Introduction
Gastric carcinoma is one of the common types of
malignant tumor that threaten human life and health (1). Gastric carcinoma has low eradication
rates during surgery, with high recurrence and metastasis rates
following surgery (2). It has a poor
sensitivity to conventional radiotherapy, and chemotherapy serves
an important role in the treatment of gastric carcinoma (3). In addition, there are not many effective
drugs for the treatment of gastric carcinoma, and these drugs are
usually associated with toxicity and unsatisfactory efficacy. By
contrast, traditional Chinese medicine has demonstrated certain
advantages in the treatment of tumors, including adverse reactions,
low toxicity, little residue and unique pharmacological actions
(4). It has been reported that
traditional Chinese medicine enhances immunity (5), activates antioxidative signaling
pathways associated with nuclear factor (NF)2 (6) and downregulates the NF-κB signaling
pathway (7). The antitumor mechanisms
include promotion of apoptosis (8),
inactivation of telomerase, antioxidation, inhibition of
angiogenesis and metastasis, and induction of differentiation of
tumor cells to normal cells (9),
increase of tumor necrosis factor receptor and Fas/Fas ligand
(FasL) expression in death receptor/ligand regulation (10), reactive oxygen species-independent
dysfunction of mitochondria and induction of reactive oxygen
species production (11), activation
of the caspase-3 signaling pathway, downregulation of Bcl-2
expression, and enhancement of apoptosis-associated protein
phosphorylation (12). These
mechanisms are associated in the treatment of tumors.
As a common plant, lily possesses high medicinal
values. It has been demonstrated that specific components extracted
from lily exhibit antitumor effects (4). However, the mechanism of action of the
effective components extracted from lily remains unclear. To the
best of our knowledge, the effect of lily on human gastric
carcinoma SGC-7901 cells has not been previously studied. In the
present study, the effect and underlying mechanisms of alkaloids,
and carbinol extracts from lily on the proliferation of SGC-7901
cells were investigated.
Materials and methods
Cells
SGC-7901 cells were purchased from the Institute of
Digestive and Experimental Research, School of Medicine, Xi'an
Jiaotong University (Xi'an, China). Cells were cultured in
RPMI-1640 medium supplemented with 10% fetal bovine serum, 100
µg/ml penicillin and 100 µg/ml streptomycin (all from Thermo Fisher
Scientific, Inc., Waltham, MA, USA) at 37°C with 5% CO2
in a humidified, sterile incubator. The medium was changed every 24
h. When 80% confluency was achieved, the cells were washed twice
with 2 ml PBS, followed by addition of 0.25% trypsin (0.5 ml). When
the cells were fully trypsinized, 2 ml fresh medium was added to
resuspend the cells, which were then divided evenly into two
culture flasks containing 6 ml medium each. Cells in the
logarithmic phase growth were chosen for subsequent
experiments.
MTT assay
To measure the proliferation of cells, an MTT assay
was performed. Single cell suspension was prepared at a
concentration of 1×105 cells/ml, and then 100 µl/well
was added into a 96-well plate. For the blank group, no cells were
added into the well. Following incubation for 24 h at 37°C, the
medium was discarded. For the blank and control groups, only fresh
medium was added. For the experimental groups, fresh medium
containing 0.05, 0.075, 0.1, 0.125 and 0.15 g/l alkaloid or 0.8,
0.9, 1.0, 1.1, 1.2, 1.3, 1.4, 1.5, and 1.6 g/l carbinol extracts
were added. To prepare these extracts, lily bulb was washed and
lyophilized to obtain a dry powder. Then, the dry powder (25 g) was
dissolved in 300 ml 90% acid ethanol solution, and extracted with
petroleum ether. The water layer was aspirated and extracted with
chloroform. Then, the chloroform layer was dried, and 0.5 g dried
powder was dissolved in 10 ml methanol to reach a concentration of
50 mg/ml. For the carbinol control groups, fresh medium containing
3.75 or 4% carbinol was added. Each group was repeated in six wells
containing 200 µl. After incubation for 24, 48 and 72 h, 20 µl MTT
(5 mg/ml) was added into each well, followed by another incubation
for 4 h at 37°C. Then, the supernatants were discarded and 150 µl
dimethyl sulphoxide (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany)
was added prior to agitating for 10–15 min at 37°C. The absorbance
was measured using a microplate reader (Model 680; Bio-Rad
Laboratories, Inc., Hercules, CA, USA) at a wavelength of 490 nm.
The measurements of the control, experimental and carbinol control
groups were adjusted according to the blank group. The cell
proliferation inhibition rate was evaluated using the following
formula: [(Absorbance of control group-absorbance of experimental
group)/absorbance of control group] ×100%. The measurements were
repeated for three times to calculate mean values. The cell
proliferation inhibition rates under all concentrations and time
points were calculated.
Light and fluorescence microscopy
Cells in the logarithmic growth
(1×105/ml) were seeded into 6-well plates with 2 ml
medium/well. For experimental groups, medium containing 0.9, 1.1
and 1.4 g/l carbinol extracts or 0.05, 0.1, and 0.15 g/l alkaloid
was used to incubate the cells. For the control group, 2 ml normal
medium was used. For the carbinol control group, 2 ml normal medium
containing 4% carbinol was used. Following incubation for 24, 48 or
72 h, the cells were washed with PBS three times, and imaged under
a light microscope (magnification, ×10; CKX41; Olympus Corporation,
Tokyo, Japan). For fluorescence microscopy, acridine
orange/ethidium bromide staining liquid was dropped on the slide
and kept at room temperature for 30 sec prior to observation under
the microscope (magnification, ×10).
Flow cytometry
Cells in the logarithmic growth were seeded into 25
ml culture flasks containing 3 ml medium. After incubation for 24
h, the medium was removed and fresh medium was added. After another
24 h of culture, the medium was discarded and fresh medium
containing 0.9, 1.1, and 1.4 g/l carbinol extracts or 0.05, 0.1,
and 0.15 g/l alkaloid was added to each experimental group. For the
control group, only fresh medium was added. After incubation for 48
h, cells were trypsinized and the cell suspension was collected.
After centrifugation at room temperature and 123 × g for 5 min, the
supernatant was discarded and the cells were washed with PBS three
times. Cell sediments were fixed with 70% pre-cold ethanol, and
kept at −20°C for 24 h. Then, the cells were washed with PBS, and
stained with propidium iodide (PI) for 30 min at room temperature
in the dark. The DNA content was measured using flow cytometry.
Cell cycles were analyzed using an excitation wavelength of 488 nm
and emission wavelength of 630 nm. The experiments were performed
in triplicate.
For Annexin V-FITC/PI staining (Beyotime Institute
of Biotechnology, Shanghai, China), 50 µl RNase (0.5 mg/ml) was
added to samples and incubated at 37°C for 30 min. After
centrifugation at room temperature and 192 × g rpm for 5 min, the
supernatant was discarded and the cells were washed with PBS three
times. Then, 10 µl Annexin V-FITC was added and incubated at room
temperature for 30 min, followed by centrifugation at room
temperature and 192 × g for 5 min. Following the removal of the
supernatant, 10 µl PI (50 µg/ml) was added. Cell apoptosis rate was
determined using flow cytometry using experiments performed in
triplicate.
Western blotting
Cells were harvested and total protein extracts were
prepared using the Total Protein Extraction kit (Wuhan Boster
Biological Technology, Ltd., Wuhan, China) according to
manufacturer's protocol. Total proteins were separated using 12%
SDS-PAGE under 80–90 V and 250 mA for 30 min, and then transferred
to polyvinylidene difluoride membranes (Bio-Rad Laboratories,
Inc.). Then, the membranes were washed with PBS-Tween 20 twice (5
min/wash), and blocked with 1% bovine serum albumin at room
temperature (Sigma-Aldrich, Merck KGaA) for 1 h. Subsequently, the
membranes were incubated with caspase-3 (cat. no. BA2142), Fas
(cat. no. BA0048), FasL (cat. no. BA0049) and GAPDH (cat. no.
BA2913) primary antibodies (1:500/600; Wuhan Boster Biological
Technology, Ltd.) at 4°C overnight. After washing with PBS-Tween 20
twice (5 min/wash), the membranes were incubated with biotinylated
goat anti-rabbit IgG secondary antibody (cat. no. BA1003; 1:40/50;
Wuhan Boster Biological Technology, Ltd.) at 37°C for 30 min. After
washing with PBS-Tween 20 three times (5 min/wash), the membranes
were incubated with streptavidin-labeled horseradish peroxidase
(1:40/50; Wuhan Boster Biological Technology, Ltd.) for 30 min,
followed by washing with PBS-Tween 20 twice (5 min/wash). Then, the
membranes were stained with DAB (1:20; Wuhan Boster Biological
Technology, Ltd.).
Statistical analysis
The results were analyzed using SPSS software
(version 22.0; IBM Corp., Armonk, NY, USA). All data are expressed
as the mean ± standard deviation. Differences among groups were
compared using one-way analysis of variance. For pairwise
comparisons, the Fisher's least significant difference t-test was
employed. Differences with P<0.05 were considered statistically
significant.
Results
Inhibition of SGC-7901 cell
proliferation is enhanced with increasing drug concentrations and
duration of exposure
To determine the proliferation rates of SGC-7901
cells, MTT assay was employed. The data demonstrated that the
proliferation inhibition rate in the carbinol control group was not
significantly different compared with that in the control group
(P>0.05; Table I). In addition,
different lily extract alkaloid concentrations (0.05 g/l vs. 0 l
g/l; 0.075 g/l vs. 0.05 g/l; 0.1 g/l vs. 0.075 g/l; 0.125 g/l vs.
0.1 g/l and 0.15 g/l vs. 0.125 g/l) or different incubation
durations (48 h vs. 24 h and 72 h vs. 48 h) resulted in
significantly different proliferation inhibition rates compared
with the control group or among alkaloid experimental groups
(P<0.05; Table II). Similarly,
different carbinol extract concentrations (0.8 g/l vs. 0 g/l; 0.9
g/l vs. 0.8 g/l; 1.0 g/l vs. 0.9 g/l; 1.1 g/l vs. 1.0 g/l; 1.2 g/l
vs. 1.1 g/l; 1.3 g/l vs. 1.2 g/l; 1.4 g/l vs. 1.3 g/l; 1.5 g/l vs.
1.4 g/l and 1.6 g/l vs. 1.5 g/l) or different incubation durations
(48 h vs. 24 h and 72 h vs. 48 h) caused significantly different
proliferation inhibition rates compared with the control group or
among carbinol extract experimental groups (P<0.05; Table III). The results suggest that
inhibition of SGC-7901 cell proliferation is enhanced as drug
concentration or time elapsed increases.
 | Table I.Inhibition of SGC-7901 cell
proliferation by different concentrations of carbinol as determined
using an MTT assay. |
Table I.
Inhibition of SGC-7901 cell
proliferation by different concentrations of carbinol as determined
using an MTT assay.
|
| Absorbance |
|---|
|
|
|
|---|
| Groups | 24 h | 48 h | 72 h |
|---|
| 0% carbinol | 0.795±0.002 | 1.120±0.003 | 1.331±0.011 |
| 3.75% carbinol | 0.806±0.014 | 1.117±0.008 | 1.342±0.009 |
| 4.00% carbinol | 0.787±0.011 | 1.118±0.006 | 1.337±0.003 |
 | Table II.Inhibition of SGC-7901 cell
proliferation by different concentrations of alkaloid as determined
using an MTT assay. |
Table II.
Inhibition of SGC-7901 cell
proliferation by different concentrations of alkaloid as determined
using an MTT assay.
|
| Inhibition rate
(%) |
|
|
|---|
|
|
|
|
|
|---|
| Treatments (g/l) | 24 h | 48 h | 72 h | F | P |
|---|
| 0 | 0 | 0 | 0 |
|
|
| 0.05 |
21.50±0.85a,d |
28.27±1.87a,b,d |
37.13±3.89a,c,d | 28.60 | 0.00 |
| 0.075 |
31.87±0.83a,d |
40.63±2.19a,c,d |
49.30±1.72a,c,d | 81.28 | 0.00 |
| 0.1 |
40.90±0.87a,d |
50.13±1.57a,c,d |
58.10±1.52a,c,d | 122.24 | 0.00 |
| 0.125 |
51.33±1.46a,d |
65.60±0.58a,c,d |
80.07±1.59a,c,d | 372.07 | 0.00 |
| 0.15 |
65.30±1.23a,d |
74.06±1.21a,c,d |
91.93±1.05a,c,d | 409.35 | 0.00 |
| F | 1630.81 | 1033.15 | 803.49 |
|
|
| P | 0.00 | 0.00 | 0.00 |
|
|
 | Table III.Inhibition of SGC-7901 cell
proliferation by different concentrations of carbinol extracts as
determined using an MTT assay. |
Table III.
Inhibition of SGC-7901 cell
proliferation by different concentrations of carbinol extracts as
determined using an MTT assay.
|
| Inhibition rate
(%) |
|
|
|---|
|
|
|
|
|
|---|
| Treatments
(g/l) | 24 h | 48 h | 72 h | F | P |
|---|
| 0 | 0 | 0 | 0 |
|
|
| 0.8 |
5.53±0.28a,c |
14.58±0.69a–c |
23.39±2.15a–c | 139.29 | 0.00 |
| 0.9 |
19.62±0.35a,c |
30.34±0.76a–c |
37.23±1.89a–c | 165.88 | 0.00 |
| 1.0 |
27.26±0.44a,c |
38.73±1.02a–c |
46.76±1.27a–c | 304.07 | 0.00 |
| 1.1 |
35.85±1.08a,c |
45.84±0.77a–c |
53.31±3.90a–c | 40.64 | 0.00 |
| 1.2 |
43.23±0.36a,c |
52.32±0.74a–c |
58.39±2.15a–c | 98.80 | 0.00 |
| 1.3 |
50.29±0.55a,c |
58.31±0.63a–c |
66.47±1.28a–c | 250.83 | 0.00 |
| 1.4 |
59.36±0.79a,c |
69.32±0.69a–c |
82.69±1.60a–c | 338.58 | 0.00 |
| 1.5 |
69.39±0.95a,c |
86.15±0.28a–c |
96.74±2.21a–c | 290.09 | 0.00 |
| 1.6 |
76.33±0.69a,c |
96.74±1.53a–c |
98.32±1.40a | 284.07 | 0.00 |
| F | 5062.53 | 4219.32 | 729.73 |
|
|
| P | 0.00 | 0.00 | 0.00 |
|
|
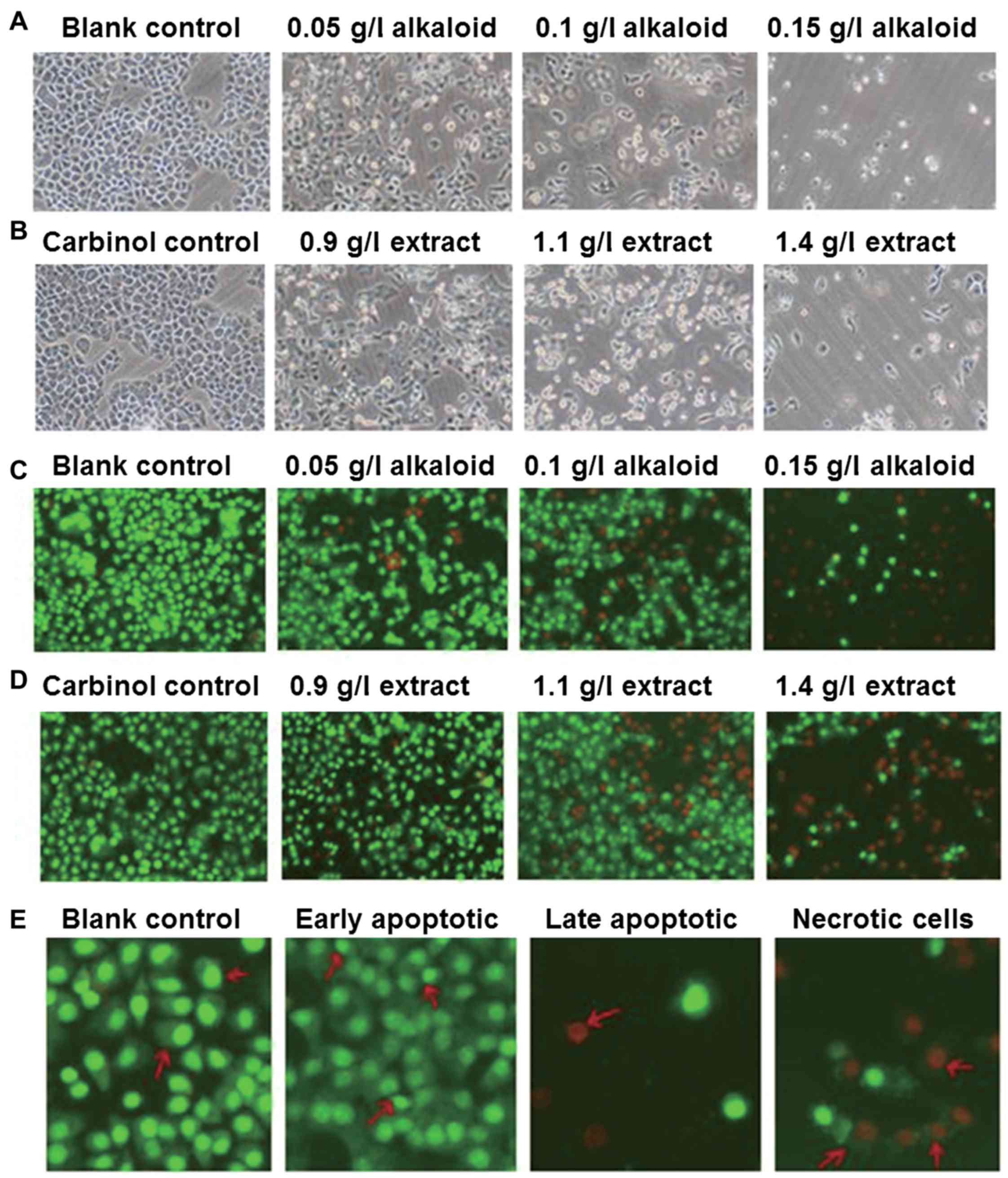
Treatment with alkaloid or carbinol
extract deteriorates the morphology of SGC-7901 cells in a
dose-dependent manner
To investigate how alkaloid or carbinol extracts
affect SGC-7901 cell morphology, the cells were observed under
inverted phase contrast and fluorescence microscopes. The images
demonstrate that SGC-7901 cells in the blank control group had
polygonal shapes and uniform sizes, with faster proliferation
compared with that in the experimental groups (Fig. 1A). In addition, the morphology of
SGC-7901 cells in the carbinol control group was not distinct
compared with that in the blank control group (Fig. 1B). After 48 h treatment with alkaloid
or carbinol extracts, SGC-7901 cells demonstrated slower growth,
deformed shapes and irregular cell edges, with the effect being
increased with higher concentrations of extract (Fig. 1A and B). Under the fluorescence
microscope, SGC-7901 cells in the blank control group revealed
uniformed sizes and even green fluorescence (Fig. 1C). The morphology of SGC-7901 cells in
the carbinol control group was not distinct compared with that in
the blank control group, also demonstrating even green fluorescence
(Fig. 1D). After 48 h treatment with
alkaloid or carbinol extracts, SGC-7901 cells demonstrated slower
growth, deformed shapes, and red fluorescence, with the effect
being increased with higher concentrations of extract (Fig. 1C and D). Furthermore, no apparent
apoptosis was observed in the blank control group, but early
apoptotic cells were observed in experimental groups, in which the
nuclei were shrunk into crescent or granular shapes, stained green,
and resided on one side of the cells. With increasing drug
concentrations, the numbers of early and late apoptotic cells were
increased, and the nuclei in the late apoptotic cells were shrunk,
stained red, and resided on one side of the cells. Furthermore, the
sizes of the necrotic cells were increased with vague edges, and
the cells were decomposed with red-stained nuclei (Fig. 1E). These results indicate that
treatment with alkaloid or carbinol extracts deteriorate the
morphology of SGC-7901 cells in a dose-dependent manner.
Treatment with alkaloid or carbinol
extracts arrests SGC-7901 cells in G2/M phase
Flow cytometry was performed in order to examine the
effects of alkaloid or carbinol extracts on the cell cycle
progression of SGC-7901 cells after 48 h treatment. Following
treatment with alkaloid or carbinol extracts for 48 h, SGC-7901
cells were arrested in the G2/M phase. With increasing
drug concentrations of either extract, cell cycle arrestment was
promoted, with the percentage of cells in the
G0/G1 phase being significantly reduced, and
that of cells in the G2/M phase being significantly
increased, compared with control groups (P<0.05; Tables IV and V). These results suggest that treatment with
alkaloid or carbinol extract arrests SGC-7901 cells in the
G2/M phase.
 | Table IV.Effect of different concentrations of
alkaloid on SGC-7901 cell cycle as determined using flow
cytometry. |
Table IV.
Effect of different concentrations of
alkaloid on SGC-7901 cell cycle as determined using flow
cytometry.
|
| Cell cycle phase
(%) |
|---|
|
|
|
|---|
| Treatments
(g/l) |
G0/G1 | S |
G2/M |
|---|
| 0 | 71.23±4.90 | 12.36±1.23 | 10.70±0.71 |
| 0.05 |
56.13±1.50a,b | 10.70±2.33 |
15.81±1.18a,b |
| 0.1 |
52.98±2.94a |
8.62±0.83a |
20.90±1.92a,b |
| 0.15 |
43.62±1.63a,b |
5.94±1.69a |
30.02±1.43a,b |
| F | 42.00 | 8.82 | 106.99 |
| P | 0.00 | 0.01 | 0.00 |
 | Table V.Effect of different concentrations of
carbinol extract on SGC-7901 cell cycle as determined using flow
cytometry. |
Table V.
Effect of different concentrations of
carbinol extract on SGC-7901 cell cycle as determined using flow
cytometry.
|
| Cell cycle phase
(%) |
|---|
|
|
|
|---|
| Treatments
(g/l) |
G0/G1 | S |
G2/M |
|---|
| 0 | 71.23±4.90 | 12.36±1.23 | 10.70±0.71 |
| 0.9 | 69.96±1.18 | 11.69±1.12 |
13.31±0.38a,b |
| 1.1 |
58.15±1.83a,b |
8.43±0.68a,b |
19.99±1.21a,b |
| 1.4 |
53.30±1.41a,b |
4.87±0.95a,b |
27.03±1.18a,b |
| F | 30.43 | 34.42 | 184.07 |
| P | 0.00 | 0.00 | 0.00 |
Treatment with alkaloid or carbinol
extracts induces apoptosis of SGC-7901 cells in a dose-dependent
manner
To examine the effect of alkaloid or carbinol
extract on SGC-7901 cell apoptosis, flow cytometry was performed in
combination with Annexin V-FITC/PI staining. Following treatment
with different concentrations of alkaloid or carbinol extract for
48 h, SGC-7901 cell apoptotic rates were enhanced with increasing
doses. The apoptotic rates of experimental groups were
significantly higher compared with that of the control groups
(P<0.05; Tables VI and VII). These results indicate that treatment
with alkaloid or carbinol extracts induces apoptosis of SGC-7901
cells in a dose-dependent manner.
 | Table VI.Effect of different concentrations of
alkaloid on SGC-7901 cell apoptotic rate as determined using flow
cytometry. |
Table VI.
Effect of different concentrations of
alkaloid on SGC-7901 cell apoptotic rate as determined using flow
cytometry.
| Treatments
(g/l) | Apoptotic rate
(%) |
|---|
| 0 | 1.57±0.64 |
| 0.05 |
6.54±0.48a,b |
| 0.1 |
11.14±2.00a,b |
| 0.15 |
20.35±1.50a,b |
| F | 111.18 |
| P | 0.00 |
 | Table VII.Effect of different concentrations of
carbinol extract on SGC-7901 cell apoptotic rate as determined
using flow cytometry. |
Table VII.
Effect of different concentrations of
carbinol extract on SGC-7901 cell apoptotic rate as determined
using flow cytometry.
| Treatments
(g/l) | Apoptotic rate
(%) |
|---|
| 0 | 1.57±0.64 |
| 0.9 |
5.36±0.62a,b |
| 1.1 |
11.78±1.13a,b |
| 1.4 |
20.32±1.21a,b |
| F | 227.22 |
| P | 0.00 |
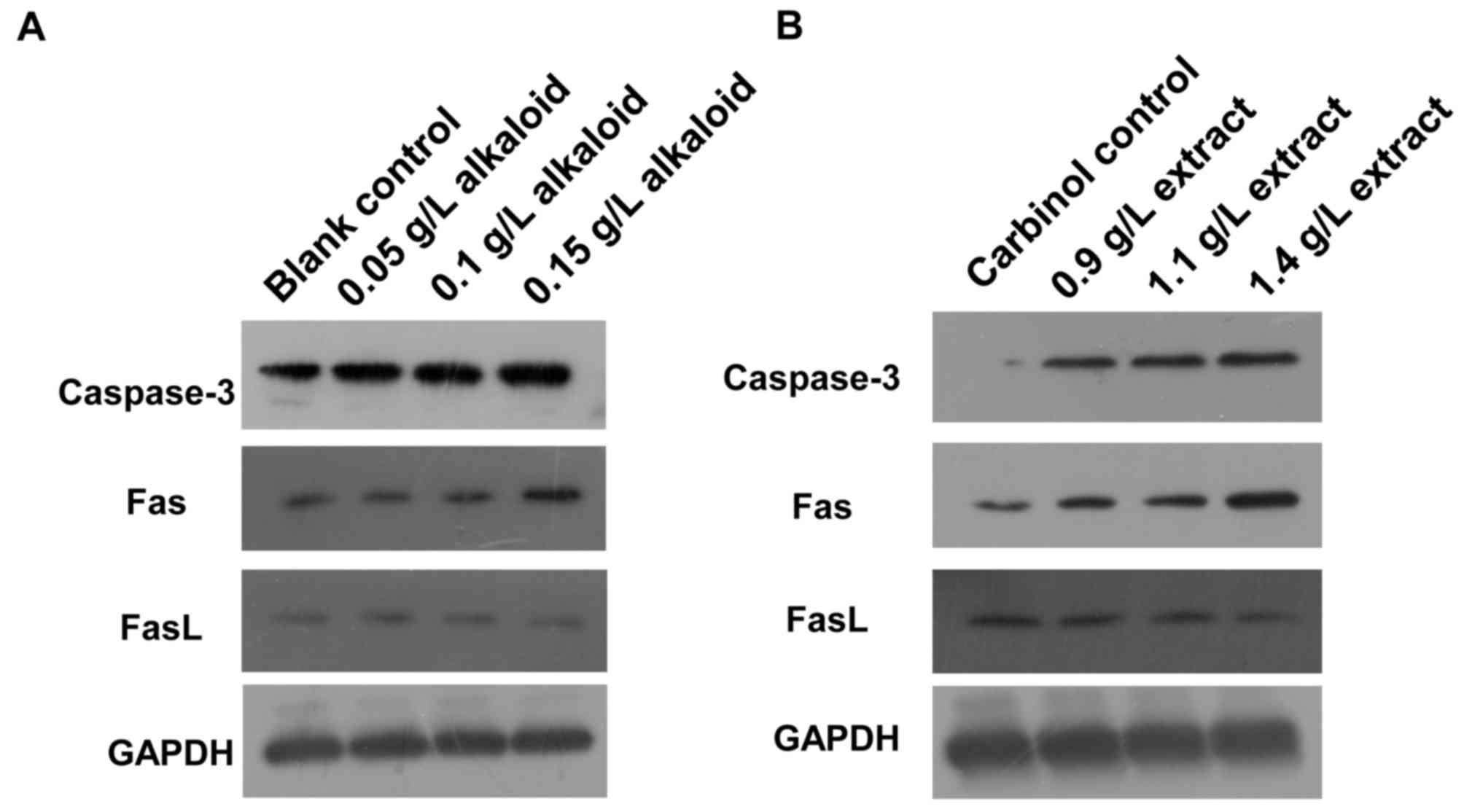
Treatment with alkaloid or carbinol
extracts enhances caspase-3 and Fas expression, but reduces FasL
expression
To determine how alkaloid or carbinol extract
affects caspase-3, Fas and FasL protein expression, western
blotting was used. The expression of caspase-3 and Fas proteins in
SGC-7901 cells of blank control group was low, but that of FasL
protein was high. After 48 h treatment with alkaloid or carbinol
extracts, the expression levels of caspase-3 and Fas proteins in
SGC-7901 cells were markedly enhanced with increasing drug
concentrations, but that of FasL protein was decreased (Fig. 2). The results suggest that treatment
with alkaloid or carbinol extracts enhances caspase-3 and Fas
expression, but reduces FasL expression.
Discussion
Apoptosis is the independent physiological
self-destructive death of cells that involves the activation,
expression and regulation of a series of genes. It is a type of
death phenomenon that adapts the organism to the environment.
Therefore, disordered apoptosis may directly or indirectly lead to
various diseases, including tumors (13). It is accepted that chemotherapy drugs
kill tumor cells directly by cytotoxicity, but it also now
understood that the primary mechanism of action of these drugs
involves the induction of apoptosis (14). It has been reported that certain
active components in lily are used for the treatment of different
cancer types, including lung cancer, lymphoma, esophageal cancer,
leukemia, skin cancer and cervical cancer. Hou et al
(15) have reported that lily
polysaccharides in combination with genistein inhibit the
proliferation of MCF-7 cells and arrest cells in the G2
phase. In the present study, treatment with alkaloid or carbinol
extracts inhibited the proliferation of SGC-7901 cells, with
increased doses and longer treatment durations exhibiting higher
inhibitory effects. In addition, the sizes of the cells were
decreased with shrunken nuclei. The cells were arrested in the
G2/M phase and apoptosis was induced. Of note, the MTT
assay results revealed that alkaloid and carbinol extracts also
exhibit direct killing effects on the cells.
Apoptosis is a gene expression cascade system
involving multiple pathways to induce apoptosis (16). There are three signal transduction
pathways that trigger apoptosis: The mitochondrial pathway,
external death receptor pathway and endoplasmic reticulum pathway
(17). Among the three pathways, Fas
and FasL serve important roles in the external death receptor
pathway (18). Fas and FasL belong to
tumor-necrosis factor/nerve-growth factor receptor family. The
cytoplasmic region of Fas has no catalytic activity prior to
binding with its ligand FasL, after which a trimer is formed to
induce the aggregation of the intracellular death domain. Following
binding of Fas-associated protein with the death domain, caspase
family cascade reactions are induced, leading to the degradation of
DNA fragments and apoptosis (19,20).
Caspase family members are involved in a protease system that leads
to the disintegration of apoptotic cells, serving a central role in
the large network of cell apoptotic mechanisms (21). It is considered that caspase-3 is the
most essential terminal cut enzyme in caspase family cascade
reactions and the deactivation or reduced expression of caspase-3
is correlated with the occurrence, and development of multiple
tumor types (22). Normally,
caspase-3 exists in an inactive zymogen form (23,24).
Following external stimulation by oxidant or ultraviolet, caspase-3
is released from the mitochondria into cytoplasm, and activated
(25). The activation of caspase-3
induces the irreversible stage of apoptosis (26). Following the activation of caspase-3,
its cascade reactions are aggravated, and a series of protein
substrates are degraded, leading to the deactivation of structural
and functional proteins, and finally, apoptosis.
It is considered that the expression levels of
caspase-3 or Fas proteins may indicate the malignancy and
development stage of tumors (27).
Reduced Fas expression inhibits the apoptosis of tumor cells, which
is induced via the Fas signaling pathway, and inhibited apoptosis
is considered to serve important roles in the occurrence and
development of tumors (28,29). FasL is the ligand of Fas that is
exclusively expressed on the surface of activated T cells. It has
been revealed that high expression of FasL exists on the surface of
numerous tumor cells, and leads to high expression of Fas on
tumor-invasive TL, inducing TL apoptosis and immune inhibition
(30). The results of the present
study demonstrated that SGC-7901 cells in the control group
exhibited low levels of caspase-3 and Fas expression, but high FasL
expression levels. In addition, alkaloid or carbinol extract
treatment enhanced the expression of caspase-3 and Fas, but reduced
the expression of FasL. These results suggest that alkaloid or
carbinol extract induces SGC-7901 cell apoptosis by upregulating
caspase-3 and Fas expression, and downregulating FasL expression.
Chinese herbal medicines have demonstrated certain advantages in
the treatment of tumors. As a type of Chinese herbal medicine, lily
has revealed specific effects in inhibiting tumor cell
proliferation and promoting tumor cell apoptosis. Further studies
on other active components of lily may also be beneficial for the
treatment of patients with tumors in the future.
Acknowledgements
The authors would like to thank Professor Yanlong
Zhang (Northwest A&F University, Xianyang, China) for his
technical support.
Funding
The present study was supported by the Yan'an
Science and Technology Project (grant no. 2013-KW03).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
AW and MW performed the experiments, analyzed the
data and wrote the manuscript. QP and LJ participated in the MTT
assay and flow cytometry analysis. JZ and MC helped with western
blot analysis and data collection. YZ conceived the idea, designed
the study and helped to draft the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Liu X, Chen Z, Zhao X, Huang M, Wang C,
Peng W, Yin J, Li J, He G, Li X and Zhu X: Effects of IGF2BP2,
KCNQ1 and GCKR polymorphisms on clinical outcome in metastatic
gastric cancer treated with EOF regimen. Pharmacogenomics.
16:959–970. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang M, Ren T and Xiao Z: Clinical
observation of paclitaxel combined with oxaliplatin in the
treatment of advanced gastric cancer. Chin J Coal Industry Med.
11:891–892. 2008.(In Chinese).
|
|
3
|
Zhong G and Song L: Status and progress of
chemotherapy for advanced gastric cancer. Clin Med J. 7:14–18.
2009.(In Chinese).
|
|
4
|
Cheng Y and Zhang L: Research progress of
anti-tumor mechanism and drug of Chinese herbal medicine. China
Pharmaceuticals. 22:103–104. 2013.(In Chinese).
|
|
5
|
Mahoney KM, Freeman GJ and McDermott DF:
The next immune-checkpoint inhibitors: PD-1/PD-L1 blockade in
melanoma. Clin Ther. 37:764–782. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Geillinger KE, Kipp AP, Schink K, Röder
PV, Spanier B and Daniel H: Nrf2 regulates the expression of the
peptide transporter PEPT1 in the human colon carcinoma cell line
Caco-2. Biochim Biophys Acta. 1840:1747–1754. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li M, Yu X, Guo H, Sun L, Wang A, Liu Q,
Wang X and Li J: Bufalin exerts antitumor effects by inducing cell
cycle arrest and triggering apoptosis in pancreatic cancer cells.
Tumour Biol. 35:2461–2471. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang H, Liu J and Dou QP: Targeting tumor
proteasome with traditional Chinese medicine. Curr Drug Discov
Technol. 7:46–53. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ma H, Cheng L, Hao K, Li Y, Song X, Zhou H
and Jia L: Reversal effect of ST6GAL 1 on multidrug resistance in
human leukemia by regulating the PI3K/Akt pathway and the
expression of P-gp and MRP1. PLoS One. 9:e851132014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dai ZJ, Tang W, Lu WF, Gao J, Kang HF, Ma
XB, Min WL, Wang XJ and Wu WY: Antiproliferative and apoptotic
effects of β-elemene on human hepatoma HepG2 cells. Cancer Cell
Int. 13:272013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yu H, Yao L, Zhou H, Qu S, Zeng X, Zhou D,
Zhou Y, Li X and Liu Z: Neuroprotection against Aβ25-35-induced
apoptosis by Salvia miltiorrhiza extract in SH-SY5Y cells.
Neurochem Int. 75:89–95. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang J, Wang J, Jiang JY, Liu SD, Fu K
and Liu HY: Tanshinone IIA induces cytochrome c-mediated caspase
cascade apoptosis in A549 human lung cancer cells via the JNK
pathway. Int J Oncol. 45:683–690. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yue Y and Zhang Y and Zhang Y: Caspase
family and apoptosis. China Healthcare Innovation. 6:25–26.
2011.(In Chinese).
|
|
14
|
Ma T: Progress of chemotherapy and
apoptosis in gastric cancer. Foreign Med Sci (Digestive Dis Sect).
23:15–18. 2003.
|
|
15
|
Hou J, Li F and Li X: Effect of Lily
polysaccharides with genistein on the proliferation of human breast
cancer cells. J Modern Oncol. 23:12–14. 2015.
|
|
16
|
Tang Z, Wen N and Zhang Y: Expression of
Caspase-3 in the apoptosis of human glioma cells in nude mice
induced by Fructus Schisandrae polysaccharide. 11:6126–6127.
2014.
|
|
17
|
Aryal P, Kim K, Park PH, Ham S, Cho J and
Song K: Baicalein induces autophagic cell death through AMPK/ULK1
activation and downregulation of mTORC1 complex components in human
cancer cells. FEBS J. 281:4644–4658. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen L, Park SM, Tumanov AV, Hau A, Sawada
K, Feig C, Turner JR, Fu YX, Romero IL, Lengyel E and Peter ME:
CD95 promotes tumour growth. Nature. 465:492–496. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tang W, Liu Q, Wang X, Wang P, Cao B, Mi N
and Zhang J: Involvement of caspase 8 in apoptosis induced by
ultrasound-activated hematoporphyrin in sarcoma 180 cells in vitro.
J Ultrasound Med. 27:645–656. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Matsuda N, Takano Y, Kageyama S,
Hatakeyama N, Shakunaga K, Kitajima I, Yamazaki M and Hattori Y:
Silencing of caspase-8 and caspase-3 by RNA interference prevents
vascular endothelial cell injury in mice with endotoxic shock.
Cardiovasc Res. 76:132–140. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gong J, Chen Z and Li W: Fas-mediated
apoptosis and Caspase family. Foreign Med Sci (Cancer Sect).
27:279–280. 2001.
|
|
22
|
Meggiato T, Calabrese F, de Cesare CM,
Baliello E, Valente M and Del Favero G: C-JUN and CPP32 (CASPASE 3)
in human pancreatic cancer: Relation to cell proliferation and
death. Pancreas. 26:65–70. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Saikumar P, Dong Z, Mikhailov V, Denton M,
Weinberg JM and Venkatachalam MA: Apoptosis: Definition,
mechanisms, and relevance to disease. Am J Med. 107:489–506. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Depraetere V and Golstein P: Dismantling
in cell death: Molecular mechanisms and relationship to caspase
activation. Scand J Immunol. 47:523–531. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xiao XL, Peng J, Su Q, Xiang SL, Tang GH,
Huang YS and Zhou XT: Diallyl trisulfide induces apoptosis of human
gastric cancer cell line MGC803 through caspase-3 pathway. Ai
Zheng. 25:1247–1251. 2006.(In Chinese). PubMed/NCBI
|
|
26
|
Cryns V and Yuan J: Proteases to die for.
Genes Dev. 12:1551–1570. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yao F, Chen Q, Tao L and Hui LI:
Expression of caspase-3 in lung cancer: Association with bcl-2 and
Bax. J Qilu Oncol. 2004, (In Chinese).
|
|
28
|
Jansson A, Arbman G and Sun XF: mRNA and
protein expression of PUMA in sporadic colorectal cancer. Oncol
Rep. 12:1245–1249. 2004.PubMed/NCBI
|
|
29
|
Tong J, Xie G, He J, Li J, Pan F and Liang
H: Synergistic antitumor effect of dichloroacetate in combination
with 5-fluorouracil in colorectal cancer. J Biomed Biotechnol.
2011:7405642011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang B, Zhang X, Xiao Y and Song Y: The
relation between Fas/FasL and tumors of digestive tract. J
Microbiol. 23:56. 2003.
|