:21340–21348. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Yang YP, Xu CX, Hou GS, Xin JX, Wang W and
Liu XX: Effects of eukaryotic expression plasmid encoding human
tumstatin gene on endothelial cells in vitro. Chin Med J
(Engl). 123:2269–2273. 2010.PubMed/NCBI
|
|
133
|
Borza CM, Pozzi A, Borza DB, Pedchenko V,
Hellmark T, Hudson BG and Zent R: Integrin alpha3beta1, a novel
receptor for alpha3(IV) noncollagenous domain and a trans-dominant
Inhibitor for integrin alphavbeta3. J Biol Chem. 281:20932–20939.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Hwang-Bo J, Park JH and Chung IS:
Tumstatin induces apoptosis mediated by Fas signaling pathway in
oral squamous cell carcinoma SCC-VII cells. Oncol Lett.
10:1016–1022. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Goto T, Ishikawa H, Matsumoto K, Nishimura
D, Kusaba M, Taura N, Shibata H, Miyaaki H, Ichikawa T, Hamasaki K,
et al: Tum-1, a tumstatin fragment, gene delivery into
hepatocellular carcinoma suppresses tumor growth through inhibiting
angiogenesis. Int J Oncol. 33:33–40. 2008.PubMed/NCBI
|
|
136
|
You Y, Xue X, Li M, Qin X, Zhang C, Wang
W, Giang C, Wu S, Liu Y, Zhu W, et al: Inhibition effect of
pcDNA-tum-5 on the growth of S180 tumor. Cytotechnology. 56:97–104.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Caudroy S, Cucherousset J, Lorenzato M,
Zahm JM, Martinella-Catusse C, Polette M and Birembaut P:
Implication of tumstatin in tumor progression of human
bronchopulmonary carcinomas. Hum Pathol. 35:1218–1222. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Yang YP, Xu CX, Hou GS, Xin JX, Wang W and
Liu XX: Effects of eukaryotic expression plasmid encoding human
tumstatin gene on endothelial cells in vitro. Chin Med J
(Engl). 123:2269–2273. 2010.PubMed/NCBI
|
|
139
|
Zhang X, Xu W, Qian H, Zhu W and Zhang R:
Mesenchymal stem cells modified to express lentivirus TNF-α
Tumstatin(45–132) inhibit the growth of prostate cancer. J Cell Mol
Med. 15:433–444. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Thevenard J, Ramont L, Mir LM,
Dupont-Deshorgue A, Maquart FX, Monboisse JC and Brassart-Pasco S:
A new anti-tumor strategy based on in vivo tumstatin
overexpression after plasmid electrotransfer in muscle. Biochem
Biophys Res Commun. 432:549–552. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Gu Q, Sun C, Luo J, Zhang T and Wang L:
Inhibition of angiogenesis by a synthetic fusion protein VTF
derived from vasostatin and tumstatin. Anticancer Drugs.
25:1044–1051. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Zhang X, Qi DD, Zhang TT, Chen QX, Wang
GZ, Sui GY, Hao XW, Sun S, Song X and Chen YL: Antitumor activity
of adenoviral vector containing T42 and 4xT42 peptide gene through
inducing apoptosis of tumor cells and suppressing angiogenesis. Mol
Med Rep. 11:2083–2091. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Trochon-Joseph V, Martel-Renoir D, Mir LM,
Thomaïdis A, Opolon P, Connault E, Li H, Grenet C, Fauvel-Lafève F,
Soria J, et al: Evidence of antiangiogenic and antimetastatic
activities of the recombinant disintegrin domain of metargidin.
Cancer Res. 64:2062–2069. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Nath D, Slocombe PM, Stephens PE, Warn A,
Hutchinson GR, Yamada KM, Docherty AJ and Murphy G: Interaction of
metargidin (ADAM-15) with alphavbeta3 and alpha5beta1 integrins on
different haemopoietic cells. J Cell Sci. 112:579–587.
1999.PubMed/NCBI
|
|
145
|
Danhier F, Le Breton A and Préat V:
RGD-based strategies to target alpha(v) beta(3) integrin in cancer
therapy and diagnosis. Mol Pharm. 9:2961–2973. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Daugimont L, Vandermeulen G, Defresne F,
Bouzin C, Mir LM, Bouquet C, Feron O and Préat V: Antitumoral and
antimetastatic effect of antiangiogenic plasmids in B16 melanoma:
Higher efficiency of the recombinant disintegrin domain of ADAM 15.
Eur J Pharm Biopharm. 78:314–319. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Spanggaard I, Snoj M, Cavalcanti A,
Bouquet C, Sersa G, Robert C, Cemazar M, Dam E, Vasseur B, Attali
P, et al: Gene electrotransfer of plasmid antiangiogenic metargidin
peptide (AMEP) in disseminated melanoma: Safety and efficacy
results of a phase I first-in-man study. Hum Gene Ther Clin Dev.
24:99–107. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Bosnjak M, Prosen L, Dolinsek T, Blagus T,
Markelc B, Cemazar M, Bouquet C and Sersa G: Biological properties
of melanoma and endothelial cells after plasmid AMEP gene
electrotransfer depend on integrin quantity on cells. J Membr Biol.
246:803–819. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Bosnjak M, Dolinsek T, Cemazar M, Kranjc
S, Blagus T, Markelc B, Stimac M, Zavrsnik J, Kamensek U, Heller L,
et al: Gene electrotransfer of plasmid AMEP, an integrin-targeted
therapy, has antitumor and antiangiogenic action in murine B16
melanoma. Gene Ther. 22:578–590. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Date K, Matsumoto K, Shimura H, Tanaka M
and Nakamura T: HGF/NK4 is a specific antagonist for pleiotrophic
actions of hepatocyte growth factor. FEBS Lett. 420:1–6. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Kubota T, Matsumura A, Taiyoh H, Izumiya
Y, Fujiwara H, Okamoto K, Ichikawa D, Shiozaki A, Komatsu S,
Nakanishi M, et al: Interruption of the HGF paracrine loop by NK4,
an HGF antagonist, reduces VEGF expression of CT26 cells. Oncol
Rep. 30:567–572. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Kishi Y, Kuba K and Nakamura T, Wen J,
Suzuki Y, Mizuno S, Nukiwa T, Matsumoto K and Nakamura T: Systemic
NK4 gene therapy inhibits tumor growth and metastasis of melanoma
and lung carcinoma in syngeneic mouse tumor models. Cancer Sci.
100:1351–1358. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Ogura Y, Mizumoto K, Nagai E, Murakami M,
Inadome N, Saimura M, Matsumoto K, Nakamura T, Maemondo M, Nukiwa T
and Tanaka M: Peritumoral injection of adenovirus vector expressing
NK4 combined with gemcitabine treatment suppresses growth and
metastasis of human pancreatic cancer cells implanted
orthotopically in nude mice and prolongs survival. Cancer Gene
Ther. 13:520–529. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Nakamura T, Sakai K, Nakamura T and
Matsumoto K: Anti-cancer approach with NK4: Bivalent action and
mechanisms. Anticancer Agents Med Chem. 10:36–46. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Matsumoto K and Nakamura T: Mechanisms and
significance of bifunctional NK4 in cancer treatment. Biochem
Biophys Res Commun. 333:316–327. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Suzuki Y, Sakai K, Ueki J, Xu Q, Nakamura
T, Shimada H, Nakamura T and Matsumoto K: Inhibition of Met/HGF
receptor and angiogenesis by NK4 leads to suppression of tumor
growth and migration in malignant pleural mesothelioma. Int J
Cancer. 127:1948–1957. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Matsumoto G, Omi Y, Lee U, Kubota E and
Tabata Y: NK4 gene therapy combined with cisplatin inhibits tumour
growth and metastasis of squamous cell carcinoma. Anticancer Res.
31:105–111. 2011.PubMed/NCBI
|
|
158
|
Taiyoh H, Kubota T, Fujiwara H, Matsumura
A, Murayama Y, Okamoto K, Ichikawa D, Ochiai T, Nakamura T,
Matsumoto K, et al: NK4 gene expression enhances
5-fluorouracil-induced apoptosis of murine colon cancer cells.
Anticancer Res. 31:2217–2224. 2011.PubMed/NCBI
|
|
159
|
Zhu Y, Cheng M, Yang Z, Zeng CY, Chen J,
Xie Y, Luo SW, Zhang KH, Zhou SF and Lu NH: Mesenchymal stem
cell-based NK4 gene therapy in nude mice bearing gastric cancer
xenografts. Drug Des Devel Ther. 8:2449–2462. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Tada Y, Hiroshima K, Shimada H, Morishita
N, Shirakawa T, Matsumoto K, Shingyoji M, Sekine I, Tatsumi K and
Tagawa M: A clinical protocol to inhibit the HGF/c-Met pathway for
malignant mesothelioma with an intrapleural injection of
adenoviruses expressing the NK4 gene. Springerplus. 4:3582015.
View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Lebrin F, Goumans MJ, Jonker L, Carvalho
RL, Valdimarsdottir G, Thorikay M, Mummery C, Arthur HM and ten
Dijke P: Endoglin promotes endothelial cell proliferation and
TGF-beta/ALK1 signal transduction. EMBO J. 23:4018–4028. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Ten Dijke P, Goumans MJ and Pardali E:
Endoglin in angiogenesis and vascular diseases. Angiogenesis.
11:79–89. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Nassiri F, Cusimano MD, Scheithauer BW,
Rotondo F, Fazio A, Yousef GM, Syro LV, Kovacs K and Lloyd RV:
Endoglin (CD105): A review of its role in angiogenesis and tumor
diagnosis, progression and therapy. Anticancer Res. 31:2283–2290.
2011.PubMed/NCBI
|
|
164
|
Tsujie M, Tsujie T, Toi H, Uneda S,
Shiozaki K, Tsai H and Seon BK: Anti-tumor activity of an
anti-endoglin monoclonal antibody is enhanced in immunocompetent
mice. Int J Cancer. 122:2266–2273. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Uneda S, Toi H, Tsujie T, Tsujie M, Harada
N, Tsai H and Seon BK: Anti-endoglin monoclonal antibodies are
effective for suppressing metastasis and the primary tumors by
targeting tumor vasculature. Int J Cancer. 125:1446–1453. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Muñoz R, Arias Y, Ferreras JM, Jiménez P,
Langa C, Rojo MA, Gayoso MJ, Córdoba-Díaz D, Bernabéu C and Girbés
T: In vitro and in vivo effects of an anti-mouse endoglin
(CD105)-immunotoxin on the early stages of mouse B16MEL4A5 melanoma
tumours. Cancer Immunol Immunother. 62:541–551. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Tabata M, Kondo M, Haruta Y and Seon BK:
Antiangiogenic radioimmunotherapy of human solid tumors in SCID
mice using (125)I-labeled anti-endoglin monoclonal antibodies. Int
J Cancer. 82:737–742. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Dolinsek T, Markelc B, Sersa G, Coer A,
Stimac M, Lavrencak J, Brozic A, Kranjc S and Cemazar M: Multiple
delivery of siRNA against endoglin into murine mammary
adenocarcinoma prevents angiogenesis and delays tumor growth. PLoS
One. 8:e587232013. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Dolinsek T, Markelc B, Bosnjak M, Blagus
T, Prosen L, Kranjc S, Stimac M, Lampreht U, Sersa G and Cemazar M:
Endoglin silencing has significant antitumor effect on murine
mammary adenocarcinoma mediated by vascular targeted effect. Curr
Gene Ther. 15:228–244. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Xu Y, Hou J, Liu Z, Yu H, Sun W, Xiong J,
Liao Z, Zhou F, Xie C and Zhou Y: Gene therapy with tumor-specific
promoter mediated suicide gene plus IL-12 gene enhanced tumor
inhibition and prolonged host survival in a murine model of Lewis
lung carcinoma. J Transl Med. 9:392011. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Stimac M, Dolinsek T, Lampreht U, Cemazar
M and Sersa G: Gene electrotransfer of plasmid with tissue specific
promoter encoding shRNA against endoglin exerts antitumor efficacy
against murine TS/A tumors by vascular targeted effects. PLoS One.
10:e01249132015. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Dolinsek T, Sersa G and Cemazar M:
Melanoma cell viability is reduced after endoglin silencing with
gene electrotransfer. Biol Med Food Environ Technol. 325–328.
2016.
|
|
173
|
Pujade-Lauraine E, Hilpert F, Weber B, et
al: Bevacizumab combined with chemotherapy for platinum-resistant
recurrent ovarian cancer: the AURELIA open-label randomized phase
III trial. J Clin Oncol. 32:1302–1308. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Heinemann V, von Weikersthal LF, Decker T,
Kiani A, Vehling-Kaiser U, Al-Batran SE, Heintges T, Lerchenmüller
C, Kahl C, Seipelt G, et al: FOLFIRI plus cetuximab versus FOLFIRI
plus bevacizumab as first-line treatment for patients with
metastatic colorectal cancer (FIRE-3): A randomised, open-label,
phase 3 trial. Lancet Oncol. 15:1065–1075. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Rini BI, Bellmunt J, Clancy J, Wang K,
Niethammer AG, Hariharan S and Escudier B: Randomized phase III
trial of temsirolimus and bevacizumab versus interferon alfa and
bevacizumab in metastatic renal cell carcinoma: INTORACT trial. J
Clin Oncol. 32:752–759. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Bear HD, Tang G, Rastogi P, Geyer CE Jr,
Liu Q, Robidoux A, Baez-Diaz L, Brufsky AM, Mehta RS, Fehrenbacher
L, et al: Neoadjuvant plus adjuvant bevacizumab in early breast
cancer (NSABP B-40 [NRG Oncology]): Secondary outcomes of a phase
3, randomised controlled trial. Lancet Oncol. 16:1037–1048. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Van Cutsem E, Tabernero J, Lakomy R,
Prenen H, Prausová J, Macarulla T, Ruff P, van Hazel GA, Moiseyenko
V, Ferry D, et al: Addition of aflibercept to fluorouracil,
leucovorin, and irinotecan improves survival in a phase III
randomized trial in patients with metastatic colorectal cancer
previously treated with an oxaliplatin-based regimen. J Clin Oncol.
30:3499–3450. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Tannock IF, Fizazi K, Ivanov S, Karlsson
CT, Fléchon A, Skoneczna I, Orlandi F, Gravis G, Matveev V, Bavbek
S, et al: Aflibercept versus placebo in combination with docetaxel
and prednisone for treatment of men with metastatic
castration-resistant prostate cancer (VENICE): A phase 3,
double-blind randomised trial. Lancet Oncol. 14:760–768. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Ramlau R, Gorbunova V, Ciuleanu TE,
Novello S, Ozguroglu M, Goksel T, Baldotto C, Bennouna J, Shepherd
FA, Le-Guennec S, et al: Aflibercept and docetaxel versus docetaxel
alone after platinum failure in patients with advanced or
metastatic non-small-cell lung cancer: A randomized, controlled
phase III trial. J Clin Oncol. 30:3640–3647. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Allen JW, Moon J, Redman M, Gadgeel SM,
Kelly K, Mack PC, Saba HM, Mohamed MK, Jahanzeb M and Gandara DR:
Southwest oncology group S0802: A randomized, phase II trial of
weekly topotecan with and without ziv-aflibercept in patients with
platinum-treated small-cell lung cancer. J Clin Oncol.
32:2463–2470. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Siu LL, Shapiro JD, Jonker DJ, Karapetis
CS, Zalcberg JR, Simes J, Couture F, Moore MJ, Price TJ, Siddiqui
J, et al: Phase III randomized, placebo-controlled study of
cetuximab plus brivanib alaninate versus cetuximab plus placebo in
patients with metastatic, chemotherapy-refractory, wild-type K-RAS
colorectal carcinoma: The NCIC clinical trials group and AGITG CO.
20 trial. J Clin Oncol. 31:2477–2484. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Lordick F, Kang YK, Chung HC, Salman P, Oh
SC, Bodoky G, Kurteva G, Volovat C, Moiseyenko VM, Gorbunova V, et
al: Capecitabine and cisplatin with or without cetuximab for
patients with previously untreated advanced gastric cancer
(EXPAND): A randomised, open-label phase 3 trial. Lancet Oncol.
14:490–499. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Hitre E, Budai B, Takácsi-Nagy Z,
Rubovszky G, Tóth E, Remenár É, Polgár C and Láng I: Cetuximab and
platinum-based chemoradio- or chemotherapy of patients with
epidermal growth factor receptor expressing adenoid cystic
carcinoma: A phase II trial. Br J Cancer. 109:1117–1122. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Massarelli E, Haddad RI, Lee JJ, Garden
AS, Blumenschein GR, William WN, Tisshler RB, Glisson BS, Gold KA,
Johnson FM, et al: Randomized phase II trial of weekly paclitaxel,
carboplatin, cetuximab (PCC) versus cetuximab, docetaxel,
cisplatin, and fluorouracil (C-TPF) in previously untreated
patients with locally advanced head and neck squamous cell
carcinoma. J Clin Oncol. 32:TPS61022014.
|
|
185
|
Wang J, Sun Y and Qin S: Endostar Phase IV
Study Group: Results of phase IV clinical trial of combining
endostar with chemotherapy for treatment of advanced non-small cell
lung cancer (NSCLC). J Clin Oncol. 28:75982010. View Article : Google Scholar
|
|
186
|
Cui C, Mao L, Chi Z, Si L, Sheng X, Kong
Y, Li S, Lian B, Gu K, Tao M, et al: A phase II, randomized,
double-blind, placebo-controlled multicenter trial of Endostar in
patients with metastatic melanoma. Mol Ther. 21:1456–1463. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Jin T, Li B and Chen XZ: AA phase II trial
of Endostar combined with gemcitabine and cisplatin chemotherapy in
patients with metastatic nasopharyngeal carcinoma (NCT01612286).
Oncol Res. 21:317–323. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Chen Z, Guo W, Cao J, Lv F, Zhang W, Qiu
L, Li W, Ji D, Zhang S, Xia Z, et al: Endostar in combination with
modified FOLFOX6 as an initial therapy in advanced colorectal
cancer patients: A phase I clinical trial. Cancer Chemother
Pharmacol. 75:547–557. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Zhu AX, Rosmorduc O, Evans TJ, Ross PJ,
Santoro A, Carrilho FJ, Bruix J, Qin S, Thuluvath PJ, Llovet JM, et
al: SEARCH: A phase III, randomized, double-blind,
placebo-controlled trial of sorafenib plus erlotinib in patients
with advanced hepatocellular carcinoma. J Clin Oncol. 33:559–566.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Brose MS, Nutting CM, Jarzab B, Elisei R,
Siena S, Bastholt L, de la Fouchardiere C, Pacini F, Paschke R,
Shong YK, et al: Sorafenib in radioactive iodine-refractory,
locally advanced or metastatic differentiated thyroid cancer: A
randomised, double-blind, phase 3 trial trial. Lancet. 384:319–328.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Bruix J, Takayama T, Mazzaferro V, Chau
GY, Yang J, Kudo M, Cai J, Poon RT, Han KH, Tak WY, et al: STORM: A
phase III randomized, double-blind, placebo-controlled trial of
adjuvant sorafenib after resection or ablation to prevent
recurrence of hepatocellular carcinoma (HCC). J Clin Oncol.
32:40062014.
|
|
192
|
Schwandt A, von Gruenigen VE, Wenham RM,
Frasure H, Eaton S, Fusco N, Fu P, Wright JJ, Dowlati A and
Waggoner S: Randomized phase II trial of sorafenib alone or in
combination with carboplatin/paclitaxel in women with recurrent
platinum sensitive epithelial ovarian, peritoneal, or fallopian
tube cancer. Invest New Drugs. 32:729–738. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Motzer RJ, Hutson TE, Tomczak P, Tomczak
P, Michaelson M, Bukowski RM, Rixe O, Oudard S, Kim ST, Baum CM and
Figlin RA: Phase III randomized trial of sunitinib malate (SU11248)
versus interferon-alfa (IFN-{alpha}) as first-line systemic therapy
for patients with metastatic renal cell carcinoma (mRCC). J Clin
Oncol. 24:LBA32006.
|
|
194
|
Socinski MA, Novello S, Brahmer JR, Rosell
R, Sanchez JM, Belani CP, Govindan R, Atkins JN, Gillenwater HH,
Pallares C, et al: Multicenter, phase II trial of sunitinib in
previously treated, advanced non-small-cell lung cance. J Clin
Oncol. 26:650–656. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
195
|
Cheng A, Kang Y, Lin D, Park J, Kudo M,
Qin S, Omata M, Lowenthal SWP, Lanzalone S, Yang L, et al: Phase
III trial of sunitinib (Su) versus sorafenib (So) in advanced
hepatocellular carcinoma (HCC). J Clin Oncol. 29:40002011.
View Article : Google Scholar
|
|
196
|
Michaelson MD, Oudard S, Ou YC, Sengeløv
L, Saad F, Houede N, Ostler P, Stenzl A, Daugaard G, Jones R, et
al: Randomized, placebo-controlled, phase III trial of sunitinib
plus prednisone versus prednisone alone in progressive, metastatic,
castration-resistant prostate cancer. J Clin Oncol. 32:76–82. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
197
|
Kantarjian HM, Shah NP, Cortes JE,
Baccarani M, Agarwal MB, Undurraga MS, Wang J, Ipiña JJ, Kim DW,
Ogura M, et al: Dasatinib or imatinib in newly diagnosed
chronic-phase chronic myeloid leukemia: 2-year follow-up from a
randomized phase 3 trial (DASISION). Blood. 119:1123–1129. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
198
|
Kluger HM, Dudek AZ, McCann C, Ritacco J,
Southard N, Jilaveanu LB, Molinaro A and Sznol M: A phase 2 trial
of dasatinib in advanced melanoma. Cancer. 117:2202–2208. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
199
|
Wong SJ, Karrison T, Hayes DN, Kies MS,
Cullen KJ, Tanvetyanon T, Argiris A, Takebe N, Lim D, Saba NF, et
al: Phase II trial of dasatinib for recurrent or metastatic c-KIT
expressing adenoid cystic carcinoma and for nonadenoid cystic
malignant salivary tumors. Ann Oncol. 27:318–123. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
200
|
Herbst RS, Giaccone G, Schiller JH, Natale
RB, Miller V, Manegold C, Scagliotti G, Rosell R, Oliff I, Reeves
JA, et al: Gefitinib in combination with paclitaxel and carboplatin
in advanced non-small-cell lung cancer: a phase III trial-INTACT 2.
J Clin Oncol. 22:785–794. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
201
|
Argiris A, Ghebremichael M, Gilbert J, Lee
JW, Sachidanandam K, Kolesar JM, Burtness B and Forastiere AA:
Phase III randomized, placebo-controlled trial of docetaxel with or
without gefitinib in recurrent or metastatic head and neck cancer:
An eastern cooperative oncology group trial. J Clin Oncol.
31:1405–1414. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Dutton SJ, Ferry DR, Blazeby JM, Abbas H,
Dahle-Smith A, Mansoor W, Thompson J, Harrison M, Chatterjee A,
Falk S, et al: Gefitinib for oesophageal cancer progressing after
chemotherapy (COG): A phase 3, multicentre, double-blind,
placebo-controlled randomised tria. Lancet Oncol. 15:894–904. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
203
|
Moore MJ, Goldstein D, Hamm J, Figer A,
Hecht JR, Gallinger S, Au HJ, Murawa P, Walde D, Wolff RA, et al:
Erlotinib plus gemcitabine compared with gemcitabine alone in
patients with advanced pancreatic cancer: A phase III trial of the
national cancer institute of canada clinical trials group. J Clin
Oncol. 25:1960–1966. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
204
|
Rosell R, Carcereny E, Gervais R,
Vergnenegre A, Massuti B, Felip E, Palmero R, Garcia-Gomez R,
Pallares C, Sanchez JM, et al: Erlotinib versus standard
chemotherapy as first-line treatment for European patients with
advanced EGFR mutation-positive non-small-cell lung cancer
(EURTAC): A multicentre, open-label, randomised phase 3 trial.
Lancet Oncol. 13:239–246. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
205
|
Sternberg CN, Davis ID, Mardiak J,
Szczylik C, Lee E, Wagstaff J, Barrios CH, Salman P, Gladkov OA,
Kavina A, et al: Pazopanib in locally advanced or metastatic renal
cell carcinoma: results of a randomized phase III trial. J Clin
Oncol. 28:1061–1068. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
206
|
van der Graaf WT, Blay JY, Chawla SP, Kim
DW, Bui-Nguyen B, Casali PG, Schöffski P, Aglietta M, Staddon AP,
Beppu Y, et al: Pazopanib for metastatic soft-tissue sarcoma
(PALETTE): A randomised, double-blind, placebo-controlled phase 3
trial. Lancet. 379:1879–1886. 2012. View Article : Google Scholar : PubMed/NCBI
|