Introduction
Intracranial schwannoma accounts for between 5 and
8% of intracranial tumors, whereas intracerebral schwannoma, a rare
disease, accounts for <1% of intracranial schwannomas (1). Intracerebral schwannoma cannot be
clearly diagnosed with preoperative clinical manifestations and/or
results generated from imaging techniques. It is clearly diagnosed
on the basis of postoperative pathology (1–4). However,
no previous study, to the best of our knowledge has systematically
summarized the disease characteristics, including the incidence of
patients, the onset age, tumor location, clinical manifestation and
prognosis. The present study not only had complied this case
report, but also summarized the characteristics of intracerebral
schwannoma, which will further deepen the knowledge and
understanding presently available of this disease. In additional to
the present case report, 84 cases reported within China and
elsewhere were summarized, and the age of the tumor onset, site of
disease, imaging results, clinical symptoms, pathological
classification and prognosis were reviewed and analyzed.
Characteristics of intracerebral schwannoma were summarized as
follows: Incidence of intracerebral schwannoma was low among cases,
the majority of cases had supratentorial tumors and the patients
were of an age ≤40, <33% of tumors were subtentorial and onset
was demonstrated in cases of an age >40; correct diagnosis was
not typically achieved preoperatively and the majority of cases
were definitively diagnosed on the basis of postoperative
pathological analysis. Furthermore, for patients whose normal brain
tissue was minimally affected by the tumor, if surgery was able to
completely resect the lesion and the prognosis was good.
Case report
The present case report was carried out in
accordance with the code of ethics outlined by the World Medical
Association (The Declaration of Helsinki) for experiments involving
humans. Informed consent was obtained for all experiments regarding
human subjects.
In the present case report, written informed consent
was obtained from the parents due to the age of the patient (12
years). The patient was admitted to the Department of Neurosurgery,
The China Japan Union Hospital of Jilin University (Jilin, China).
The female patient was of Han nationality and did not have a family
history of this particular genetic disease. Symptoms upon
presentation included dizziness (lasting for 1 year before
examination), headache, nausea, vomiting and require assistance
with walking. Physical examination demonstrated that binocular
vision was normal, bilateral pupils were of the same size, eyes
were sensitive to light reflection and eyeballs moved freely in all
directions. Furthermore, facial sensation was normal, binocular
horizontal nystagmus was present and the reflex of bilateral
corneas was slow. Muscle tension of the four limbs was normal and
the finger-nose test of the left side was positive with no
pathological reflex detected. Preoperative magnetic resonance
imaging (MRI) of the head demonstrated an abnormal cystic signal in
the cerebellar vermis, and parenchyma parts presented nodular
isointensity shadows above the cystic wall; additionally, there was
no obstructive dilatation in bilateral lateral ventricles or the
third ventricle. Preoperatively, the patient was diagnosed with
glioma in the cerebellar vermis, and therefore a cerebellar
hemisphere tumor resection from the median suboccipital approach
was organized for the patient. As a brainstem parenchymal tumor was
not considered, and there were no obvious hydrocephalus symptoms
prior to the operation, no neurophysiological monitoring or
ventricular drainage was performed. However, during surgery, the
tumor was revealed to have originated from the brainstem parenchyma
(Fig. 1). Further analysis of the
preoperative MRI film confirmed results reported during
surgery.
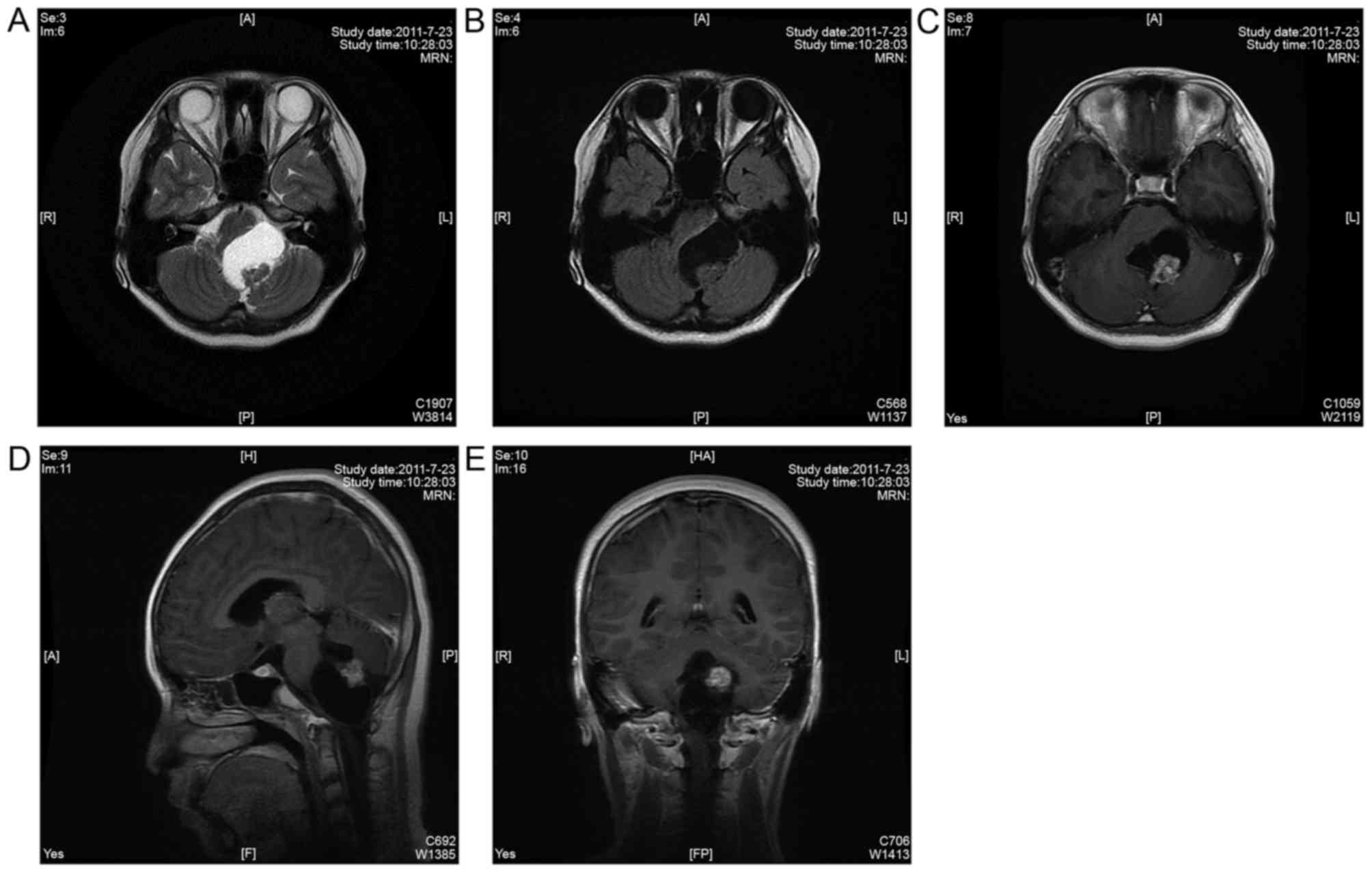 | Figure 1.(A) MRI T2 sequence and (B) MRI T1
sequence demonstrated that a cystic abnormal signal was observed in
the brainstem parenchyma, and parenchyma parts exhibited nodular
isointensity shadows above the cystic wall. (C) MRI T1-weighted
axial enhanced sequence, (D) MRI T1- weighted sagittal enhanced
sequence, (E) MRI T1-weighted coronary enhanced sequence
demonstrated that nodular lesions were mainly isointensity shadows,
and the mass effect of the lesion was significant. Additionally,
lesions were heterogeneously enhanced following injection of
contrast material. A, anterior; P, posterior; R, right; L, left; H,
head; F, feet; HA, head anterior; FP, foot posterior; MRN, magnetic
resonance neurography |
The origin of intracerebral schwannoma and
intracranial schwannoma display differences in their pathogenic
sites; however, in patient's MRIs no differences were detected. The
fourth ventricle was deformed due to tumor oppression. The tumor
surface consisted of a layer of parenchymal tissue of the
brainstem; 10 ml light yellow liquid was extracted by puncturing
the cyst while the dorsal brainstem was collapsed. The left side of
the cerebellar tonsils was stripped to locate the parenchymal tumor
tissue above the cyst wall. The tumor tissue was revealed to be
rich in blood supply, hard in texture and gray-red with
calcification inside and a clear border, and the resected solid
tumor was 1.5×1.5×1.5 cm in size. Notably, the cyst wall was
excised completely and the patient did not develop transient
post-surgery hydrocephalus. MRI of the head was reviewed 10 days
after surgery and demonstrated that the tumor was completely
resected and the brainstem was restored to its original position
(Fig. 2). Results obtained from
pathological analysis demonstrated that the patient had a
schwannoma within the brainstem.
 | Figure 2.(A) MRI T2-weighted axial sequence,
(B) MRI T1-weighted axial sequence, (C) MRI T1-weighted sagittal
sequence, (D) MRI T1-weighted coronary sequence reviewed on the
tenth day following surgery demonstrating that the tumor was
completely resected, and the brainstem was back in the original
position. MRI, magnetic resonance imaging. A, anterior; P,
posterior; R, right; L, left; H, head; F, feet; MRN, magnetic
resonance neurography. |
For immunohistochemistry (IHC), tissue sections were
treated with 2% H2O2 in methanol for 1 h at
120°C to inactivate endogenous peroxidase. The sections were washed
twice with PBS and then incubated with blocking serum (MXB
Biotechnologies, Fuzhou, China; http://www.maxim.com.cn/) for 1 h. The sections were
then incubated with cytokeratin 20 (cat. no. Ks20.8),
carcinoembryonic antigen (cat. no. ZC23) and cell proliferation
factor Ki67 (MIB-1) pre-diluted primary antibodies (all purchased
from MXB Biotechnologies) for 1 h at 37°C. Then sections were then
washed with PBS and incubated with a biotinylated goat anti-rabbit
secondary antibody (ready to use dilution; Kit-0014; MXB
Biotechnologies) for 1 h at 37°C. Sections were then treated with
ABC solution (MXB Biotechnologies) for 1 h at 23°C, washed with
PBS, and incubated with DAB (MXB Biotechnologies) for 10 min at
23°C. The IHC results were observed using a light microscope
(magnification, ×100) and demonstrated that S-100 (+), epithelial
membrane antigen (EMA; -), glial fibrillary acidic protein (GFAP;
-), Ki-67 (1% +), epidermal growth factor receptor (−), cluster of
differentiation (CD)34 (vascular +), CD31 (vascular +) and vascular
endothelial growth factor (−; Fig.
3). Follow-up of the patient was carried out 6 months after
surgery and revealed that the recovery of the patient was good,
binocular horizontal nystagmus was significantly relieved compared
with that prior to the surgery, bilateral corneal reflexes were
present, outreach activity barriers existed in the left eye, the
patient had no cough when drinking water, speech was smooth and
limb activity was free. The 5-year follow-up demonstrated no
residual tumor or recurrence, whereas the neurological dysfunction
of the patient was completely improved with no dysfunction
(Fig. 4).
Discussion
Demographics and prevalence
Intracranial schwannoma accounts for between 5 and
8% of intracranial tumors, whereas intracerebral schwannoma, a rare
disease, accounts for <1% of intracranial schwannoma. A total of
84 cases previously reported within China and elsewhere were
reviewed and summarized (Table I)
(1–56). Gibson et al (5) reported a case of a 6-year-old male with
a schwannoma in the temporal lobe that was surgically resected
successfully for the first time in 1966. Erongun et al
(33) summarized 35 cases (including
the 1996 case), the youngest patient was 4 years old, whereas the
oldest patient was 63 years old, with a median age of 21 years; the
number of males and females was 18 and 17, respectively.
Furthermore, brain schwannomas were predominantly identified in
children and young people, including 26 patients <30 years old,
accounting for 74.3% of total cases. Andrade et al (37) summarized and analyzed 55 cases
reported in 2002, including 31 males and 24 females; the proportion
of men was slightly higher at a male/female ratio of 1.29:1.
Additionally, the median age was 21 years, and the age demonstrated
a bimodal distribution, with 40 cases <30 years, accounting for
72.7% of total cases. The median age of the 36 cases with
supratentorial tumors was 18 years, with a male/female ratio of
1.31:1, whereas the median age of patients with subtentorial tumors
was 45 years, with a male/female ratio of 1.25:1. Patients with
supratentorial intracerebral schwannomas were typically younger,
whereas patients with subtentorial intracerebral schwannomas were
older; however, no significant difference was identified between
the incidence in men and women irrespective of supratentorial or
subtentorial intracerebral schwannoma. A total of 84 cases reported
within China and elsewhere were reviewed and analyzed, including 47
males and 37 females with a male/female ratio of 1.27:1.
Furthermore, the youngest case was 6 months old, whereas the oldest
case was 84 years old, with a median age of 23 years. Notably, the
age demonstrated a bimodal distribution, including 55 cases <30
years, accounting for 65.5% of total cases. Additionally, the
median age of the 61 cases with supratentorial intracerebral
schwannomas was 21 years; however, the median age of patients with
subtentorial intracerebral schwannomas was 48 years (Fig. 5). Therefore, this suggests that
intracerebral schwannoma occurred at no specific age and
demonstrated bimodality, with a peak at ~20 years; however, the age
of the majority of patients was <30 years. Furthermore, the
proportion of males was higher compared with females, which is
consistent with the characteristics of intracranial tumors.
Additionally, the incidence of supratentorial intracerebral
schwannoma was increased at a younger age (median of 21 years),
whereas the incidence of subtentorial intracerebral schwannoma was
increased at an older age (median of 48 years). However, the
present case report describes a 12-year-old female who was
diagnosed with a subtentorial schwannoma in the brainstem. To the
best of our knowledge, this present case report is the second to
diagnose the disease in a patient ≤12 years of age.
 | Table I.Data for 84 cases obtained from
previous studies. |
Table I.
Data for 84 cases obtained from
previous studies.
| Author(s) | Year of
diagnosis | Age, years | Sex | Brain location | Site | (Refs.) |
|---|
| Gibson et
al, 1966 | 1966 | 6 | M | Supratentorial | Temporal | (5) |
| New, 1972 | 1972 | 8 | M |
| Parietal | (6) |
| Ghatak et
al, 1975 | 1975 | 63 | F |
| Parietal | (7) |
| Pialat et
al, 1975 | 1975 | 24 | F |
| Frontal | (8) |
| Rensburg et
al, 1975 | 1975 | 21 | M |
| Temporal | (9) |
| Hockley and
Hendrick, 1975 | 1975 | 11 | M |
| Temporal | (10) |
| Hahn and Netsky,
1977 | 1977 | 26 | M |
| Frontal | (11) |
| Russel and
Rubinstein, 1989 | 1977 | 17 | M |
| Frontal | (1) |
|
| 1977 | 17 | F |
| Frontal |
|
| Kasantikul et
al, 1981 | 1981 | 21 | M |
| Temporal | (12) |
|
| 1981 | 23 | M |
| Parietal |
|
| Auer et al,
1982 | 1982 | 15 | M |
| Frontal | (13) |
| Shalit et
al, 1982 | 1982 | 29 | F |
|
Parieto-occipital | (14) |
| Gokay et al,
1984 | 1984 | 16 | F |
|
Fronto-parietal | (15) |
| Rodriguez-Salazar
et al, 1984 | 1984 | 10 | F |
| Frontal | (23) |
| Bruni et al,
1984 | 1984 | 39 | M |
| Frontal | (16) |
| Bruner et
al, 1984 | 1984 | 18 | M |
| Frontal | (24) |
| Deng and Wu,
1985 | 1985 | 53 | F |
| Parietal | (25) |
| Stefanko et
al, 1986 | 1986 | 15 | M |
|
Parieto-occipital | (17) |
| Schwartz and
Sotrel, 1988 | 1988 | 20 | F |
|
Fronto-parietal | (18) |
| Ezura et al,
1992 | 1992 | 13 | F |
| Frontal | (19) |
| Ghosh and Chandy,
1992 | 1992 | 27 | M |
| Frontal | (20) |
| Frim et al,
1992 | 1992 | 11 | F |
| Temporal | (21) |
| Casadei et
al, 1993 | 1993 | 16 | M |
| Temporal | (2) |
|
| 1993 | 17 | M |
| Temporal |
|
|
| 1993 | 21 | M |
| Parietal |
|
|
| 1993 | 23 | F |
| Temporal |
|
|
| 1993 | 49 | F |
| Temporal |
|
|
| 1993 | 84 | F |
| Temporal |
|
| Di Biasi et
al, 1994 | 1994 | 19 | M |
| Parietal | (3) |
| Zhao et al,
1994 | 1994 | 38 | M |
| Occipital | (32) |
| Sharma et
al, 1996 | 1996 | 19 | F |
| Occipital | (34) |
|
| 1996 | 8 | M |
| Temporal |
|
|
| 1996 | 0.5 | F |
| Temporal |
|
|
| 1996 | 21 | M |
| Frontal |
|
| Erongun et
al, 1996 | 1996 | 4 | F |
|
Parieto-occipital | (33) |
| Tsuiki et
al, 1997 | 1997 | 17 | M |
| Frontal | (35) |
|
| 1997 | 21 | M |
| Frontal |
|
| Wang et al,
2000 | 2000 | 45 | M |
| Frontal | (36) |
|
| 2000 | 34 | M |
| Frontal |
|
|
| 2000 | 12 | F |
| Rear upper part of
the optic chiasm |
|
|
| 2000 | 10 | M |
| Parietal |
|
| Andrade et
al, 2002 | 2002 | 17 | M |
| Hypothalamus | (37) |
| Xu and Wang,
2002 | 2002 | 61 | F |
| Occipital | (38) |
| Zhong et al,
2004 | 2004 | 26 | M |
| Frontal | (40) |
| Celikoglu et
al, 2007 | 2007 | 23 | F |
| Falx, right
parasagittal parietal | (44) |
| Xu et al,
2007 | 2007 | 31 | M |
| Temporal | (45) |
|
| 2007 | 17 | F |
| Frontal |
|
|
| 2007 | 22 | F |
| Temporal |
|
| Yi et al,
2008 | 2008 | 43 | F |
| Frontal | (46) |
| Ishihara et
al, 2009 | 2009 | 5 | M |
| Occipital | (47) |
| Zhu et al,
2010 | 2010 | 30 | M |
| Frontal | (49) |
| Consales et
al, 2010 | 2010 | 7 | M |
|
Parieto-occipital | (50) |
| Paredes et
al, 2012 | 2011 | 19 | M |
| Occipital | (4) |
|
| 2011 | 32 | M |
| Occipital |
|
| Guha et al,
2012 | 2012 | 51 | F |
| Temporal | (51) |
| Srinvias et
al, 2013 | 2013 | 16 | F |
|
Fronto-parietal | (52) |
| Lee et al,
2013 | 2013 | 25 | M |
| Frontal | (53) |
| Ma et al,
2013 | 2013 | 24 | F |
| Frontal | (54) |
| AlBatly et
al, 2014 | 2014 | 49 | F |
| Temporal | (55) |
| Wilson et
al, 2016 | 2016 | 34 | M |
| Temporal | (56) |
| Sarkar et
al, 1987 | 1987 | 24 | M | Subtentorial | Cerebellum | (22) |
| Aryanpur and Long
et al, 1988 | 1988 | 50 | F |
| Medulla
oblongata | (26) |
| Schwartz and
Sotrel, 1988 | 1988 | 48 | M |
| Cerebellum | (18) |
| Ladouceur et
al, 1989 | 1989 | 46 | F |
| Brainstem | (27) |
| Redekop et
al, 1990 | 1990 | 7 | M |
| Fourth
ventricle | (28) |
| Tran-Dinh et
al, 1991 | 1991 | 64 | F |
| Cerebellum | (29) |
| Casadei et
al, 1993 | 1993 | 52 | F |
| Cerebellum | (2) |
|
| 1993 | 55 | M |
| Cerebellum |
|
|
| 1993 | 79 | F |
| Cerebellar
vermis |
|
| Sharma et
al, 1993 | 1993 | 73 | M |
| Cerebellum | (30) |
| Sharma and Newton,
1993 | 1993 | 73 | F |
| Cerebellum | (31) |
| Sharma et
al, 1996 | 1996 | 14 | M |
| Brainstem | (34) |
|
| 1996 | 14 | M |
| Pontine |
|
|
| 1996 | 45 | M |
| Cerebellum |
|
|
| 1996 | 24 | M |
| Cerebellum |
|
| Tsuiki et
al, 1997 | 1997 | 64 | F |
| Cerebellum | (35) |
| Wang et al,
2000 | 2000 | 16 | F |
| Brainstem | (36) |
|
| 2000 | 49 | F |
| Cerebellum |
|
| Lin et al,
2003 | 2003 | 48 | M |
| Medulla
oblongata | (39) |
| Maiuri et
al, 2004 | 2004 | 29 | F |
| Cerebellar | (42) |
| Zeng et al,
2005 | 2005 | 15 | M |
| Medulla
oblongata | (41) |
| Muzzafar et
al, 2010 | 2010 | 68 | M |
| Brainstem | (48) |
| Present case
report | 2016 | 12 | F |
| Medulla
oblongata |
|
Pathogenic site
The majority of intracerebral schwannomas are
supratentorial, which typically occur in the superficial sections
of the brain parenchyma or near the ventricle; however, previous
studies have demonstrated that these are likely to occur in the
frontal and temporal lobes of the cerebral hemisphere, as well as
within the cerebellar hemisphere, cerebellar vermis and the fourth
ventricle (4,39). Erongun et al (33) summarized that 27/35 cases (77.1%) with
supratentorial intracerebral schwannomas occurred at a younger age
with a distribution of 9 cases in the frontal lobe, 5 cases in the
parietal lobe, 5 cases in the temporal lobe, 3 cases in the top
occipital lobe, 1 case in the frontotemporal lobe and 1 case in the
cerebral hemisphere. Andrade et al (37) demonstrated that 36/55 cases (65.45%)
had supratentorial intracerebral schwannomas, including 13 cases in
the frontal lobe, 12 cases in the temporal lobe, 9 cases in the
parietal lobe, 1 case in the occipital lobe and 1 case in the
cerebral hemisphere. Furthermore, of the 19 cases with subtentorial
intracerebral schwannomas, 6 were located in the brainstem, and 13
were in the cerebellar hemisphere and vermis. In addition, Consales
et al (50) provided further
evidence for the distribution of intracerebral schwannoma and
summarized 12 cases in 2010, including 3 cases in the frontal lobe,
4 cases in the temporal lobe, 1 case in the parietal lobe, 2 cases
in the cerebellum, 1 case in the brainstem and 1 case in the
hypothalamus. In total, 84 cases (Fig.
6) were summarized, including 61 cases of supratentorial
intracerebral schwannomas (72.6%), of which 24 were in the frontal
lobe, 17 were in the temporal lobe, 12 were in the parietal lobe, 6
were in the occipital lobe and 2 were in the hypothalamus.
Furthermore, 15 cases with subtentorial intracerebral schwannomas
were in the cerebellar hemisphere and 8 cases were in the
brainstem. Therefore, it can be concluded that intracerebral
schwannomas are primarily supratentorial, typically located in the
frontotemporal lobe, whereas subtentorial intracerebral schwannoma
accounts for <33% of the total intracerebral schwannomas, of
which schwannomas within the brainstem exhibit an increased
incidence. It is inferred that this part is close to the cranial
nerves of the brainstem, which may be associated with the origin of
intracerebral schwannoma.
Clinical manifestations
Intracerebral schwannoma has no specific clinical
manifestation, and it is not classified by age. Its main clinical
manifestations include epilepsy, increased intracranial pressure
and local neurological dysfunction. Clinical manifestations in 84
patients were summarized and analyzed. The primary manifestations
for patients <25 years old were headaches and epilepsy; however,
elderly patients primarily suffered from acute local neurological
dysfunction.
Imaging analysis
Results obtaining from imaging analysis demonstrated
that characteristics of intracerebral schwannomas were similar to
those of normal intracranial schwannomas. A typical imaging feature
(57) of intracranial schwannomas was
cystic masses with a clear boundary. Tumors were primarily located
in the superficial part of the brain parenchyma, or the brain
parenchyma near the ventricle with calcification. Additionally,
edema was observed in the surrounding brain tissue, and the solid
part of the tumor was homogeneously enhanced. However, not all
intracranial schwannomas presented with cystic degeneration. It has
been previously reported that 25.7% of tumors presented with cystic
degeneration; however, calcification was rare (33). Results obtained from imaging analysis
of the 12-year-old female included cystic mass lesions in the
brainstem parenchyma, in which MRI T1 sequence and MRI T2 sequence
mixed signal solid signal shadows were observed on the posterior
capsule wall, and long T1 and short T2 signal shadows were observed
in the tumor parenchyma. A combination of the aforementioned
imaging results with high-density head computer tomography results
demonstrated calcified shadow, deformation of the brainstem and
cerebellar tissues around the tumor due to compression and mild
edema. Parenchyma was strengthened following enhancement. Thus, no
significant difference was reported between intracerebral
schwannoma and ordinary schwannoma in MRI; however, there was a
difference in the pathogenic site of origin (57).
Origin
The origin of the intracerebral nerve sheath remains
unclear. It is well-documented that Schwann cells do not exist in
the brain parenchyma; they are primarily in the choroidal tissues
of the subarachnoid space, and the intracranial peripheral vascular
plexus of the ventricle. Certain researchers believe that specific
soft membrane cells have the potential to transform into Schwann
cells, which are the origin of intracerebral schwannomas (58). Although the development of
intracerebral schwannomas remains unclear, the majority of
researchers claim that intracerebral schwannomas originate from the
subarachnoid space and peripheral venous plexus (59). Nerve fibers of the trigeminal nerve
that dominate the dura mater are considered to be the origin of
intracerebral schwannomas near to the dural convexity; however,
certain intracerebral schwannomas are located deep in the brain
parenchyma and peripheral vascular plexus of the cerebral arteries.
This is because the majority of intracerebral schwannomas are
located in superficial sections of the brain surface or near the
ventricle adjacent to the brain surface. Therefore, Schwann cells
in the peripheral venous plexus of the subarachnoid space may
proliferate inward and further transform into intracerebral
schwannomas (60,61). Several previous studies have
investigated molecular markers and confirmed that Schwann cells
originate from neural crest cells. SRY-related HMG-box 10 (SOX10)
exists throughout the maturation process, activator protein 2α
(AP2α) exists in the precursor stage prior to neural crests
transforming into Schwann cells and cadherin-19 exists only in the
precursor stage of Schwann cells. Brain fatty acid-binding protein
and P0 markers exist in the precursor stage and later stages of
Schwann cell maturation. GFAP and S-100 are expressed in the
immature stage of Schwann cells but not in Schwann cell precursors.
Early developmental markers including paired box (PAX)3 and PAX7
are often associated with malignancy, whereas AP2α and markers of
late SOX10 are present in relatively benign tumors including
schwannomas and gangliomas. Notably, PAX3 and PAX7 are present in
the mesoderm, whereas AP2α and SOX10 are expressed in the ectoderm.
However, IHC staining of intracerebral schwannomas revealed S-100
(+), GFAP (−) and EMA (−). Neural crest cells directly
differentiate into Schwann cell precursors that further transform
into immature Schwann cells, which at the stage of fetal birth
differentiate into medulla or demyelinated Schwann cells (60–63).
Therefore, it has been suggested in previous studies that neural
crest cells trapped in the brain at the stage of embryonic
development are the cause of the occurrence of intracerebral
schwannomas (64). Russel and
Rubinstein (1) considered that
mesenchymal cells with a soft membrane and Schwann cells exhibit
tissue similarity, and the histological transformation may lead to
schwannomas of the central nervous system.
Pathology
Intracranial schwannoma accounts for ~8% of
intracranial tumors and is not age-specific; however, previous
results suggest a peak incidence at an age between 40 and 60 years,
without sex orientation in the incidence rate. However,
intracerebral schwannoma is a rare disease and accounts for <1%
of intracranial schwannomas. In addition, the number of males is
greater compared with the number of females, which may be due to
the fact that, overall, more male patients are diagnosed with
intracranial tumors compared with females. Typical schwannomas have
two types of tissue conformations: The dense Antoni A and the loose
Antoni B (65). Histopathological
analysis of the 12-year-old case revealed a staggered arrangement
of sparse and dense areas of the Schwann cell, which was associated
with local focal calcification. Tumor cells were oval with small
nuclei; however, the mitotic figure was difficult to observe. IHC
staining demonstrated that S-100 was expressed in tumor cells,
negative expression of EMA and GFAP was able to distinguish
meningiomas and gliomas, and the proliferation index of Ki-67 was
not increased (1% of positive expression) indicating that tumor
cell proliferation was slow.
Prognosis
The prognosis for the majority of cases reviewed in
the present case report was classified as being good; however, 3
cases (23,33,42)
demonstrated a markedly high level of malignancy (spread to other
organs) and Rodriguez et al (23) reported 1 case of moderate malignancy
(intracranial relapse), and, despite reoccurrence of the tumor, the
prognosis was good following a second round of surgery. Bruner
(24) reported a patient who
succumbed 5 months after surgery due to postoperative secondary
meningitis. Erongun et al (33) reported a 4-year-old female who
presented with a tumor in the left parietal lobe and a high level
of malignancy, as demonstrated using postoperative pathological
analysis. Furthermore, the patient received surgery three times
within an 8-month period and died from complications generated from
having multiple surgeries 1 month after the last operation. The
5-year follow-up on the 12-year-old female revealed no reoccurrence
of the tumor and also the neurological dysfunction of the patient
had improved.
Conclusion
The incidence of intracerebral subtentorial
schwannoma is low, with the majority of cases being supratentorial
and primarily occurring at a young age. In total, 33% of patients
exhibit subtentorial schwannoma, occurring predominantly at an
older age; however, the 12-year-old female described in the present
case report exhibited a very rare condition: Subtentorial
schwannoma located in the brainstem. Usually, a correct diagnosis
cannot be achieved prior to the operation because intracerebral
schwannoma is a rare disease. Therefore, subtentorial schwannoma is
typically diagnosed on the basis of postoperative pathological
analysis. If the tumor can be completely removed, the prognosis of
the patient is good.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XJ conceived and designed the study. YG, ZQ, DL, WY,
LS and NL acquired the data, acquired and managed the patients and
provided the radiology images. CZ, BZ, YH and DS contributed to the
study design and analyzed and interpreted the data. XJ supervised
the study.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the China-Japan Union Hospital of Jilin University.
The patient provided written informed consent for the present
study.
Patient consent for publication
The patient and his father consented to contribute
his radiology images, hematology and pathological sections to
medical research, for copyright and ethics without controversy.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
EMA
|
epithelial membrane antigen
|
|
GFAP
|
glial fibrillary acidic protein
|
|
MRI
|
magnetic resonance imaging
|
References
|
1
|
Russel DS and Rubinstein LJ: Pathology of
tumors of the nervous system. Edward Arnold; London: 1989, 1.
|
|
2
|
Casadei GP, Komori T, Scheithauer BW,
Miller GM, Parisi JE and Kelly PJ: Intracranial parenchymal
schwannoma. A clinicopathological and neuroimaging study of nine
cases. J Neurosurg. 79:217–222. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Di Biasi C, Trasimeni G, Iannilli M,
Polettini E and Gualdi GF: Intracerebral schwannoma: CT and MR
findings. AJNR Am J Neuroradiol. 15:1956–1958. 1994.PubMed/NCBI
|
|
4
|
Paredes I, Jimenez Roldán L, Ramos A,
Lobato RD and Ricoy JR: Intraparenchymal schwannomas: Report of two
new cases studied with MRI and review of the literature. Clin
Neurol Neurosurg. 114:42–46. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gibson AA, Hendrick EB and Conen PE: Case
reports. Intracerebral schwannoma. Report of a case. J Neurosurg.
24:552–557. 1966. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
New PF: Intracerebral schwannoma. Case
report. J Neurosurg. 36:795–797. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ghatak NR, Norwood CW and Davis CH:
Intracerebral schwannoma. Surg Neurol. 3:45–47. 1975.PubMed/NCBI
|
|
8
|
Pialat J, Sindou M, Courjon J, Tommasi M
and Mansuy L: Un cas de neurinome intracerebral frontal. Lyon Med.
234:129–134. 1975.(In French).
|
|
9
|
Van Rensburg MJ, Proctor NS, Danziger J
and Orelowitz MS: Temporal lobe epilepsy due to an intracerebral
Schwannoma: Case report. J Neurol Neurosurg Psychiatry. 38:703–709.
1975. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hockley AD and Hendrick EB: Unilateral
proptosis and intracranial schwannoma. Surg Neurol. 4:509–512.
1975.PubMed/NCBI
|
|
11
|
Hahn J and Netsky M: Brain tumors of mixed
tissue origin: Staining procedures to distinguish glial from
connective tissue. South Med J. 70:539–542. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kasantikul V, Brown WJ and Cahan LD:
Intracerebral neurilemmoma. J Neurol Neurosurg Psychiatry.
44:1110–1115. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Auer RN, Budny J, Drake CG and Ball MJ:
Frontal lobe perivascular schwannoma. Case report. J Neurosurg.
56:154–157. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shalit MN, Toledo E and Sandbank U:
Intracerebral schwannoma. Acta Neurochir (Wein). 64:253–258. 1982.
View Article : Google Scholar
|
|
15
|
Gokay H, Izgi N, Barlas O and Erseven G:
Supratentorial intracerebral schwannomas. Surg Neurol. 22:69–72.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bruni P, Esposito S, Greco R and Oddi G:
Solitary intracerebral schwannoma in von Recklinghausen's disease.
Surg Neurol. 22:360–364. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Stefanko SZ, Vuzevski VD, Maas AI and van
Vroonhoven CC: Intracerebral malignant schwannoma. Acta
Neuropathol. 71:321–325. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schwartz AM and Sotrel A: Intracerebral
and intracerebellar neurilemoma. South Med J. 81:385–388. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ezura M, Ikeda H, Ogawa A and Yoshimoto T:
Intracerebral schwannoma: Case report. Neurosurgery. 30:97–100.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ghosh S and Chandy MJ: Solitary ectopic
intracerebral schwannoma. Br J Neurosurg. 6:163–166. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Frim DM, Ogilvy CS, Vonsattal JP and
Chapman PH: Is intracerebral schwannoma a developmental tumor of
children and young adults? Case report and review. Pediatr
Neurosurg. 18:190–194. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sarkar C, Mehta VS and Roy S:
Intracerebellar schwannoma. Case report. J Neurosurg. 67:120–123.
1987. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rodriguez-Salazar A, Carrillo R and de
Miguel J: Intracerebral schwannoma in a child: Report of a case.
Childs Brain. 11:69–72. 1984.PubMed/NCBI
|
|
24
|
Bruner JM, Hunphreys JH and Armstrong OL:
Intracerebral nerve sheath tumor. J Neuropathol Exp Neurol.
43:2961984. View Article : Google Scholar
|
|
25
|
Deng C and Wu P: Intracranial ectopic
neurilemoma: Report of two cases. J Nantong University. 1:46–47.
1985.
|
|
26
|
Aryanpur J and Long DM: Schwannoma of the
medulla oblongata. Case report. J Neurosurg. 69:446–449. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ladouceur D, Bergeron D, Lamarche JB and
Lamontagne L: Cystic schwannoma of the brainstem. Can J Neurol Sci.
16:357–360. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Redekop G, Elisevich K and Gilbert J:
Fourth ventricular schwannoma. Case report. J Neurosurg.
73:777–781. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tran-Dinh HD, Soo YS, O'Neil P and
Chaseling R: Cystic cerebellar schwannoma: Case report.
Neurosurgery. 29:296–299; discussion 299–300. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sharma RR, Gurusinghe NT, Lynch PG, Parekh
HC and Bertolis G: Intraparenchymatous schwannoma of the
cerebellum. Br J Neurosurg. 7:83–89. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sharma V and Newton G: Schwannoma of the
medulla oblongata. Br J Neurosurg. 7:427–429. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhao X, Wang Y and Duan M: Mixed brain
cysticercosis with intracerebral schwannoma: A case study. Chin J
Neurosurg. 10:2801994.(In Chinese).
|
|
33
|
Erongun U, Ozkal E, Acar O, Uygun A,
Kocaoğullar Y and Gungor S: Intracerebral schwannoma: Case report
and review. Neurosurg Rev. 19:269–274. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sharma MC, Karak AK, Gaikwad SB, Mahapatra
AK, Mehta VS and Sudha K: Intracranial intraparenchymal
schwannomas: A series of eight cases. J Neurol Neurosurg
Psychiatry. 60:200–203. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tsuiki H, Kuratsu J, Ishimaru Y, Nakahara
T, Kishida K, Takamura M, Marubayashi T and Ushio Y: Intracranial
intraparenchymal schwannoma: Report of three cases. Acta Neurochir
(Wein). 139:756–760. 1997. View Article : Google Scholar
|
|
36
|
Wang H, Shi J, Tan Q and Sun K: Case
report: Intracerebral schwannoma without the relationship of the
brain nerve trunk. JiangSu Med J. 26:869–870. 2000.(In
Chinese).
|
|
37
|
Andrade GC, Neto Paiva MA and Braga FM:
Thalamic intracerebral schwannoma: Case report. Arq Neuropsiquiatr.
60:308–313. 2002.(In Portuguese). View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Xu J and Wang Y: One case of intracerebral
schwannoma. Chin J Neurosurg. 18:862002.(In Chinese).
|
|
39
|
Lin J, Feng H, Li F, Zhao B and Guo Q:
Intraparenchymal schwannoma of the medulla oblongata. Case report.
J Neurosurg. 98:621–624. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhong Q, Zhang X and Zhang Y: A case of
schwannoma in brain parenchyma. Chin J Radiol. 38:1119–1120.
2004.(In Chinese).
|
|
41
|
Zeng E, Yuan X, Jiang W and Chen F:
Brainstem schwannoma: One case report. Chin J Minima Invas
Neurosur. 10:4732005.
|
|
42
|
Maiuri F, Colella G, D'Acunzi G and De
Caro Mdel B: Malignant intracerebellar schwannoma. J Neurooncol.
66:191–195. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Anton T, Guttierez J and Rock J: Tentorial
schwannoma: A case report and review of the literature. J
Neurooncol. 76:307–311. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Celikoğlu E, Hakan T and Bozbuğa M: Cystic
schwannoma of the falx cerebri. J Clin Neurosci. 14:589–592. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Xu D, Chen D and Zhang Y: Magnetic
resonance imaging of schwannoma in brain parenchyma (report of 3
cases). J Rare Uncom Dis. 14:7–10. 2007.
|
|
46
|
Yi Y, Zhou J and Lv S: A case of
schwannoma in brain parenchyma. 19:300–301. 2008.
|
|
47
|
Ishihara M, Miyagawa-Hayashino A,
Nakashima Y, Haga H, Takahashi JA and Manabe T: Intracerebral
schwannoma in a child with infiltration along perivascular spaces
resembling meningioangiomatosis. Pathol Int. 59:583–587. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Muzzafar S, Ketonen L, Weinberg JS and
Schellingerhout D: Imaging and clinical features of an intra-axial
brain stem schwannoma. AJNR Am J Neuroradiol. 31:567–569. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhu Q, Jiang X and Ren Z: Schwannoma in
brain parenchyma and literature review. Anhui Med J. 31:156–158.
2010.(In Chinese).
|
|
50
|
Consales A, Rossi A, Nozza P, Ravegnani M,
Garrè ML and Cama A: Intracerebral schwannoma in a child. Br J
Neurosurg. 24:306–308. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Guha D, Kiehl TR, Krings T and Valiante
TA: Intracerebral schwannoma presenting as classic temporal lobe
epilepsy. J Neurosurg. 117:136–140. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Srinivas R, Krupashankar D and Shasi V:
Intracerebral schwannoma in a 16-year-old girl: A case report and
review of the literature. Case Rep Neurol Med.
2013:1714942013.PubMed/NCBI
|
|
53
|
Lee S, Park SH and Chung CK:
Supratentorial intracerebral schwannoma: Its fate and proper
management. J Korean Neurosurg Soc. 54:340–343. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Ma L, Yang SX and Wang YR: Intracerebral
schwannoma mimicking parasagittal meningioma. J Craniofac Surg.
24:e541–e543. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
AlBatly AA, Zakzouk RS and Alhaidey AK: An
atypical case of intracerebral schwannoma. Pan Afr Med J.
18:3422014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Wilson BR, Steinberg JA, Snyder V, Jiang
MN and Carter BS: Histologic evidence for arteriovenous
malformation-like vasculature occurring within an intracerebral
Schwannoma: A case report and review of the literature. World
Neurosurg. 92:582 e589–582, e513. 2016. View Article : Google Scholar
|
|
57
|
Osborn AB and Salzman K: Diagnostic
imaging: Brain Amirsys. Salt Lake City: 2007
|
|
58
|
Feigin I and Ogata J: Schwann cells and
peripheral myelin within human central nervous tissues: The
mesenchymal character of Schwann cells. J Neuropathol Exp Neurol.
30:603–612. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Nelson E and Rennels M: Innervation of
intracranial arteries. Brain. 93:475–490. 1970. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Scherer SS: The biology and pathobiology
of Schwann cells. Curr Opin Neurol. 10:386–397. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Woodhoo A and Sommer L: Development of the
Schwann cell lineage: From the neural crest to the myelinated
nerve. Glia. 56:1481–1490. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Mirsky R, Woodhoo A, Parkinson DB,
Arthur-Farraj P, Bhaskaran A and Jessen KR: Novel signals
controlling embryonic Schwann cell development, myelination and
dedifferentiation. J Peripher Nerv Syst. 13:122–135. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Trotter J: The development of
myelin-forming glia: Studies with primary cell cultures and
immortalized cell lines. Perspect Dev Neurobiol. 1:149–154.
1993.PubMed/NCBI
|
|
64
|
Iu Chelyshev A and Saitkulov KI: The
development, phenotypic characteristics and communications of
Schwann cells. Usp Fiziol Nauk. 31:54–69. 2000.(In Russian).
|
|
65
|
Li Q and Xu Z: Nervous system tumor
pathology and genetics. People's Medical Publishing House Co.,
LTD.;
|




















