Introduction
Lung cancer, is the most frequently diagnosed cancer
and the leading cause of cancer-associated mortalities among males
and females worldwide. Small-cell lung carcinoma (SCLC) is an
aggressive malignancy with a high mortality, accounting for 15% of
all lung cancer cases (1). There has
been a distinct lack of significant advances in SCLC therapy over
the last 30 years (2). Therefore, new
ways to treat or prevent SCLC are therefore needed.
In many types of cancer, Wnt signaling, which plays
a fundamental role in proliferation and development, is
inappropriately activated (3–5). A series of studies mentioned that Wnt
signaling may be a potential therapeutic target for lung cancer
(6–9).
β-catenin, a key downstream effector of the signaling pathway,
mediates the canonical Wnt signals. The degradation of β-catenin is
regulated by glycogen synthase kinase-3, in a complex with
adenomatous polyposis coli (APC) and axin. Cytosolic β-catenin
levels are kept low by the destruction complex in the absence of
Wnt (10–13). Effective pharmacological inhibitors of
the Wnt signaling pathway have only recently been available. These
inhibitors are poly-ADP-ribose polymerase (PAPR) enzymes, namely
tankyrase (TNKS) 1 and TNKS2, which regulate canonical Wnt activity
by promoting the stabilization of axin to increase the destruction
of β-catenin (14). It has been
reported that TNKS1 is upregulated in many types of cancer,
including breast cancer, colon and bladder cancer as well as
high-grade non-Hodgkin's lymphoma (15–19).
XAV939, a small molecule inhibitor of the
dysregulated Wnt signaling pathway, is characterized as a potent
inhibitor of TNKS (14). A number of
recent studies have demonstrated that XAV939 is able to inhibit the
growth of breast, colon and non-small cell lung cancer cells by
blocking the Wnt signaling pathway (20–22).
However, there rarely have been similar studies on SCLC.
The purpose of the present study was to investigate
if XAV939 is able to inhibit the proliferation of SCLC cells and
the underlying mechanisms. H446 cells were treated with
XAV939/cisplatin (DDP) alone or combined for 24 or 48 h. The
inhibition of cell proliferation was detected by Cell Counting
Kit-8 (CCK-8). The mRNA and protein expression of β-catenin and
cyclin D1 were detected by reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) and western blotting. The
results of the present study demonstrated that the
anti-proliferative effects of TNKS inhibitor XAV939 may be
associated with the downregulation of the Wnt/β-catenin signaling
pathway in H446 cells. The present study indicated that TNKS may be
a potential molecular target for the treatment of SCLC.
Materials and methods
Chemicals
XAV939 was purchased from Sigma-Aldrich (Merck KGaA,
Darmstadt, Germany). DDP was purchased from Hospira Australia Pty
Ltd. (Pfizer, Inc., New York, NY, USA).
Cell culture
The human H446 SCLC cells were purchased from the
Institute of Biochemistry and Cell Biology (Shanghai Institutes for
Biological Sciences, Chinese Academy of Sciences, Shanghai, China).
The cells were cultured in RPMI 1640 medium (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) and supplemented with 10% fetal
bovine serum, penicillin G sodium (100 U/ml; all purchased from
Gibco; Thermo Fisher Scientific, Inc.) and streptomycin sulfate
(100 mg/ml) at 37°C under an atmosphere of 5% CO2.
CCK-8 cell viability analysis
The effect of XAV939 on the viability of H446 cells
was analyzed by CCK-8. The experiment was divided into three
groups: XAV939, DDP and combination group. There were 5
concentration gradients for each treatment group. H446 cells
(1×105) were plated and treated in 96-well plates. The
cells were treated with the corresponding drug for 24 or 48 h.
Then, 10 µl CCK8 was added to each well. The absorbance was
measured at 490 nm using a microplate reader (Multiskan™ GO
microplate spectrophotometer; Thermo Fisher Scientific, Inc.). The
experiment was performed in triplicate. The data was analyzed using
the efficiency equation fa/fu=(D/Dm)m (fu, tumor cell survival
rate; fa, tumor cell inhibition rate; fu=1-fa; D, drug
concentration; Dm, effect concentration). Combination Index (CI)
values were calculated using the D1/Dx1+D2/Dx2+αD1D2/Dx1Dx2 method.
Synergism was considered when CI <1.
RT-qPCR analysis
The N-H446 cells were plated in 6-well plates and
treated with XAV939 (10, 20 and 40 µM) for 24 h. Total RNA
extraction was performed according to the TRIZOL manufacturer's
protocol (Takara Bio, Inc., Otsu, Japan). The synthesis of cDNAs
was performed by reverse transcription reactions using a
PrimeScript™ RT reagent transcription kit (Takara Bio,
Inc.). cDNA samples were subjected to qPCR using the SYBR Premix Ex
Taq kit (Takara Bio, Inc.). PCR was performed with the following
primers: β-catenin forward, 5′-CATTACAACTCTCCACAACC-3′, reverse
5′-CAGATAGCACCTTCAGCAC-3′; cyclin D1 forward,
5′-CATTGATTCAGCCTGTTTGG-3′ and reverse, 5′-GAATTCATCGGAACCGAACT-3′.
GAPDH expression was used to normalize the Cq values. The
expression of each gene was analyzed in triplicate. The data was
analyzed using the 2−ΔΔCq method (23).
Western blot analysis
Following treatment with XAV939 (10, 20 and 40 µM)
for 24 h, the H446 cells were collected and lysed using
radioimmunoprecipitation assay buffer (Beyotime Institute of
Biotechnology, Shanghai, China) and BCA Protein Assay Kit for
determination of protein concentration (Beyotime Institute of
Biotechnology). A total of 20 µg protein was separated on 8%
SDS-PAGE and transferred to a PVDF membrane. The membranes were
blocked at room temperature for 2h with 5% non-fat milk. The
membranes were then incubated with the following primary
antibodies: Anti-β-catenin (cat no. 6387; Cell Signaling
Technology, Inc., Danvers, MA, USA), anti-cyclin D1 (cat no. 2922;
Cell Signaling Technology, Inc.) and anti-β-actin (cat no. AF0003;
Beyotime Institute of Biotechnology) at a dilution of 1:1,000 at
4°C overnight. Following three washes with TBST, the membranes were
incubated with horseradish peroxidase-conjugated secondary
antibodies (goat anti-rabbit IgG: dilution, 1:10,000; cat no. 7074;
anti-mouse: dilution, 1:10,000; cat no. 7076; Cell Signaling
Technology, Inc.) for 2 h at 37°C. The blots were visualized using
the ECL Plus system (Thermo Fisher Scientific, Inc.).
Statistics analysis
The SPSS 17.0 software (SPSS, Inc., Chicago, IL,
USA) was used to analyze the data. The data were presented as the
mean ± standard deviation. One-way analysis of variance and paired
Student's t-test, along with the post-hoc tests least significant
difference test and Dunnett's test, were used for comparison
between the groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effect of XAV939 on the proliferation
of H446 cells
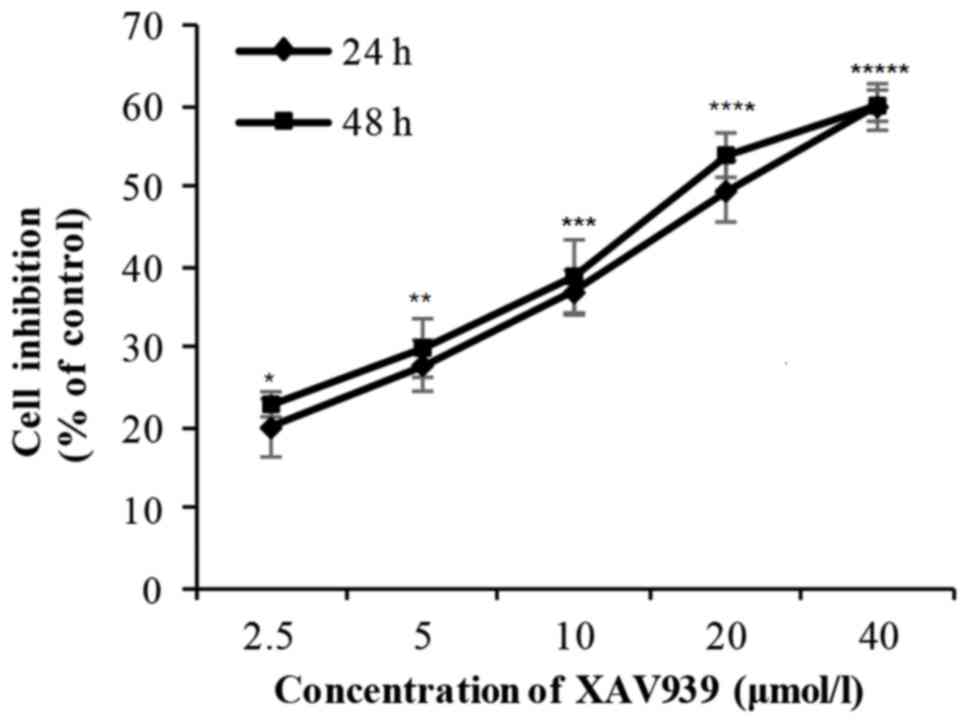
H446 cells were treated with different concentration
(2.5–40 µM) of XAV939 for 24 or 48 h. There was a significant
difference in the cell inhibition rate with the increase of drug
concentration (P<0.01; Table I).
However, there was no significant difference in cell inhibition
rate between 24 and 48 h (P>0.05; Table I). Collectively, these results
indicate that the inhibitory effect of XAV939 on the proliferation
of H446 cells is dose-dependent but not time-dependent (Fig. 1 and Table
I). The IC50 value for XAV939 is 21.56 µM.
 | Table I.Inhibition of H446 cells by
XAV939. |
Table I.
Inhibition of H446 cells by
XAV939.
| Groups (µM) | 24 h | 48 h | t | P-value (Comparison
between treatment durations) |
|---|
| 2.5 | 19.97±3.63 | 22.90±1.56 | −0.985 | 0.428 |
| 5.0 | 27.72±3.19 | 29.92±3.65 | −2.133 | 0.167 |
| 10.0 | 36.82±2.80 | 38.85±4.55 | −0.519 | 0.655 |
| 20.0 | 49.43±3.81 | 53.95±2.77 | −2.451 | 0.134 |
| 40.0 | 59.96±2.90 | 60.12±1.95 | −0.066 | 0.954 |
| F | 72.175 | 77.284 | – | – |
| P-value | <0.0001 | <0.0001 | – | – |
Effect of DDP on the proliferation of
H446 cells
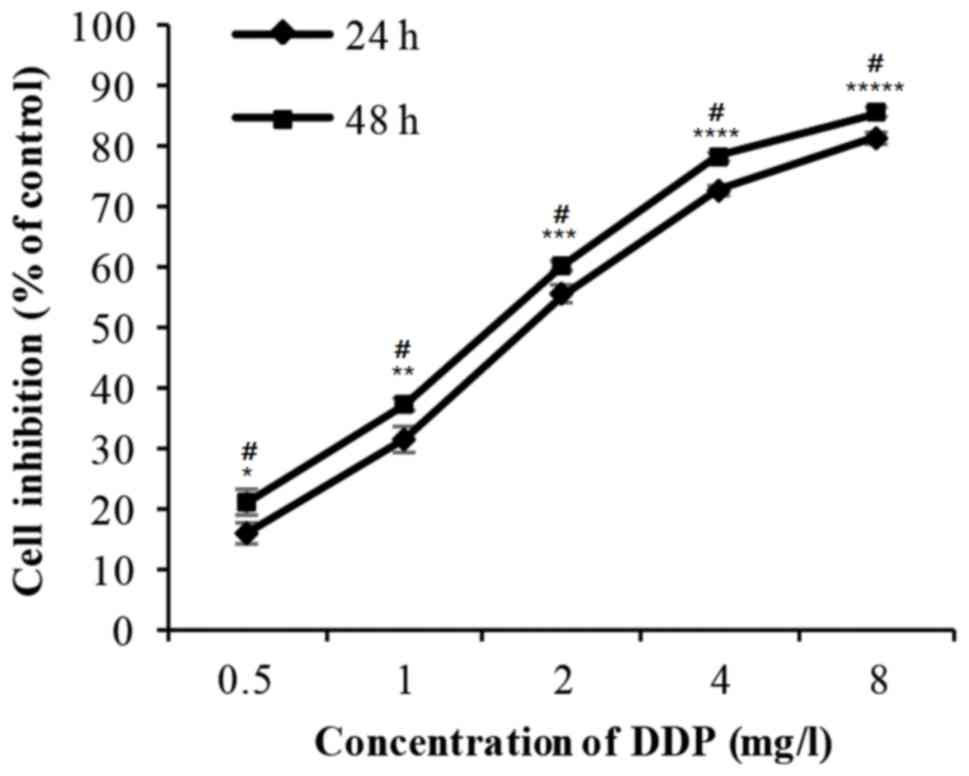
The cells were incubated with 0.5–8 mg/l DDP for 24
or 48 h. The cell inhibition rate increased markedly in a dose- and
time-dependent manner (Fig. 2 and
Table II). The IC50 value
for DDP is 7.91 µM.
 | Table II.Inhibition of H446 cells by
cisplatin. |
Table II.
Inhibition of H446 cells by
cisplatin.
| Groups (mg/l) | 24 h | 48 h | t | P-value (Comparison
between treatment durations) |
|---|
| 0.5 | 17.53±1.74 | 22.61±2.14 | −4.69 | 0.043 |
| 1.0 | 29.84±2.15 | 36.54±1.03 | −10.267 | 0.009 |
| 2.0 | 50.15±1.48 | 56.34±0.80 | −4.795 | 0.041 |
| 4.0 | 64.91±0.81 | 71.29±0.70 | −7.669 | 0.017 |
| 8.0 | 72.59±0.97 | 77.47±0.76 | −4.922 | 0.039 |
| F | 703.827 | 1091.974 | – | – |
| P-value | <0.0001 | <0.0001 | – | – |
Effect of XAV939 and DDP combination
treatment on the proliferation of H446 cells
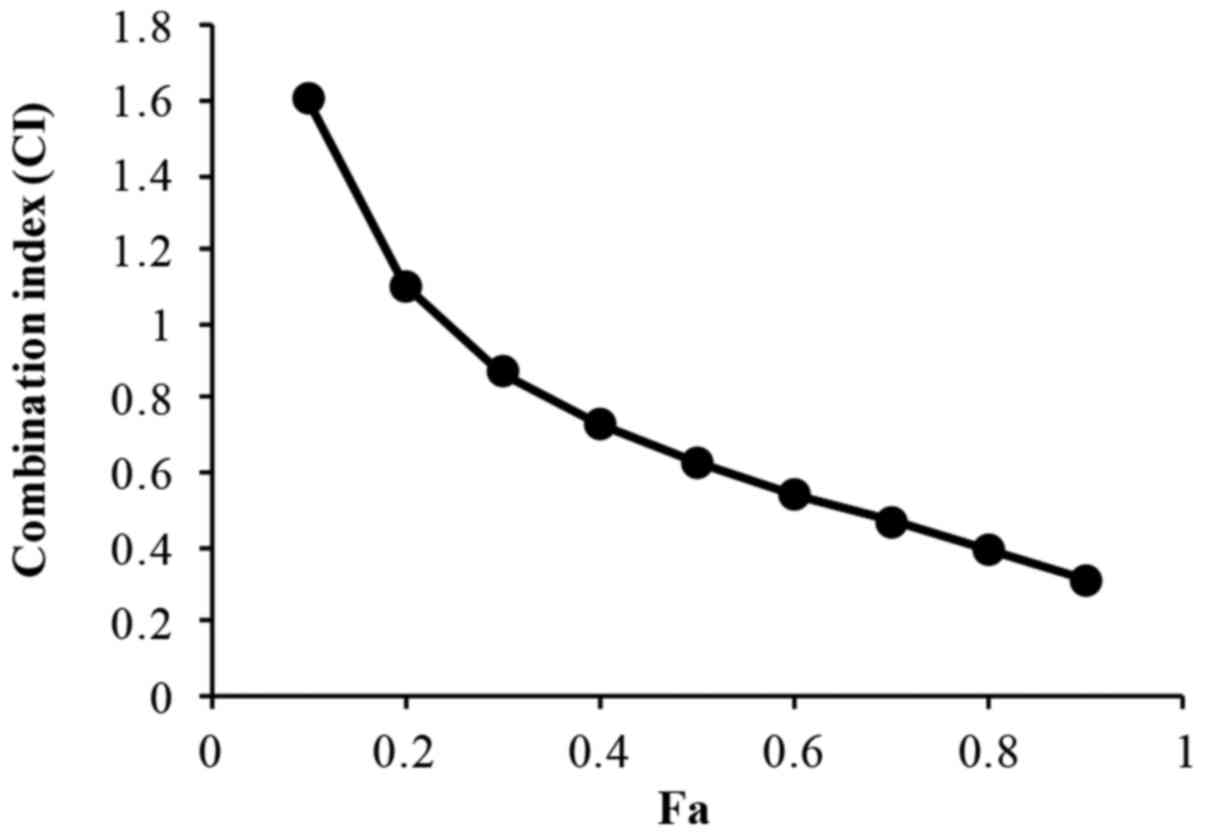
The treatment of H446 cells with a combination of
XAV939 and DDP (2.5–40 µM XAV939 and 0.5–8 mg/l DDP; 1:1 ratio) for
24 or 48 h resulted in an increase in cell inhibition rate in a
dose- and time-dependent manner (P<0.01; Table III). The IC50 value for
XAV939 and DDP combination treatment is 7.98 µM. However, the
effect of combination treatment with XAV939 and DDP was
antagonistic at low doses in H446 cells (CI>1), whilst at higher
concentrations the effect was synergistic (CI<1) (Fig. 3 and Table
IV).
 | Table III.Inhibition of H446 cells by a
combination of XAV939 and DDP. |
Table III.
Inhibition of H446 cells by a
combination of XAV939 and DDP.
| Groups | 24 h | 48 h | t | P-value (Comparison
between treatment durations) |
|---|
| 0.5 mg/l DDP + 2.5
µM XAV939 | 15.90±1.22 | 21.09±0.82 | −12.189 | 0.007 |
| 1 mg/l DDP + 5 µM
XAV939 | 31.48±0.71 | 37.33±1.15 | −10.818 | 0.008 |
| 2 mg/l DDP + 10 µM
XAV939 | 55.68±1.17 | 60.34±1.69 | −2.869 | 0.103 |
| 4 mg/l DDP + 20 µM
XAV939 | 72.79±1.92 | 78.40±1.85 | −20.774 | 0.002 |
| 8 mg/l DDP + 40 µM
XAV939 | 81.49±0.93 | 85.85±0.81 | −4.482 | 0.046 |
| F | 1437.682 | 1248.72 | – | – |
| P-value | <0.0001 | <0.0001 | – | – |
 | Table IV.Inhibition of H446 cells with single
or combination treatments of XAV939 and DDP. |
Table IV.
Inhibition of H446 cells with single
or combination treatments of XAV939 and DDP.
| Groups | Combination
index | Correlation
index | IC50
(µM) |
|---|
| XAV939 | 0.652 | 0.997 | 21.56465 |
| DDP | 0.940 | 0.965 | 7.910076 |
| XAV939 + DDP | 1.162 | 0.996 | 7.980187 |
Effect of XAV939 on the mRNA
expression levels of β-catenin and cyclin D1 in H446 cell
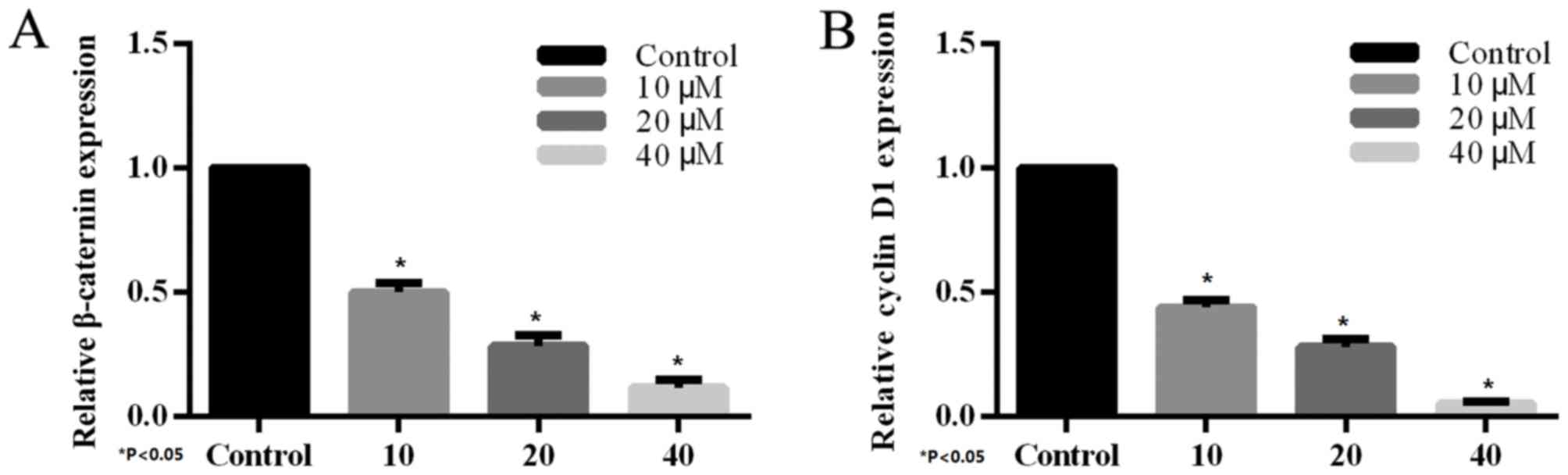
The effect of XAV939 on the expression of endogenous
Wnt-regulated target genes, β-catenin and cyclin D1 was determined
by RT-PCR. H446 cells were treated with various concentrations (10,
20 and 40 µM) of the tankyrase inhibitor, XAV939, for 24 h.
Compared with the control group, treatment with XAV939 was able to
inhibit Wnt-associated expression of target genes in H446 cells.
The effects on gene expression were dose-dependent (Fig. 4) and the differences were
statistically significant.
Effect of XAV939 on the expression of
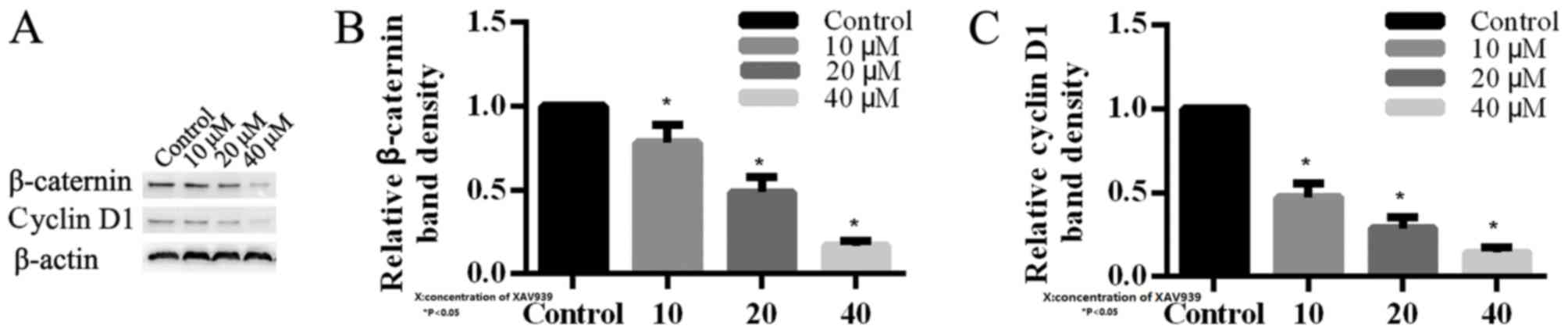
β-catenin and cyclin D1 proteins in H446 cells
In order to investigate the mechanism of XAV939 in
H446 cells, the expression levels of key proteins involved in Wnt
signaling pathway were analyzed by western blotting. β-catenin was
observed to be reduced in H446 cells that were treated with 10, 20
and 40 µM XAV939 compared with the control (Fig. 5). Additionally, cyclin D1 was
downregulated in H446 cells following treatment with XAV939, which
is an inhibitor of the Wnt signaling pathway. The effects of XAV939
on the levels of expression of β-catenin and cyclin D1 proteins
were dose-dependent (Fig. 5).
Altogether these results demonstrated that XAV939 may affect
associated target genes in order to block Wnt transcriptional
responses in SCLC cells.
Discussion
As an increasing number of targeted drugs are to be
used for the treatment of non-small cell lung carcinoma (NSCLC),
the overall survival and disease-free survival (DFS) of patients
are markedly prolonged. On the contrary, the treatments for
patients with SCLC are progressing slowly (2). Therefore, there is an urgent requirement
for the identification of effective targets in order to improve
patient survival.
In 1973, Sharma et al (24) identified the Wingless gene (Wg) that
leads to a wingless phenotype in drosophila embryo research. Nusse
et al (25) identified the
Int-1 gene in mouse breast cancer in in 1982. In 1987, the study
confirmed that Wg is the homologous gene of Int-1 (26), therefore Wg and Int-1 are named as Wnt
genes. Aberrant WNT signaling pathway is associated with a wide
array of tumor types, including colorectal cancer, acute myeloid
leukemia, breast cancer, ovarian cancer and NSCLC (3,5,27,28).
Therefore, Wnt signaling pathway may provide a potential
therapeutic target for SCLC.
A family of secreted lipid-modified Wnt protein
ligands activate the pathway in order to promote the nuclear
accumulation of β-catenin by binding to a family of 7-transmembrane
Frizzled (in the canonical Wnt signaling pathway (29). β-catenin forms complexes with the
transcription factors T-cell factors (TCFs) and lymphoid
enhancer-binding factor in the nucleus, and this reduces the
expression of TCF responsive target genes, including critical
growth-regulators, such as cyclin D1, and c-Myc (30,31). The
β-catenin destruction complex, which consists of APC, axin, casein
kinase 1 and glycogen synthase kinase-3β, downregulates the level
of β-catenin (12). XAV939 is a small
molecule inhibitor of the WNT signaling pathway, which is able to
block WNT signaling through upregulating the destruction of
β-catenin and stabilizing the axin protein.
In order to demonstrate that XAV939 is able to
inhibit the growth of SCLC cells, CCK-8 assay was employed. A
significant difference was observed in the rate of proliferation
following treatment with XAV939. The effect of XAV939 was
dose-dependent but not time-dependent.
DDP, a common chemical anti-tumor drug is still used
in the clinic for the treatment of SCLC. Due to serious side
effects, DDP is limited in clinical use. Therefore, there is a
requirement to identify a drug that is able to achieve the
therapeutic effect of the original dose of DDP that can be used in
combination with a lower dosage of DDP.
Consistent with the findings of the XAV939 treatment
group, a significant difference in the inhibitory rate of H446
cells following treatment with DDP was observed. However, the
effect of DDP was dose-dependent and time-dependent. Following
treatment with a combination of XAV939 and DDP, it was observed
that the effects were antagonistic at low doses and synergistic at
high doses. The drugs played their own role, and no marked
synergistic effect was observed when the dose of XAV939 was low. It
is possible to attain the optimum curative effect and the least
adverse reactions when an appropriate dosage ratio is
identified.
In order to further elucidate the mechanism of
XAV939 in SCLC, Wnt-associated target genes were analyzed by
RT-qPCR, and the expression of the associated proteins were
examined by western blotting. In the present study, the levels of
β-catenin and cyclin D1 were downregulated following the treatment
of XAV939 for 24 h. All of these results suggested that XAV939 is
able to downregulate β-catenin, the primary Wnt signaling effector
and reduce the critical growth regulator cyclin D1.
In summary, the present study confirmed that the
inhibition of XAV939 is marked in SCLC cells. Additionally, the
mechanism of XAV939 may be associated with the suppression of
Wnt/β-catenin signaling pathway. Further studies of the small
molecule inhibitor, XAV939, in vivo are needed.
Acknowledgements
Not applicable.
Funding
Financial assistance was provided by the Affiliated
Hospital of Qingdao University.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Author's contributions
FP, LJY and LJZ analyzed and collected the data
regarding the MTT and the western blot analysis. FZS, WXG and JXT
analyzed and collected the data regarding the PCR. FP and FZS made
major contributors in writing the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Byers LA and Rudin CM: Small cell lung
cancer: Where do we go from here? Cancer. 121:664–672. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Polakis P: Wnt signaling in cancer. Cold
Spring Harb Perspect Biol. 4:a0080522012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Clevers H and Nusse R: Wnt/β-catenin
signaling and disease. Cell. 149:1192–1205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
MacDonald BT, Tamai K and He X:
Wnt/beta-catenin signaling: Components, mechanisms, and diseases.
Dev Cell. 17:9–26. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Snow GE, Kasper AC, Busch AM, Schwarz E,
Ewings KE, Bee T, Spinella MJ, Dmitrovsky E and Freemantle SJ: Wnt
pathway reprogramming during human embryonal carcinoma
differentiation and potential for therapeutic targeting. BMC
Cancer. 9:3832009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
You L, He B, Xu Z, Uematsu K, Mazieres J,
Mikami I, Reguart N, Moody TW, Kitajewski J, McCormick F and
Jablons DM: Inhibition of Wnt-2-mediated signaling induces
programmed cell death in non-small-cell lung cancer cells.
Oncogene. 23:6170–6174. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pacheco-Pinedo EC, Durham AC, Stewart KM,
Goss AM, Lu MM, Demayo FJ and Morrisey EE: Wnt/β-catenin signaling
accelerates mouse lung tumorigenesis by imposing an embryonic
distal progenitor phenotype on lung epithelium. J Clin Invest.
121:1935–1945. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nguyen DX, Chiang AC, Zhang XH, Kim JY,
Kris MG, Ladanyi M, Gerald WL and Massagué J: WNT/TCF signaling
through LEF1 and HOXB9 mediates lung adenocarcinoma metastasis.
Cell. 138:51–62. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
He TC, Sparks AB, Rago C, Hermeking H,
Zawel L, da Costa LT, Morin PJ, Vogelstein B and Kinzler KW:
Identification of c-MYC as a target of the APC pathway. Science.
281:1509–1512. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tetsu O and McCormick F: Beta-catenin
regulates expression of cyclin D1 in colon carcinoma cells. Nature.
398:422–426. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jho EH, Zhang T, Domon C, Joo CK, Freund
JN and Costantini F: Wnt/beta-catenin/Tcf signaling induces the
transcription of Axin2, a negative regulator of the signaling
pathway. Mol Cell Biol. 22:1172–1183. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rousset R, Mack JA, Wharton KA Jr, Axelrod
JD, Cadigan KM, Fish MP, Nusse R and Scott MP: Naked cuticle
targets dishevelled to antagonize Wnt signal transduction. Genes
Dev. 15:658–671. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang SM, Mishina YM, Liu S, Cheung A,
Stegmeier F, Michaud GA, Charlat O, Wiellette E, Zhang Y, Wiessner
S, et al: Tankyrase inhibition stabilizes axin and antagonizes Wnt
signalling. Nature. 461:614–620. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gelmini S, Poggesi M, Distante V, Bianchi
S, Simi L, Luconi M, Raggi CC, Cataliotti L, Pazzagli M and Orlando
C: Tankyrase, a positive regulator of telomere elongation, is over
expressed in human breast cancer. Cancer Lett. 216:81–87. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gelmini S, Poggesi M, Pinzani P, Mannurita
SC, Cianchi F, Valanzano R and Orlando C: Distribution of
Tankyrase-1 mRNA expression in colon cancer and its prospective
correlation with progression stage. Oncol Rep. 16:1261–1266.
2006.PubMed/NCBI
|
|
17
|
Gelmini S, Quattrone S, Malentacchi F,
Villari D, Travaglini F, Giannarini G, Della Melina A, Pazzagli M,
Nicita G, Selli C and Orlando C: Tankyrase-1 mRNA expression in
bladder cancer and paired urine sediment: Preliminary experience.
Clin Chem Lab Med. 45:862–866. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
MacNamara B, Wang W, Chen Z, Hou M, Mazur
J, Gruber A and Porwit-MacDonald A: Telomerase activity in relation
to pro- and anti-apoptotic protein expression in high grade
non-Hodgkin's lymphomas. Haematologica. 86:386–393. 2001.PubMed/NCBI
|
|
19
|
Klapper W, Krams M, Qian W, Janssen D and
Parwaresch R: Telomerase activity in B-cell non-Hodgkin lymphomas
is regulated by hTERT transcription and correlated with
telomere-binding protein expression but uncoupled from
proliferation. Br J Cancer. 89:713–719. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bao R, Christova T, Song S, Angers S, Yan
X and Attisano L: Inhibition of tankyrases induces Axin
stabilization and blocks Wnt signalling in breast cancer cells.
PLoS One. 7:e486702012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Waaler J, Machon O, Tumova L, Dinh H,
Korinek V, Wilson SR, Paulsen JE, Pedersen NM, Eide TJ, Machonova
O, et al: A novel tankyrase inhibitor decreases canonical Wnt
signaling in colon carcinoma cells and reduces tumor growth in
conditional APC mutant mice. Cancer Res. 72:2822–2832. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Busch AM, Johnson KC, Stan RV, Sanglikar
A, Ahmed Y, Dmitrovsky E and Freemantle SJ: Evidence for tankyrases
as antineoplastic targets in lung cancer. BMC Cancer. 13:2112013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sharma RP and Chopra VL: Effect of the
Wingless (wg1) mutation on wing and haltere development in
Drosophila melanogaster. Dev Biol. 48:461–465. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nusse R, van Ooyen A, Cox D, Fung YK and
Varmus H: Mode of proviral activation of a putative mammary
oncogene (int-1) on mouse chromosome 15. Nature. 307:131–136. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Siegfried E and Perrimon N: Drosophila
wingless: A paradigm for the function and mechanism of Wnt
signaling. Bioessays. 16:395–404. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Moon RT: Wnt/beta-catenin pathway. Sci
STKE. 2005:cm12005.PubMed/NCBI
|
|
28
|
Klaus A and Birchmeier W: Wnt signalling
and its impact on development and cancer. Nat Rev Cancer.
8:387–398. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Holland JD, Klaus A, Garratt AN and
Birchmeier W: Wnt signaling in stem and cancer stem cells. Curr
Opin Cell Biol. 25:254–264. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dang CV: c-Myc target genes involved in
cell growth, apoptosis, and metabolism. Mol Cell Biol. 19:1–11.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shtutman M, Zhurinsky J, Simcha I,
Albanese C, D'Amico M, Pestell R and Ben-Ze'ev A: The cyclin D1
gene is a target of the beta-catenin/LEF-1 pathway. Proc Natl Acad
Sci USA. 96:5522–5527. 1999. View Article : Google Scholar : PubMed/NCBI
|